Abstract
Investigators have recently turned to the soil nematode Caenorhabditis elegans as a small animal infection model to study infectious disease. To extrapolate findings concerning bacterial pathogenesis from non-mammals to mammals, virulence factors should be conserved in function, independent of the infection model. Emerging from these studies is the observation that bacterial virulence regulatory networks function in a conserved manner across multiple hosts, including nematodes, mice, and plants. Several regulatory networks have been implicated in nematode innate immune function, and are being exploited in the C. elegans infection model to develop novel chemical therapies against bacterial pathogens.
Caenorhabditis elegans as a model host for infectious disease
Researchers require model infection systems in order to understand microbe-host interactions, study pathogenesis, and develop anti-microbial therapies and vaccines. For many investigators, the mouse Mus musculus is the workhorse of our trade. However, in recent years laboratories have looked to small, invertebrate models of infection such as the wax moth Galleria mellonella, Drosophila melanogaster, the soil nematode Caenorhabditis elegans, and plants such as alfalfa seeds or Arabidopsis thaliana, to name a few. Assessing virulence in multiple hosts is often useful for comparing the utility of different model organisms, identifying and understanding host response systems that are conserved through evolution, relating observed phenotypes to disease in humans and addressing the universality of bacterial virulence factors and mechanisms.
Over the past decade researchers have demonstrated that many bacteria pathogenic to humans, other animals and plants are also harmful to C. elegans. A natural bacteriophore, C. elegans is an attractive small animal infection model because of its transparent body amenable to microscopy, short generation time (∼ three days), large number of progeny from each nematode, simple and well-studied anatomy and development, genetic tractability and fully sequenced genome. Pathogenic bacteria are seeded on agar, nematodes, usually as fourth-stage (L4) larva, are placed on bacterial lawns, and then nematode viability, intestinal colony forming units (CFUs) and persistence of the bacteria are monitored over time. Two general phenotypes are observed: a slowly killing (slow kill) phenotype involving intestinal infection that occurs over several days and a fast killing (fast kill) phenotype whereby nematodes die over a few hours, usually mediated by a diffusible toxin. Additional phenotypes, and extra-intestinal infections have also been observed. For example, the nematode pathogen Microbacterium nematophilum, upon adherence to the post-anal cuticle, causes gross swelling of the underlying hypodermal tissues [1]. The bacterium Leucobacter chromiireducens subsp. solipictus enters through the vulva causing uterine infection and is correlated with reduced lifespan [2].
Approximately 35 Gram-positive, Gram-negative and fungal pathogens have been described to harm C. elegans, and a comprehensive listing and review of these organisms has been published elsewhere [3]. This review addresses a theme that has emerged from the growing body of literature on modeling bacterial disease in C. elegans. The regulatory networks of bacterial pathogens are conserved across multiple infection models and can therefore be studied effectively in C. elegans. Some of these bacterial regulators, particularly quorum sensing molecules, interact with innate immune function and this information is being exploited to develop novel antibacterial chemotherapies.
Regulatory networks
Quorum sensing
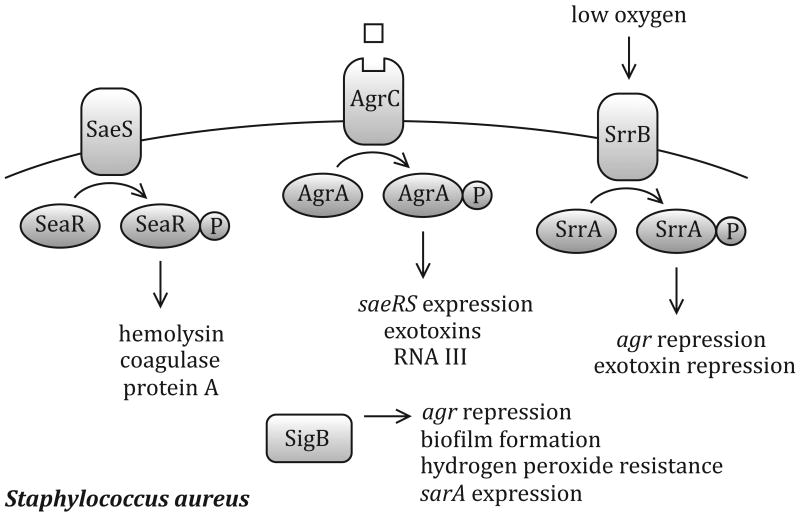
Many bacterial genes are activated by threshold quantities of small-molecule signals produced by individual cells of the same or different species. For several infections modeled in the C. elegans, quorum sensing is essential for virulence (Table 1). In Gram-positive bacteria, quorum sensing, or cell density signaling is mediated by small peptides. These peptides are generally secreted via ATP-binding cassette (ABC) transporters and sensing of these signal molecules occurs via two-component regulatory circuitry. The Gram-positive bacterium Staphylococcus aureus exhibits a slow kill phenotype over several days, and killing is attenuated in a strain deleted for the agr quorum sensing system [4]. AgrB and AgrD produce an octapeptide pheromone necessary for quorum sensing signaling, and agr mutants are attenuated in multiple animal infection models, including the mouse and a rabbit endocarditis model. AgrA and AgrC constitute a two-component response regulator that responds to the octapeptide, stimulating expression of the RNAIII effector molecule (Figure 1). RNAIII alters expression of a large number of virulence factors, including exoproteins, cell wall components, and regulatory loci in S. aureus. Similarly, the fsr quorum sensing system of Enterococcus faecalis is necessary for full virulence in the nematode [5, 6]. The fsr system shares homology with agr of S. aureus. The E. faecalis proteins FsrA and FsrC make up the two-component response regulator that senses the extracellular accumulation of a peptide lactone encoded at the C terminus of the FsrB protein. An fsrB mutant of E. faecalis is also attenuated for virulence in a mouse model of infection [6]. These data suggest that for Gram-positive pathogens, quorum-sensing control of virulence occurs in multiple hosts, including C. elegans.
Table 1.
Bacterial regulatory genes necessary for virulence in C. elegans
| Type of Regulator | Gene(s) | Organism | Non-Nematode Modelsa | Refs |
|---|---|---|---|---|
| Quorum Sensing | agr | S. aureus | Mouse, Rabbit | [4] |
| fsr | E. faecalis | Mouse | [5, 6] | |
| lasR | P. aeruginosa | Mouse, Arabidopsis | [9, 10] | |
| rhlR | P. aeruginosa | [9, 11] | ||
| phoB | P. aeruginosa | [12] | ||
| cep | Burkholderia sp. | Mouse, Wax Moth, Alfalfa | [13, 14] | |
| Alternate Sigma Factors | rpoS | S. entericab | Mouse | [15, 16] |
| rpoN | P. aeruginosa | Mouse, Arabidopsis | [17] | |
| sigB | S. aureus | Mousec | [4, 18] | |
| Two-Component Systems | saeRS | S. aureus | Mouse | [20, 48] |
| srrAB | S. aureus | Rabbitd | [20, 23] | |
| ompR/envZ | S. entericab | [16] | ||
| phoPQ | S. entericab | Mouse | [25, 26] | |
| gacAS | P. aeruginosa | Mouse, Arabidopsis | [10, 30, 32, 48] | |
| PA3946e | P. aeruginosa | [31] | ||
| PA4380e | P. aeruginosa | [32] |
Other models, in addition to C. elegans, in which the gene was also necessary for full virulence.
serovar Typhimurium.
sigB was necessary infection in a mouse catheter infection model, but not in an intravenous model.
In contrast to the nematode model, overexpression of srrAB resulted in decreased virulence in the rabbit model.
Putative gene identified in P. earuginosa.
Figure 1.
Regulatory networks required for infection of C. elegans by Staphyolococcus aureus. The two-component system SaeRS regulates hemolysin, coagulase and protein A production [49], while SrrAB represses agr and exotoxin expression in response to low oxygen levels [22]. The AgrAC quorum sensing system controls saeRS and exotoxin expression [50, 51] in response to an octapeptide pheromone (light green square). The alternative sigma factor SigB is involved in regulation of agr [52], biofilm formation [53], hydrogen peroxide resistance [54] and sarA [55].
For Gram-negative bacteria, multiple quorum sensing systems have been described. Acylhomoserine lactones (AHL) are the best described of these and they control a wide range of phenotypes. AHLs were first elucidated in Vibrio fischeri, where the molecules, synthesized by LuxI, freely diffuse through the bacterial membrane. When the AHL concentration reaches a threshold level reflecting a high cell density they bind, in this case, to the LuxR regulator stimulating bioluminescence. Homologs of the LuxI AHL autoinducer synthase and the LuxR regulator have been identified in many Gram-negative bacteria. Interestingly, orphaned LuxR-type regulators, in the absence of a cognate LuxI-type synthase have also been described, and are hypothesized, in some instances, to be involved in sensing interspecies population densities [7, 8].
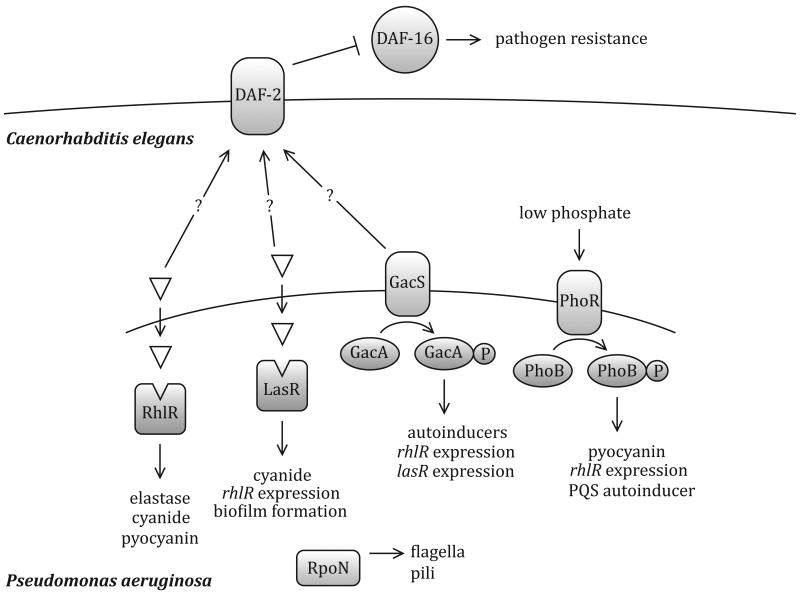
Quorum sensing signaling is required for Pseudomonas aeruginosa virulence in the nematode model. This Gram-negative pathogen can exhibit either a fast kill or slow kill phenotype in the C. elegans infection model depending on the type of medium on which the nematodes are propagated. Both the LasR and RhlR quorum sensing regulators, which bind to the N-3-oxo-dodecanoyl-L-homoserine lactone (3O-C12-AHL) and N-buytryl-L-homoserine lactone (N-butyryl-AHL), respectively, are necessary for fast or paralytic killing, occurring over several hours [9, 10]. In a slow kill assay where death occurs at approximately three days, mutation in both the LasR and RhlR quorum sensing systems of P. aeruginosa were also necessary to attenuate killing [11]. Interestingly, Zaborin et al. describe a ‘red death’ phenotype caused by P. aeruginosa where the gut tube turns red during phosphate depletion, and the signaling systems involved were PhoB (Figure 2), a MvfR-PQS quorum sensing pathway and the siderophore pyoverdin [12].
Figure 2.
Regulatory networks required for infection of C. elegans by Pseudomonas aeruginosa. GacAS regulates rhlR, lasR, and AHL autoinducers [29]. PhoRB also regulates the quorum sensing components rhlR and the PQS autoinducer, as well pyocyanin production [56]. The quorum sensing regulator LasR binds to the autoinducer produced by LasI, and regulates biofilm formation [57]. LasR is also necessary for cyanide production [58] as well as expression of another quorum sensing system rhlR [59]. RhlR regulates production of cyanide [58], elastase and pyocyanin [60]. The alternative sigma factor RpoN is involved in regulation of flagella and pili [61]. Light green triangles represent AHLs used in quorum sensing. P. aeruginosa overcomes C. elegans host immune defenses by downregulating (blunt arrow) the DAF-16 transcription factor [36]. This phenotype requires the nematode insulin-like receptor DAF-2, and the P. aeruginosa GacA protein and quorum-sensing regulators LasR and RhlR.
Introduction of the associate iron complex in the intestine of C. elegans or a mouse model caused death in both organisms by epithelial cell disruption and apoptosis.
Similar to P. aeruginosa, Burkholderia cepacia complex (BCC) are a group of ubiquitous Gram-negative bacteria that cause opportunistic infections in the lungs of cystic fibrosis patients. B. cepacia kills nematodes over a two to three day period, and the cep quorum sensing system is necessary for killing [13, 14]. With the goal of identifying virulence factors necessary for disease in four host organisms, Uehlinger et al. found that quorum sensing signaling was necessary for full virulence in all of those tested, including C. elegans, the wax moth, alfalfa and mice [14]. Consistent with observations concerning Gram-positive pathogens, quorum sensing is necessary for P. aeruginosa and BCC-mediated death observed in C. elegans and is conserved in this function across multiple infection systems.
Alternative sigma factors
Alternative sigma factors confer upon the RNA polymerase holoenzyme the ability to bind to different promoters. This allows for the spatiotemporal control of specific regulons, or collections of genes controlled by a common regulatory protein. Alternative sigma factors often control large numbers of genes, and as such, have been implicated in the regulation of pathogenesis. A few alternative sigma factors are necessary for full virulence in the C. elegans model (Table 1). In Salmonella enterica serovar Typhimurium, the alternative sigma factor σS, encoded by rpoS, regulates gene expression in response to environmental stresses such as starvation, oxidation and low pH, signals relevant to pathogenesis, and σs also regulates the expression of Salmonella plasmid-encoded virulence genes [15]. rpoS mutants are less virulent in both C. elegans and in mouse models of disease [15, 16]. In P. aeruginosa, the alternative sigma factor σN, encoded by rpoN, is necessary for full virulence in C. elegans, mice, and Arabidopsis thaliana [17].
In S. aureus, the alternative sigma factor σB, encoded by sigB, positively regulates a variety of virulence related phenotypes, such as biofilm formation and hydrogen peroxide resistance, in response to environmental stress (Figure 1). Mutations in sigB lead to moderate attenuation of virulence in the C. elegans model [4] and have varying effects in mammalian hosts. For example, in mice, sigB mutants were less virulent in a catheter infection model, but not in an intravenous model [18]. Interestingly, although most clinical isolates of S. aureus are sigB positive, some are sigB deficient, indicating that although σB appears to contribute to virulence, it is not required for infection [19]. In contrast, the σS and σN alternate sigma factors of Salmonella and P. aeruginosa, respectively, are required for virulence in nematodes and mice. These regulators enable the bacterium to respond to environmental stresses such as oxidative damage, low pH and nutrient deprivation that are encountered in otherwise unrelated host organisms. The specific nature of at least some of these stresses perhaps could be best elucidated in the nematode.
Two-component systems
Two-component systems, consisting of a sensor kinase and a response regulator, are one of the main systems bacteria use to sense and respond to their environment. The membrane-associated sensor kinase undergoes autophosphorylation in response to a chemical or physical extracellular signal, and then transfers its phosphate group to the response regulator. Phosphorylation of the response regulator modifies the function of the protein and affects change within in the cell, often by altering gene expression or, for example, flagellar motility.
Two-component regulatory systems are necessary for virulence in many pathogenic bacteria. In S. aureus, two two-component regulatory systems, saeRS and srrAB, are necessary for full virulence in C. elegans [20]. SaeRS interacts with the agr quorum sensing system to integrate environmental information in the cell and regulates the transcription of many virulence factors [21]. saeRS mutants are less virulent in mice than wild-type S. aureus, indicating that this regulatory system is conserved across hosts. However, the SrrAB two-component system regulates virulence in S. aureus by downregulating several virulence factors in response to low oxygen conditions [22], and the overexpression of srrAB results in reduced virulence in a rabbit endocarditis model [23]. Thus two-component regulatory systems, sensing environmental and quorum sensing signals, play a pivotal role in the ability of S. aureus to cause disease in C. elegans and mammalian models of infection (Table 1).
In the PhoPQ system of Salmonella, PhoQ undergoes autophosphorylation in response to low Mg2+ concentration, a signal indicative of being within a host environment. PhoQ then phosphorylates PhoP, which in its activated form induces expression of multiple virulence factors [24]. The PhoPQ regulatory system has long been known to be involved in Salmonella virulence, and phoP mutants also exhibit reduced virulence in mammalian models [25]. Both a phoP-phoQ double knockout mutant and a mutant with a point mutation in phoQ exhibit reduced killing of C. elegans in a slow kill assay [26], indicating that it is also conserved across hosts. In addition to PhoPQ, the response regulator OmpR, which controls expression of the type III secretion system encoded within pathogenicity island 2 (SPI-2) [27] necessary for survival inside host cells and systemic infection in mice [28], is also necessary for full virulence of Salmonella in the C. elegans model [16].
Multiple two-component systems are required for virulence in P. aeruginosa as well (Figure 2). One such system, GacAS, regulates the expression of multiple virulence factors, including the quorum-sensing molecule N-butyryl-AHL [29]. GacAS is also required for full virulence in the mouse and Arabidopsis thaliana models of infection [30], again highlighting the extent to which two-component systems are conserved in the virulence of multiple hosts. In addition to GacAS, two other genes encoding putative two-component regulatory systems, PA3946 and PA4380, are necessary for full virulence in C. elegans [31, 32]. Similar to quorum sensing networks and alternative sigma factors, two component systems that have a role in mammalian infections are repeatedly identified in C. elegans screens of very different pathogens. These regulatory networks can be effectively investigated using the C. elegans infection system, which offers an unexploited advantage. The specific in vivo triggers for many virulence-associated two-component systems and alternate sigma factors are not known. The simplicity of the C. elegans intestine and the availability of complete mutant sets, interaction maps and RNA interference (RNAi) tools could make it possible to elucidate the ligands for many sensor kinases in this model.
Innate immunity
The nematode infection model has been instrumental in deciphering host innate immunity (for reviews see Ref. [33, 34]), and several examples now exist whereby gene-gene interactions between host innate immune components and bacterial regulatory circuitry have been observed. In Salmonella, the PhoPQ regulon and SPI-1 and SPI-2 pathogenicity islands encoding type III secretion systems are induced during infection, and are required for persistence in the nematode gut [35]. Defects in persistence due to mutation in SPI-2 and phoP could be rescued by RNAi-directed downregulation of the nematode gene spp-1. The gene spp-1 encodes an anti-microbial peptide that is a member of the saposin-like protein family, part of nematode innate immunity.
In another well-studied example, nematode resistance to P. aeruginosa killing requires the insulin-like receptor DAF-2 and transcription factor DAF-16 (Figure 2), while downregulation of DAF-16 and associated genes causes nematodes to be sensitive to P. aeruginosa, a phenotype requiring the two-component response regulator GacA and the LasR and RhlR quorum sensing regulators [36]. The nematode DAF-16 transcriptional factor controls multiple phenotypes including resistance to pathogens, part of innate immunity, but also aging and dauer formation [37]. These two examples in Salmonella and P. aeruginosa represent the beginnings of our understanding of interkingdom signaling between bacteria and the nematode model host.
Interestingly, although bacterial regulatory networks identified in the nematode model are important in multiple host systems and C. elegans has been used to identify host innate immune system components that are involved in resistance to multiple pathogens, available information suggests that the specific bacterial effectors and their nematode receptors may not be so conserved. Additionally, C. elegans has been used to characterize bacterial surface proteins and toxins, and it also appears well suited to identify and evaluate conserved mechanisms of global significance in both bacteria and hosts.
Exploiting regulatory networks for therapeutic drug discovery
Some researchers might question the use of a small animal drug screening system since the molecular mechanisms of nematode killing are largely unknown, and, in most instances, disease symptoms are clearly dissimilar to disease observed in humans. For example, E. faecalis merely causes distention of the nematode gut lumen [5] and a specific mode of killing has not been described. Normally a harmless member of the intestinal commensal flora, E. faecalis causes nosocomial bacteremia, and surgical wound and urinary tract infections in humans. Yersinia spp. kill C. elegans by forming biofilms over the head of the nematode [38], ultimately starving them to death. This mechanism is allied to ‘flea-blocking’, the formation of biofilms in the gut of the flea, which blocks the flea digestive tract and stimulates them to bite new hosts [39], while in humans, the symptoms of bubonic plaque are swollen lymph glands, fever, chills, headache, and exhaustion. P. aeruginosa is an opportunistic pathogen that infects immunocompromised individuals, e.g. affecting the lungs of cystic fibrosis patients, causing urinary tract infections and sepsis. In humans with cystic fibrosis, P. aeruginosa forms biofilms, producing large quantities of alginate, stimulating an immune response that ultimately leads to tissue destruction. One known mechanism of nematode death of C.elegans by P. aeruginosa is the quorum sensing dependent production of hydrogen cyanide, resulting in fast killing [31]. Hydrogen cyanide production by P. aeruginosa is an uncommon exception: the specific molecular mechanism of nematode death caused by bacterial pathogens, for both the fast and slow kill phenotypes, remains largely obscure and unrelated to infectious disease observed in humans.
In spite of the differences in pathogenesis mechanisms between nematodes and mammals, there are significant advantages in using the C. elegans infection model to develop chemotherapeutic agents against certain bacteria. Not the least is the limitations of larger mammalian models: high throughput chemical library screens cannot be performed in mice. Targeting quorum sensing signaling and other global regulatory pathways seems particularly prudent because of the extensive list of virulence-associated phenotypes regulated via this mechanism. Biofilms are thought to be an integral part of most, if not all, bacterial diseases, and require cell-to-cell signaling, or quorum sensing for maturation. In addition to biofilms, a wide range of virulence factors and phenotypes involved in disease of humans are controlled by quorum sensing, including capsule expression, siderophores, pilus assembly, and toxin production to list a few [40]. Because regulation of virulence determinants occurs in a manner conserved across hosts, independent of the specific mechanisms that ultimately cause damage, targeting of signaling pathways can be effectively pursued in the C. elegans model. The nematode is amenable to high-throughput screening, it provides a built-in check for drug toxicity to a whole eukaryotic organism, and regulatory networks controlling bacterial virulence are conserved across invertebrate and vertebrate hosts. Thus the C. elegans infection model is a powerful tool for drug discovery.
An emerging utility of the C. elegans infection model is the ability to identify and study anti-infective agents targeted at specific bacterial regulatory networks [41-43]. The current restricted pipeline for antimicrobial drugs means that new drugs are sorely needed and new screening technologies could help to identify alternate strategies for dealing with infecting pathogens. Anti-infective molecules target virulence factors or associated regulatory networks necessary for causing host damage and disease. They do not exert broad-based selective pressure like bacteriostatic and bacteriocidal drugs and therefore are less likely to select for resistance in non-target organisms. Since methods for identifying such compounds are only being developed today, there is a good chance that existing chemical libraries will contain compounds with anti-infective activity. Robotics and high throughput technologies have allowed researchers to screen libraries of compounds and plant extracts for anti-infectives in the nematode model. In one such study, 37,000 compounds and extracts were screened, giving 28 hits, including 6 new structural classes of drugs that enhance survival of C. elegans when infected by E. faecalis [42]. In addition to anti-virulence compounds, performing screens of this nature in a simple organism such as C. elegans makes it possible to identify hits with in vivo activity, which could be missed in an in vitro screen.
C. elegans can also be used to test the in vivo effects of specific classes of anti-infectives as with the evaluation of quorum sensing quenchers against P. aeruginosa [44] and Burkholderia [43]. Here acylases, and related enzymes, that degrade AHL molecules extend the lifespan of nematodes in the presence of these Gram-negative pathogens. The PvdQ protein degrades the 3O-C12-AHL of P. aeruginosa while a lactonase, AiiA, from Bacillus thuringiensis was used to inhibit AHL-mediated killing of C. elegans by Burkholderia cepacia. An antagonist of a LuxN-type receptor protected C. elegans from quorum sensing dependent killing by Chromobacterium violaceum [45], furthering the notion that therapeutic drug development can be achieved in this small, invertebrate infection model.
Concluding remarks
In developing infection models to study bacterial diseases, researchers typically must choose animals that faithfully mimic related illnesses in humans. On the bacterial side, virulence factors necessary for causing clinical signs and symptoms ideally should be conserved of function within the laboratory animal host. As an example, the type III secretion systems of Gram-negative pathogens are important virulence factors in human disease and these systems inject proteins into host cells to subvert their function. For Salmonella, both type III secretion systems, encoded within SPI-1 and SPI-2, are required for disease in humans and for persistent infection in C. elegans [35]. However, unlike those of Salmonella, the type III secretion systems of P. aeruginosa and enteropathogenic E. coli, both thought to be necessary for virulence in humans, appear to be host specific as they are not necessary for infection of C. elegans [46, 47]. Thus type III secretion system function cannot be universally modeled in the nematode infection system.
One might hypothesize that type III secretion systems, adhesins and other factors that interact directly with host cells are host-specific virulence factors produced in response to direct in vivo selective pressures. However, after a little over a decade of using C. elegans to study bacterial disease it has become clear that regulatory networks are highly conserved through evolution, across hosts even when downstream effectors may differ. Studying the genetic basis for quorum sensing signaling, alternative sigma factors, and two-component systems in the nematode model has been enlightening because the regulatory network[GT1] probably derives from the conserved nature of environmental stresses encountered by bacterial pathogens within a host and the requirement for regulatory networks to respond to these signals. Yet many questions concerning bacterial regulators within the nematode host remain (Box 1). Furthermore, targeting regulatory networks offers hope of developing anti-infective therapies to fight infection in the current era of increasing antibiotic resistance. Finally, the exciting discovery of bacterial regulatory networks interacting with C. elegans innate immunity strikes at the essence of infectious disease, communication between microbe and host.
Box 1. Questions for future research.
What additional bacterial regulatory networks interact with C. elegans innate immune components?
Can in vivo biofilms be modeled effectively in the nematode? If so, what additional regulators control their formation?
What are the ligands for the sensor kinases of the bacterial two-component regulators within the nematode intestine?
Do quorum sensing regulators control pathogenesis during mixed infections with multiple pathogens, or pathogens and commensal organisms?
Are there additional regulatory networks that are amenable for targeting in developing anti-infective therapies?
Acknowledgments
We thank Drs. Iruka Okeke and Creg Darby for editorial suggestions on the manuscript, and members of our lab for helpful discussion. Work for developing the C. elegans infection model for E. coli pathotypes was supported by DARPA grant DAAD19-03-1-0055 awarded to J.L.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hodgkin J, et al. A novel bacterial pathogen, Microbacterium nematophilum, induces morphological change in the nematode C. elegans. Curr Biol. 2000;10:1615–1618. doi: 10.1016/s0960-9822(00)00867-8. [DOI] [PubMed] [Google Scholar]
- 2.Muir RE, Tan MW. Virulence of Leucobacter chromiireducens subsp. solipictus to Caenorhabditis elegans: characterization of a novel host-pathogen interaction. Appl Environ Microbiol. 2008;74:4185–4198. doi: 10.1128/AEM.00381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sifri CD, et al. The worm has turned--microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 2005;13:119–127. doi: 10.1016/j.tim.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Sifri CD, et al. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect Immun. 2003;71:2208–2217. doi: 10.1128/IAI.71.4.2208-2217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garsin DA, et al. A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci U S A. 2001;98:10892–10897. doi: 10.1073/pnas.191378698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sifri CD, et al. Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect Immun. 2002;70:5647–5650. doi: 10.1128/IAI.70.10.5647-5650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malott RJ, et al. A Burkholderia cenocepacia orphan LuxR homolog is involved in quorum-sensing regulation. J Bacteriol. 2009;191:2447–2460. doi: 10.1128/JB.01746-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patankar AV, Gonzalez JE. An orphan LuxR homolog of Sinorhizobium meliloti affects stress adaptation and competition for nodulation. Appl Environ Microbiol. 2009;75:946–955. doi: 10.1128/AEM.01692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darby C, et al. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1999;96:15202–15207. doi: 10.1073/pnas.96.26.15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan MW, et al. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci U S A. 1999a;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steindler L, et al. LasI/R and RhlI/R quorum sensing in a strain of Pseudomonas aeruginosa beneficial to plants. Appl Environ Microbiol. 2009;75:5131–5140. doi: 10.1128/AEM.02914-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaborin A, et al. Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. Proc Natl Acad Sci U S A. 2009;106:6327–6332. doi: 10.1073/pnas.0813199106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kothe M, et al. Killing of Caenorhabditis elegans by Burkholderia cepacia is controlled by the cep quorum-sensing system. Cell Microbiol. 2003;5:343–351. doi: 10.1046/j.1462-5822.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 14.Uehlinger S, et al. Identification of specific and universal virulence factors in Burkholderia cenocepacia strains by using multiple infection hosts. Infect Immun. 2009;77:4102–4110. doi: 10.1128/IAI.00398-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nickerson CA, Curtiss R., 3rd Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect Immun. 1997;65:1814–1823. doi: 10.1128/iai.65.5.1814-1823.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labrousse A, et al. Caenorhabditis elegans is a model host for Salmonella typhimurium. Curr Biol. 2000;10:1543–1545. doi: 10.1016/s0960-9822(00)00833-2. [DOI] [PubMed] [Google Scholar]
- 17.Hendrickson EL, et al. Differential roles of the Pseudomonas aeruginosa PA14 rpoN gene in pathogenicity in plants, nematodes, insects, and mice. J Bacteriol. 2001;183:7126–7134. doi: 10.1128/JB.183.24.7126-7134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenz U, et al. The alternative sigma factor sigma B of Staphylococcus aureus modulates virulence in experimental central venous catheter-related infections. Microbes Infect. 2008;10:217–223. doi: 10.1016/j.micinf.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson-Kanth A, et al. Natural human isolates of Staphylococcus aureus selected for high production of proteases and alpha-hemolysin are sigmaB deficient. Int J Med Microbiol. 2006;296:229–236. doi: 10.1016/j.ijmm.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 20.Bae T, et al. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci U S A. 2004;101:12312–12317. doi: 10.1073/pnas.0404728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogasch K, et al. Influence of the two-component system SaeRS on global gene expression in two different Staphylococcus aureus strains. J Bacteriol. 2006;188:7742–7758. doi: 10.1128/JB.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yarwood JM, et al. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J Bacteriol. 2001;183:1113–1123. doi: 10.1128/JB.183.4.1113-1123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pragman AA, et al. Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J Bacteriol. 2004;186:2430–2438. doi: 10.1128/JB.186.8.2430-2438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller SI, et al. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aballay A, et al. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr Biol. 2000;10:1539–1542. doi: 10.1016/s0960-9822(00)00830-7. [DOI] [PubMed] [Google Scholar]
- 27.Feng X, et al. Dual regulation by phospho-OmpR of ssrA/B gene expression in Salmonella pathogenicity island 2. Mol Microbiol. 2003;48:1131–1143. doi: 10.1046/j.1365-2958.2003.03502.x. [DOI] [PubMed] [Google Scholar]
- 28.Ochman H, et al. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci U S A. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reimmann C, et al. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol. 1997;24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- 30.Rahme LG, et al. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher LA, Manoil C. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J Bacteriol. 2001;183:6207–6214. doi: 10.1128/JB.183.21.6207-6214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garvis S, et al. Caenorhabditis elegans semi-automated liquid screen reveals a specialized role for the chemotaxis gene cheB2 in Pseudomonas aeruginosa virulence. PLoS Pathog. 2009;5:e1000540. doi: 10.1371/journal.ppat.1000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DH, Ausubel FM. Evolutionary perspectives on innate immunity from the study of Caenorhabditis elegans. Curr Opin Immunol. 2005;17:4–10. doi: 10.1016/j.coi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Kurz CL, Ewbank JJ. Caenorhabditis elegans: an emerging genetic model for the study of innate immunity. Nat Rev Genet. 2003;4:380–390. doi: 10.1038/nrg1067. [DOI] [PubMed] [Google Scholar]
- 35.Alegado RA, Tan MW. Resistance to antimicrobial peptides contributes to persistence of Salmonella typhimurium in the C. elegans intestine. Cell Microbiol. 2008;10:1259–1273. doi: 10.1111/j.1462-5822.2008.01124.x. [DOI] [PubMed] [Google Scholar]
- 36.Evans EA, et al. Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog. 2008;4:e1000175. doi: 10.1371/journal.ppat.1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurz CL, Tan MW. Regulation of aging and innate immunity in C. elegans. Aging Cell. 2004;3:185–193. doi: 10.1111/j.1474-9728.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 38.Darby C, et al. Caenorhabditis elegans: plague bacteria biofilm blocks food intake. Nature. 2002;417:243–244. doi: 10.1038/417243a. [DOI] [PubMed] [Google Scholar]
- 39.Darby C, et al. Identification of gmhA, a Yersinia pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect Immun. 2005;73:7236–7242. doi: 10.1128/IAI.73.11.7236-7242.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 41.Ball AR, et al. Conjugating berberine to a multidrug efflux pump inhibitor creates an effective antimicrobial. ACS Chem Biol. 2006;1:594–600. doi: 10.1021/cb600238x. [DOI] [PubMed] [Google Scholar]
- 42.Moy TI, et al. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem Biol. 2009;4:527–533. doi: 10.1021/cb900084v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wopperer J, et al. A quorum-quenching approach to investigate the conservation of quorum-sensing-regulated functions within the Burkholderia cepacia complex. Appl Environ Microbiol. 2006;72:1579–1587. doi: 10.1128/AEM.72.2.1579-1587.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papaioannou E, et al. Quorum-quenching acylase reduces the virulence of Pseudomonas aeruginosa in a Caenorhabditis elegans infection model. Antimicrob Agents Chemother. 2009;53:4891–4897. doi: 10.1128/AAC.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swem LR, et al. A quorum-sensing antagonist targets both membrane-bound and cytoplasmic receptors and controls bacterial pathogenicity. Mol Cell. 2009;35:143–153. doi: 10.1016/j.molcel.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mellies JL, et al. The global regulator Ler is necessary for enteropathogenic Escherichia coli colonization of Caenorhabditis elegans. Infect Immun. 2006;74:64–72. doi: 10.1128/IAI.74.1.64-72.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wareham DW, et al. The Pseudomonas aeruginosa PA14 type III secretion system is expressed but not essential to virulence in the Caenorhabditis elegans-P. aeruginosa pathogenicity model. FEMS Microbiol Lett. 2005;242:209–216. doi: 10.1016/j.femsle.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 48.Tan MW, et al. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci U S A. 1999b;96:2408–2413. doi: 10.1073/pnas.96.5.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giraudo AT, et al. The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Arch Microbiol. 1997;168:53–58. doi: 10.1007/s002030050469. [DOI] [PubMed] [Google Scholar]
- 50.Dinges MM, et al. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novick RP, Jiang D. The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology. 2003;149:2709–2717. doi: 10.1099/mic.0.26575-0. [DOI] [PubMed] [Google Scholar]
- 52.Bischoff M, et al. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J Bacteriol. 2001;183:5171–5179. doi: 10.1128/JB.183.17.5171-5179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lauderdale KJ, et al. Interconnections between Sigma B, agr, and proteolytic activity in Staphylococcus aureus biofilm maturation. Infect Immun. 2009;77:1623–1635. doi: 10.1128/IAI.01036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kullik I, et al. Deletion of the alternative sigma factor sigmaB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheung AL, et al. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect Immun. 1999;67:1331–1337. doi: 10.1128/iai.67.3.1331-1337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jensen V, et al. RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and -independent pathways. J Bacteriol. 2006;188:8601–8606. doi: 10.1128/JB.01378-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davies DG, et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 58.Pessi G, Haas D. Transcriptional control of the hydrogen cyanide biosynthetic genes hcnABC by the anaerobic regulator ANR and the quorum-sensing regulators LasR and RhlR in Pseudomonas aeruginosa. J Bacteriol. 2000;182:6940–6949. doi: 10.1128/jb.182.24.6940-6949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pesci EC, Iglewski BH. The chain of command in Pseudomonas quorum sensing. Trends Microbiol. 1997;5:132–134. doi: 10.1016/S0966-842X(97)01008-1. [DOI] [PubMed] [Google Scholar]
- 60.Brint JM, Ohman DE. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Totten PA, et al. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol. 1990;172:389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]