Abstract
Objective
We examined the temporal relationship between scleroderma development and malignancy, and evaluated whether this differed by autoantibody status among affected patients.
Methods
Participants had a diagnosis of scleroderma, cancer, an available serum sample, and a cancer pathology specimen. Sera were tested for autoantibodies against topoisomerase I, centromere, and RNA polymerase I/III by immunoprecipitation and/or ELISA. Clinical and demographic characteristics were compared across autoantibody categories. Expression of RNA polymerases I and III was evaluated by immunohistochemistry using cancerous tissue from patients with anti-RNA polymerase antibodies.
Results
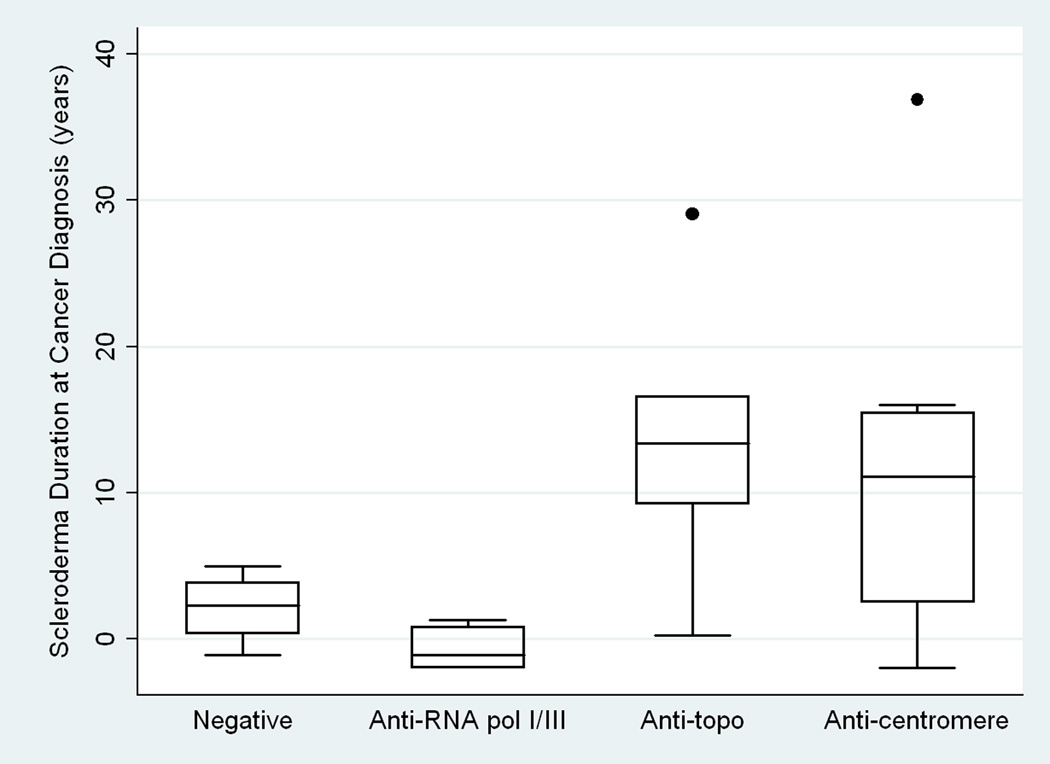
Twenty three subjects were enrolled. Six subjects tested positive for anti-RNA polymerase I/III (Pol), 5 for anti-topoisomerase I (Topo), 8 for anti-centromere (CENP), and 4 recognized none of these antigens (Negative). The median duration of scleroderma at cancer diagnosis differed significantly between groups: −1.2 years (Pol), +13.4 years (Topo), +11.1 years (CENP), and +2.3 years (Negative) (p=0.027). RNA polymerase III demonstrated a robust nucleolar staining pattern in 4 of 5 available tumors from patients with antibodies to RNA polymerase I/III. In contrast, nucleolar RNA polymerase III staining was not detected in any of 4 examined tumors in the RNA polymerase antibody-negative group (p=0.048).
Conclusions
There is a close temporal relationship between onset of cancer and scleroderma in patients with antibodies to RNA polymerase I/III, which is distinct from scleroderma patients with other autoantibody specificities. In this study, autoantibody response and tumor antigen expression are associated. We propose that malignancy may initiate the scleroderma-specific immune response and drive disease in a subset of scleroderma patients.
Introduction
Patients with scleroderma may have an increased risk of malignancy compared to the general population (1–6). A wide array of cancers has been reported in scleroderma, although lung and breast cancers are thought to be the most common (3, 4, 6, 7). Although it is controversial whether malignancy risk is truly increased in scleroderma patients, reports detailing a close, at times concurrent onset of scleroderma and malignancy raise the possibility of malignancy triggering an autoimmune disease process in a subset of scleroderma patients (8–10). Among scleroderma patients, this tight temporal association is most striking in breast cancer, with the majority of cases developing scleroderma within 18 months of cancer diagnosis (11–14). In 2 case series reviewing scleroderma patients with breast cancer, it has been estimated that up to 50% of breast cancer cases closely preceded or were diagnosed simultaneously with scleroderma (12, 14). Additionally, it is reported that prompt therapy of a malignancy can abrogate the scleroderma disease process (8, 9, 15), suggesting that in these unique cases the biological response to the malignancy or the malignant process itself may be driving the expression of scleroderma.
Despite this reported association between malignancy and scleroderma onset, few studies have evaluated scleroderma disease characteristics that associate with the presence or risk of malignancy, and little is known about potential mechanisms underlying this connection. We hypothesize that scleroderma-specific autoantibody production in a subset of patients with scleroderma is a manifestation of the immune response to tumor antigens that may associate with or induce the scleroderma disease process. In this study, we evaluated whether clinical characteristics, including the temporal relationship between scleroderma and malignancy onset, differed by autoantibody status among patients with scleroderma and cancer. After demonstrating a temporal clustering between cancer onset and scleroderma in the RNA polymerase antibody-positive group, we investigated the expression of RNA polymerases I and III in cancerous tissue of these scleroderma patients compared to cancers from RNA polymerase antibody-negative patients, as well as noncancerous tissue from controls.
Patients and Methods
Patients
Participants were scleroderma patients followed at the Johns Hopkins Scleroderma Center who had (i) a new or past diagnosis of malignancy, (ii) an available serum sample, and (iii) an existing cancer pathology specimen available for histologic confirmation of cancer diagnosis. Among established patients, subjects were identified as having had a prior diagnosis of malignancy from the Center’s research database. Eligibility included informed consent and meeting either American College of Rheumatology criteria for scleroderma (16), having at least 3 of 5 features of the CREST syndrome (calcinosis, Raynaud's, esophageal dysmotility, sclerodactyly, telangiectasias), or having definite Raynaud’s phenomenon, abnormal nailfold capillaries and the presence of a scleroderma-specific autoantibody. For all patients, the closest available serum sample to cancer diagnosis was studied.
Demographic data, scleroderma subtype (limited versus diffuse skin disease), disease duration, date of cancer diagnosis, smoking status (never, former, or current), most recent Medsger disease severity scores (17), peak modified Rodnan skin scores (18), medication use prior to cancer diagnosis, autoantibody status, pulmonary function tests, and echocardiogram data were obtained from the Center’s database and, when necessary, medical chart review. Patients were classified according to LeRoy et al (19) as having limited cutaneous disease if scleroderma skin changes were noted only on the face and/or distal to the knees and elbows; diffuse cutaneous disease involved the trunk and/or proximal extremities. Disease duration was defined as the period of time from the first non-Raynaud’s phenomenon symptom to the date of cancer diagnosis. Cancer onset was defined by the date of cancer diagnosis. All forced vital capacity (FVC) and diffusion capacity (DLCO) results were standardized by age, gender and height according to NHANES criteria and Knudson et al., respectively (20, 21). Interstitial lung disease (ILD) was defined by an FVC < 70% predicted, and pulmonary hypertension (PH) was defined by a right ventricular systolic pressure (RVSP) ≥ 45mmHg on resting echocardiography (22) or by right heart catheterization evidence of pulmonary arterial hypertension.
All studies on human materials were performed on samples provided in compliance with Johns Hopkins IRB and HIPAA regulations. Surgical procedures were performed for patient management; the research tissue used in our studies was in excess of the biopsied tissue required for routine diagnostic purposes. Serum samples were collected under an IRB approved protocol. Each serum was tested for autoantibodies against topoisomerase I by ELISA using commercially available kits (Inova Diagnostics). The presence of antibodies against RNA polymerase I/III in each patient serum was assessed using two different assays. Sera were tested by immunoprecipitation using radiolabeled Hela cell extracts. The presence of anti-RNA polymerase I/III antibodies was determined based on co-migration of immunoprecipitated bands with those detected using an RNA polymerase I/III scleroderma reference serum, which was included in each precipitation set (data not shown). The findings for RNA polymerase III were also validated using a commercially available RNA polymerase III ELISA kit (Inova Diagnostics); in all cases, the identified sera had antibodies against RNA polymerase using both assays. The presence of anti-centromere antibodies was determined by immunoprecipitation using in vitro transcription translated 35S-methionine labeled centromere protein B as described (23).
Immunohistochemistry
Paraffin sections from affected cancerous tissue in 6 patients with RNA polymerase antibodies were initially available for study. Since the patient tissues were obtained and paraffin embedded by various pathology units at different times, we first assessed fixation variation (and subsequent loss of antigenicity) by staining sections of each tissue with a monoclonal antibody to CD31 (Dako). This evaluation confirmed excellent tissue preservation and comparable antigenicity in tissues from 4 different patients (subjects 1 and 35 (both breast cancer), subject 2 (lung cancer) and subject 4 (ovarian cancer)). As 2 of the original 6 patients with RNA polymerase antibodies did not have adequate tissue preservation, tissue from 1 additional patient (subject 42) with anti-RNA polymerase antibodies and breast cancer was obtained for further validation of our preliminary findings. Normal breast and ovary paraffin sections were purchased from US Biomax (Rockville, MD). Cancerous and normal tissue sections were stained with a monoclonal antibody against RNA polymerase I (polypeptide C) (Abnova, Taipei, Taiwan) or a polyclonal antibody against RNA polymerase III (POLR3A, Santa Cruz Biotechnology, CA). Staining was visualized with diaminobenzidine per the manufacturer’s directions (Dako, Carpinteria, CA), and all sections were counterstained with Mayers’ hematoxylin.
Statistical analyses
Clinical and demographic characteristics were compared across autoantibody categories. Statistical significance testing included the Kruskal Wallis test for continuous variables and the Fisher’s exact test for binomial and categorical variables. Comparison of tissue nucleolar RNA polymerase III antigen expression by serum anti-RNA polymerase III antibody status was performed by the Fisher’s exact test. Statistical analyses were performed using Stata 10.0 (Stata Corporation, College Station, TX, USA). P values are 2-sided and were considered significant at α = 0.05.
Results
Among 2367 patients seen at the Johns Hopkins Scleroderma Center, 210 have a known history of malignancy. Thirty seven of these patients were seen between February and August of 2008 and were screened for entry into our study. Both serum and pathology samples could be obtained in 23 of these individuals. These 23 cases therefore comprised our study population. The mean ages at scleroderma and cancer diagnosis were 50.1 years (SD 12.1 years) and 57.3 years (SD 11.0 years), respectively. The mean duration of scleroderma at cancer diagnosis was +7.2 years (SD 10.4), and the average duration of Raynaud’s phenomenon (RP) at cancer diagnosis was +10.1 years (SD 14.1). The majority of subjects were female (95.7%) and white (95.7%). Nineteen subjects (82.6%) met ACR criteria for diagnosis of systemic sclerosis, and the remaining 4 met CREST criteria. Fourteen subjects (60.9%) had limited cutaneous disease, and 9 subjects (39.1%) had diffuse skin involvement. The mean modified Rodnan skin score was 15.0 (SD 14.6), and 5 individuals had ILD, 2 had scleroderma renal crisis, and 3 had a myopathy suggestive of inflammatory myositis. The types of malignancies were varied, but the majority (91.3%) were epithelial cell tumors with breast being the primary site in 13 cases (Table 1).
Table 1.
Cancer Site and Histology of Study Participants, N=23
| Cancer site and histology | Number (%) |
|---|---|
| Breast, no. (%) | 13 (56.5) |
| Ductal carcinoma in situ, no. | 4 |
| Invasive ductal carcinoma, no. | 5 |
| High grade adenocarcinoma, no. | 1 |
| Invasive lobular carcinoma, no. | 3 |
| Lung, no. (%) | 2 (8.7) |
| Small cell carcinoma, no. | 1 |
| Adenocarcinoma, no. | 1 |
| Lymphomas, no. (%) | 2 (8.7) |
| Skin – squamous cell carcinoma, no. (%) | 1 (4.3) |
| Ovary – poorly differentiated carcinoma, no. (%) | 1 (4.3) |
| Tongue – squamous cell carcinoma, no. (%) | 1 (4.3) |
| Uterus – endometrial adenocarcinoma, no. (%) | 1 (4.3) |
| Anus – squamous cell carcinoma, no. (%) | 1 (4.3) |
| Vagina – squamous cell carcinoma, no. (%) | 1 (4.3) |
Among these 23 individuals, 6 tested positive for anti-RNA polymerase I/III, 5 for anti-topoisomerase I, 8 for anti-centromere, and 4 for none of these 3 antibodies (Table 2). No individual produced antibodies to more than one of these tested autoantigens. Age, gender, race, smoking status, disease severity indices, medication use prior to cancer diagnosis, and the frequency of ILD or evidence for PH did not differ statistically between groups. In contrast, the median duration of scleroderma at cancer diagnosis differed significantly between groups: −1.2 years in the anti-RNA polymerase I/III group (range −2 to +1.3 years), +13.4 years in the anti-topoisomerase I group (range +0.25 to +29 years), +11.1 years in the anti-centromere group (range −2.0 to +36.9 years), and +2.3 years in the group negative for all of these antibodies (hereafter referred to as the “antibody negative group”) (range −1.2 to +5.0 years) (p=0.027) (Figure 1). The median duration of RP at cancer diagnosis followed a similar trend with a duration of +0.25 years in the anti-RNA polymerase I/III group (range −2.4 to +1 years), +13.2 years in the anti-topoisomerase I group (range +0.25 to +34 years), +23.8 years in the anti-centromere group (range −5.0 to +36.9 years), and +4.0 years in the antibody negative group (range −1.2 to +7.9 years) (p=0.113). Patients in the anti-RNA polymerase I/III group exclusively had diffuse disease, whereas 40% of the anti-topoisomerase I, none of the anti-centromere, and 25% of subjects in the antibody negative group had diffuse cutaneous disease (p<0.001). Correspondingly, the median modified Rodnan skin score was significantly higher (36) in the anti-RNA polymerase I/III group than in the anti-topoisomerase I (9), anti-centromere (4) or antibody negative (6) groups (p=0.012).
Table 2.
Characteristics of Study Participants by Autoantibody Status
| Variables | Pol I/III (N=6) |
Topo (N=5) |
CENP (N=8) |
Negative (N=4) |
P-value |
|---|---|---|---|---|---|
| Age at Scleroderma Diagnosis (years), median | 51.8 | 45.1 | 48.9 | 54.6 | 0.511 |
| Age at Cancer Diagnosis (years), median | 51.0 | 64.9 | 60.9 | 56.9 | 0.337 |
| Female sex, no. (%) | 6 (100) | 5 (100) | 7 (87.5) | 4 (100) | 1 |
| Race, no. (%) | 0.391 | ||||
| White | 6 (100) | 4 (80) | 8 (100) | 4 (100) | |
| African-American | 0 (0) | 1 (20) | 0 (0) | 0 (0) | |
| ACR Criteria Met, no. (%)* | 6 (100) | 5 (100) | 5 (62.5) | 3 (75) | 0.176 |
| Scleroderma Classification, no. (%) | <0.001 | ||||
| Limited | 0 (0) | 3 (60) | 8 (100) | 3 (75) | |
| Diffuse | 6 (100) | 2 (40) | 0 (0) | 1 (25) | |
| Scleroderma Duration at Cancer Diagnosis (years), median |
−1.2 | 13.4 | 11.1 | 2.3 | 0.027 |
| Raynaud’s Duration at Cancer Diagnosis (years), median |
0.25 | 13.2 | 23.8 | 4.0 | 0.113 |
| Smoking, no. (%) | 0.832 | ||||
| Never | 4 (66.7) | 2 (40) | 5 (62.5) | 3 (75) | |
| Former | 2 (33.3) | 3 (60) | 3 (37.5) | 1 (25) | |
| Disease Severity, no. (%) | |||||
| Severe Raynaud’s Phenomenon ** | 1 (16.7) | 3 (60) | 5 (62.5) | 0 (0) | 0.100 |
| Abnormal General Severity Score § | 4 (66.7) | 2 (40) | 3 (37.5) | 1 (25) | 0.681 |
| History of renal crisis | 2 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0.123 |
| History of myopathy | 2 (33.3) | 0 (0) | 0 (0) | 1 (25) | 0.206 |
| Modified Rodnan Skin Score, median | 36 | 9 | 4 | 6 | 0.012 |
| Interstitial lung disease,╪ no. (%) | 2 (33.3) | 2 (40) | 0 (0) | 1 (25) | 0.231 |
| Pulmonary arterial hypertension,¥ no. (%) | 1 (16.7) | 1 (20) | 2 (25) | 1 (25) | 1 |
| Medications Prior to Cancer Diagnosis, no. (%) | |||||
| Hormone replacement therapy | 1 (16.7) | 1 (20) | 2 (25) | 0 (0) | 0.892 |
| Prednisone | 1 (16.7) | 1 (20) | 1 (12.5) | 0 (0) | 1 |
| Methotrexate | 1 (16.7) | 0 (0) | 1 (12.5) | 0 (0) | 1 |
| Azathioprine | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 0.391 |
| Cyclophosphamide | 0 (0) | 2 (40) | 0 (0) | 0 (0) | 0.063 |
| Mycophenolate mofetil | 0 (0) | 1 (20) | 0 (0) | 1 (25) | 0.202 |
Pol I/III: anti-RNA polymerase I/III, topo: anti-topoisomerase I, CENP: anti-centromere, negative: no autoantibody
Remaining patients met CREST criteria.
Defined as Medsger Raynaud’s Severity Score ≥2.
Defined as Medsger General Severity Score ≥1; based on presence of anemia or weight loss.
Defined by FVC < 70% predicted
Defined by RVSP ≥ 45mmHg or right heart catheterization evidence of PAH.
Figure 1. Distribution of Scleroderma Duration at Cancer Diagnosis by Autoantibody Status.
There is a tight temporal relationship between malignancy diagnosis and scleroderma onset in patients producing anti-RNA polymerase I/III antibodies (Anti-RNA pol I/III) compared to patients with anti-topoisomerase I antibodies (Anti-topo) and patients with antibodies to centromere (Anti-centromere). The antibody negative group (Negative) has a similar close relationship between scleroderma and cancer onset.
Characteristics of these 6 patients with anti-RNA polymerase I/III and a tight temporal clustering of scleroderma onset and malignancy diagnosis are provided in Table 3. Five of the malignancies were epithelial cell tumors, 3 of which were breast in origin. Cancer diagnosis closely preceded scleroderma onset in 4 individuals, and the range of scleroderma duration at cancer diagnosis was −2 to +1.3 years. Similarly, cancer preceded RP onset in 2 individuals and occurred concurrently in one subject. These subjects had aggressive skin disease with skin scores ranging from 14 to 48. Scleroderma renal crisis developed in 2 patients.
Table 3.
Characteristics of Initial 6 Anti-RNA polymerase I/III positive subjects
| Subject # |
Gender | Malignancy | Scleroderma Duration at Cancer Diagnosis (years) |
RP Duration at Cancer Diagnosis (years) |
mRSS* | Scleroderma Complications** |
|---|---|---|---|---|---|---|
| 1 | Female | Breast invasive ductal carcinoma |
−2.0 | 0.7 | 42 | Renal crisis ILD |
| 2 | Female | Lung small cell carcinoma |
−1.0 | 0 | 47 | ILD |
| 4 | Female | Ovarian poorly differentiated metastatic carcinoma |
−1.3 | −0.3 | 21 | Renal crisis Myopathy |
| 9 | Female | Non-Hodgkin lymphoma |
0.8 | 0.5 | 14 | Myopathy PAH |
| 13 | Female | Breast invasive ductal carcinoma |
1.3 | 1.0 | 30 | None |
| 35 | Female | Breast ductal carcinoma in situ |
−2.0 | −2.4 | 48 | None |
Maximal mRSS during disease course
Complications evaluated include renal crisis, myopathy, ILD, and PAH.
Abbreviations: RP – Raynaud’s phenomenon, mRSS – modified Rodnan skin score, ILD – interstitial lung disease, PAH – pulmonary arterial hypertension
As adequate tissue preservation permitted only 4 of these 6 subjects’ tissues to be studied further, one additional patient with scleroderma, cancer, an available cancer pathology specimen, and anti-RNA polymerase I/III autoantibodies was recruited (subject 42). Subject 42 had a breast ductal carcinoma that was detected 1.5 years after scleroderma onset.
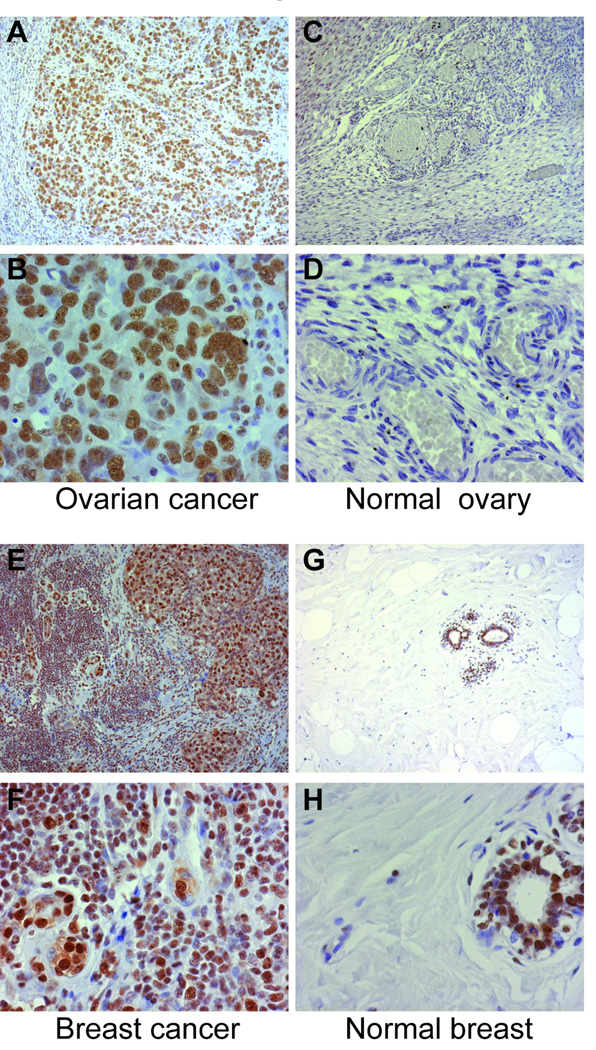
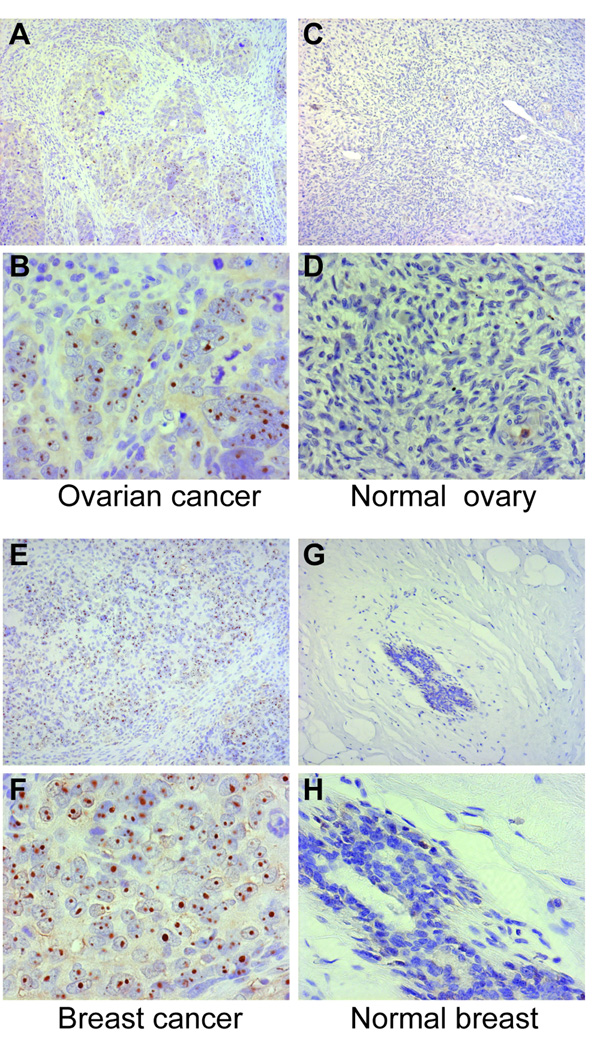
To evaluate levels of RNA polymerase I and RNA polymerase III expression in vivo, paraffin sections from cancerous breast (n=3; subjects # 1, #35, and #42), cancerous lung (n=1; subject # 2) and cancerous ovarian (n=1; subject #4) tissue were analyzed by immunohistochemistry (selection of these tissues is detailed in the Methods section). Tumors from 4 RNA polymerase antibody-negative patients were also evaluated (cancerous breast, n=3 and cancerous lung, n=1; 3 of these 4 subjects had anti-topoisomerase I antibodies). Breast, ovarian and lung paraffin sections were also obtained from normal individuals for the purposes of comparison. Robust and extensive nuclear staining was detected in all of the cancerous tissue sections stained with anti-RNA polymerase I antibody, irrespective of the subject’s RNA polymerase antibody status (Figure 2, panels A, B, E and F and data not shown). When staining was performed under identical conditions using an isotype matched IgG1 antibody instead of the RNA polymerase I monoclonal antibody, no staining was detected (data not shown). In contrast to the prominent staining noted in cancerous tissues, normal breast, ovarian and lung paraffin sections showed minimal/limited RNA polymerase I staining (Figure 2, panels C, D, G and H and data not shown). Of note, in normal breast, RNA polymerase I staining was restricted to ductal cells (Figure 2, panels G and H). The pattern of staining of RNA polymerase III in cancers was strikingly different. An exclusively nucleolar staining pattern with the anti-RNA polymerase III antibody was detected in 4 of the 5 cancerous tissues from RNA polymerase antibody-positive patients (Figure 3, panels A, B, E and F, and data not shown). In contrast, this nucleolar pattern was absent in all 4 of the tumors from RNA polymerase antibody-negative patients (p=0.048). Additionally, it was also not detected in the normal tissue sections, nor in the cancerous sections when staining was performed under identical conditions except that normal goat serum was substituted for the anti-RNA polymerase III goat polyclonal antibody (Figure 3, panels C, D, G and H and data not shown). Tumor RNA polymerase III staining and not tumor RNA polymerase I staining is therefore strikingly associated with the scleroderma patient’s RNA polymerase antibody status.
Figure 2. RNA polymerase I staining is prominent in cancerous ovary and breast tissue from scleroderma patients compared to normal ovary and breast. Paraffin sections from cancerous ovary (panels A and B) and breast (panels E and F) from scleroderma patients with cancer, as well as normal ovary (panels C and D) and normal breast (panels G and H) were stained with antibodies against RNA polymerase I as described in the methods section.
In all panels, the brown color represents RNA polymerase I staining, with nuclei in blue (Mayers’ hematoxylin counterstain). Magnifications are 10× (upper panels of each set - panels A, C, E and G) and 40× (lower panels of each set - panels B, D, F and H). In each set, the 40× panel is a magnification of part of the field shown at10×. The cancer sections shown were from subjects # 4 (ovarian cancer) and # 42 (breast cancer).
Figure 3. RNA polymerase III staining is prominent in cancerous ovary and breast tissue from scleroderma patients compared to normal ovary and breast. Paraffin sections from cancerous ovary (panels A and B) and breast (panels E and F) from scleroderma patients with cancer, as well as normal ovary (panels C and D) and normal breast (panels G and H) were stained with antibodies against RNA polymerase III as described in the methods section.
In all panels, the brown color represents RNA polymerase III staining, with nuclei in blue (Mayers’ hematoxylin counterstain). Magnifications are 10× (upper panels of each set - panels A, C, E and G) and 40× (lower panels of each set - panels B, D, F and H). In each set, the 40× panel is a magnification of part of the field shown at10×. The cancer sections shown were from subjects # 4 (ovarian cancer) and # 42 (breast cancer).
Discussion
In this pilot study, we evaluated whether clinical characteristics, including the temporal relationship between scleroderma and malignancy onset, differed by autoantibody status among patients with scleroderma and cancer. After identifying all patients with a history of cancer seen in our center, we evaluated the first 23 patients in whom histologic confirmation of cancer diagnosis was possible. Within this group, we found that RNA polymerase I/III autoantibodies strongly associate with malignancy that occurs contemporaneously with scleroderma onset. In all patients who produced anti-RNA polymerase I/III antibodies, scleroderma developed within 2 years of cancer diagnosis. Because of this association, we evaluated expression of RNA polymerases I and III in the tumors of patients with anti-RNA polymerase I/III antibodies compared to those patients without these antibodies. Interestingly, we found that nucleolar RNA polymerase III expression was enhanced exclusively in patients with RNA polymerase antibodies. Although RNA polymerase I was expressed at high levels in tumors, this was not restricted to tumors from patients with the anti-RNA polymerase immune response. This association of tumor RNA polymerase III expression with autoantibodies in those patients suggests that RNA polymerase III could be driving the immune response in these individuals. These preliminary findings require confirmation in a larger patient sample with an expanded control population. It is of interest that another subset of patients, the autoantibody “negative” group, also had a similar close relationship between scleroderma and cancer onset. This group may elaborate unique autoantibodies and express novel tumor antigens that remain to be identified.
Our study suggests that cases of paraneoplastic scleroderma may demonstrate hallmark scleroderma specific reactivity. Prior studies have not investigated or detected this association between contemporaneous onset of scleroderma and malignancy and anti-RNA polymerase I/III antibodies in scleroderma patients with cancer. This relationship may have been missed because: (i) RNA polymerase I/III antibody testing was not commercially available until recently and (ii) prior investigations have focused on whether the relationship between scleroderma and cancer differed by scleroderma subtype or tumor origin and histology. By seeking whether the relationship between scleroderma and malignancy onset differed by autoantibody status among patients with scleroderma and cancer, we were able to detect this association even in a relatively small group of patients.
This striking temporal relationship between scleroderma and malignancy onset among anti-RNA polymerase I/III subjects with cancer is similar to that observed in dermatomyositis (24–26) and systemic lupus erythematosus (27) and suggests that cancer and autoimmunity onset might be mechanistically related. There is strong evidence that anti-cancer immunity and autoimmunity are related. For example, effective initiation of anti-cancer immunity during immunotherapy is often accompanied by autoimmunity (28–33). Multiple immune effector pathways are likely involved in causing tissue damage, with prominent involvement of cytotoxic killing pathways. We therefore hypothesize that tumors expressing high concentrations of RNA polymerase III initiate an immune response to these autoantigens. In the appropriate setting, possibly involving enhanced expression of the same autoantigens in damaged or perturbed blood vessels, this anti-tumor immune response may also be directed against specific host tissues, with consequent tissue damage that generates the ongoing rheumatic phenotype. Direct visualization of specific autoantigen expression in tissues targeted in scleroderma is an important future priority.
Although the groups of patients with cancer and autoantibodies to centromere and topoisomerase-1 had a prolonged interval between scleroderma onset and cancer diagnosis, there were outliers in each group (2 anti-centromere and 1 anti-topoisomerase I) in whom cancer and scleroderma onset occurred close together in time. It is of interest that increased topoisomerase I expression has been detected in a variety of cancers (34–36), and in two cases of patients with pre-existing scleroderma, anti-topoisomerase I titers markedly increased in patients at the time of lung cancer diagnosis, recognizing distinct epitopes (37). The data suggest that in some cases, anti-topoisomerase I antibody production might also be driven by malignancy (37). In our series of patients, this group appears to be a minority.
A variety of other mechanisms could explain the relationship between malignancy and scleroderma. Immunosuppressive therapy for autoimmune disease could account for the increase in malignancy risk in a subset of patients. Additionally, treatment of malignancy could result in the development of scleroderma. For example, multiple chemotherapeutic agents have been implicated as potential causes of scleroderma, scleroderma-like disease, or severe Raynaud’s phenomenon (38–42). Radiation therapy may also result in severe skin thickening in patients with scleroderma (43) or cause localized scleroderma in patients without a prior history of connective tissue disease (44). If cancer therapy were the inciting agent that triggered the development of scleroderma, we would not expect our findings to segregate by autoantibody status. Chronic inflammation and repair due to the scleroderma disease process may predispose cells to malignant transformation; this may especially be true of late lung cancers and esophageal adenocarcinomas in the setting of pulmonary fibrosis and longstanding gastroesophageal reflux disease, respectively. Other possible explanations for the relationship between cancer and scleroderma include genetic susceptibility to both malignancy and the development of autoimmune disease, or a common inciting exposure.
We propose that in scleroderma patients who produce anti-RNA polymerase antibodies but have not been diagnosed with cancer that the full expression of an underlying malignancy was aborted by the now scleroderma-specific (originally anti-tumor) immune response. In the paraneoplastic neurological diseases, available data suggest that a patient’s immune response recognizes antigens expressed in the tumors and the target tissue, and that often patients have very small or undetectable tumors at disease diagnosis (45–47). Further investigation is needed to determine whether anti-RNA polymerase I/III antibodies are a marker for increased malignancy risk in scleroderma and whether more aggressive cancer screening should be performed in this patient population.
It is important to note that our small sample size limits the generalizability of these conclusions, and these results need validation in a larger patient sample with nonscleroderma cancer controls. The association of cancer RNA polymerase III expression, RNA polymerase III autoantibodies and interval between cancer and scleroderma diagnosis observed in scleroderma patients does not in any way predict that such antigen expression patterns are restricted to scleroderma-associated cancers. Indeed, it is likely that similar RNA polymerase III expression patterns occur in tumors from patients who do not have scleroderma, and that additional pathways and events are required to generate both the scleroderma-specific immune response and clinical phenotype. We acknowledge that we cannot establish a causal relationship between cancer and scleroderma with our retrospective study design that focused on scleroderma patients with a history of malignancy. Another limitation of our study was that in some cases, we lacked serum samples that were concurrent with malignancy onset. To address this, we evaluated the closest available serum sample to cancer diagnosis; the median duration between cancer diagnosis and serum sample studied was 2.3 years. There are many issues that can only be addressed in a prospective study including changes in autoantibody profiles in response to cancer therapy (range of autoantibodies targeted and titers).
We have demonstrated a tight temporal relationship between scleroderma onset and malignancy diagnosis in scleroderma patients with cancer who produce anti-RNA polymerase autoantibodies. Expression of RNA polymerase III is enhanced exclusively in the tumors from patients with RNA polymerase antibodies, demonstrating that tumor antigen expression and scleroderma autoantibodies are strongly associated, and highlighting RNA polymerase III as the tumor-associated antigen target. These findings argue for a mechanistic relationship between malignancy, the immune response and development of scleroderma, and raise the possibility that RNA polymerase I/III autoantibodies are markers of malignancy in newly diagnosed scleroderma patients. These findings may have both important diagnostic and therapeutic implications.
Acknowledgments
These studies were supported in part by the Scleroderma Research Foundation and the Stabler Foundation; the Karen Brown Scleroderma Foundation and the American College of Rheumatology Research and Education Foundation’s Clinical Investigator Fellowship Award (Dr. Shah); and by the following NIH grants: P30 AR058885 (Dr. Shah), 1K23AR052742 (Dr. Hummers), 1P50 HL084946-01 (Drs. Wigley and Hummers), RO1-AR-044684 (Dr Casciola-Rosen), and R37-DE-12354 (Dr Rosen). We thank the Rheumatic Diseases Research Core Center (P30-AR-053503) for performing the ELISA assays, and Tonie Hines for excellent technical assistance.
References
- 1.Abu-Shakra M, Guillemin F, Lee P. Cancer in systemic sclerosis. Arthritis Rheum. 1993 Apr;36(4):460–464. doi: 10.1002/art.1780360405. [DOI] [PubMed] [Google Scholar]
- 2.Derk CT, Rasheed M, Artlett CM, Jimenez SA. A cohort study of cancer incidence in systemic sclerosis. J Rheumatol. 2006 Jun;33(6):1113–1116. [PubMed] [Google Scholar]
- 3.Hill CL, Nguyen AM, Roder D, Roberts-Thomson P. Risk of cancer in patients with scleroderma: A population based cohort study. Ann Rheum Dis. 2003 Aug;62(8):728–731. doi: 10.1136/ard.62.8.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters-Golden M, Wise RA, Hochberg M, Stevens MB, Wigley FM. Incidence of lung cancer in systemic sclerosis. J Rheumatol. 1985 Dec;12(6):1136–1139. [PubMed] [Google Scholar]
- 5.Rosenthal AK, McLaughlin JK, Gridley G, Nyren O. Incidence of cancer among patients with systemic sclerosis. Cancer. 1995 Sep 1;76(5):910–914. doi: 10.1002/1097-0142(19950901)76:5<910::aid-cncr2820760528>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Roumm AD, Medsger TA., Jr Cancer and systemic sclerosis. an epidemiologic study. Arthritis Rheum. 1985 Dec;28(12):1336–1340. doi: 10.1002/art.1780281204. [DOI] [PubMed] [Google Scholar]
- 7.Pontifex EK, Hill CL, Roberts-Thomson P. Risk factors for lung cancer in patients with scleroderma: A nested case-control study. Ann Rheum Dis. 2007 Apr;66(4):551–553. doi: 10.1136/ard.2006.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasegawa M, Sato S, Sakai H, Ohashi T, Takehara K. Systemic sclerosis revealing T-cell lymphoma. Dermatology. 1999;198(1):75–78. doi: 10.1159/000018070. [DOI] [PubMed] [Google Scholar]
- 9.Juarez M, Marshall R, Denton C, Evely R. Paraneoplastic scleroderma secondary to hairy cell leukaemia successfully treated with cladribine. Rheumatology (Oxford) 2008 Nov;47(11):1734–1735. doi: 10.1093/rheumatology/ken367. [DOI] [PubMed] [Google Scholar]
- 10.Reyes CM, Rudinskaya A, Kloss R, Girardi M, Lazova R. Scleroderma-like illness as a presenting feature of multiple myeloma and amyloidosis. J Clin Rheumatol. 2008 Jun;14(3):161–165. doi: 10.1097/RHU.0b013e3181775a15. [DOI] [PubMed] [Google Scholar]
- 11.Forbes AM, Woodrow JC, Verbov JL, Graham RM. Carcinoma of breast and scleroderma: Four further cases and a literature review. Br J Rheumatol. 1989 Feb;28(1):65–69. doi: 10.1093/rheumatology/28.1.65. [DOI] [PubMed] [Google Scholar]
- 12.Launay D, Le Berre R, Hatron PY, Peyrat JP, Hachulla E, Devulder B, et al. Association between systemic sclerosis and breast cancer: Eight new cases and review of the literature. Clin Rheumatol. 2004 Dec;23(6):516–522. doi: 10.1007/s10067-004-0940-5. [DOI] [PubMed] [Google Scholar]
- 13.Lu TY, Hill CL, Pontifex EK, Roberts-Thomson PJ. Breast cancer and systemic sclerosis: A clinical description of 21 patients in a population-based cohort study. Rheumatol Int. 2008 Feb 16; doi: 10.1007/s00296-008-0540-9. [DOI] [PubMed] [Google Scholar]
- 14.Scope A, Sadetzki S, Sidi Y, Barzilai A, Trau H, Kaufman B, et al. Breast cancer and scleroderma. Skinmed. 2006 Jan–Feb;5(1):18–24. doi: 10.1111/j.1540-9740.2006.04448.x. [DOI] [PubMed] [Google Scholar]
- 15.Bachleitner-Hofmann T, Machold K, Knobler R, Drach J, Grumbeck E, Gisslinger H. Marked and sustained improvement of systemic sclerosis following polychemotherapy for coexistent multiple myeloma. Clin Exp Rheumatol. 2002 Jan–Feb;20(1):85–88. [PubMed] [Google Scholar]
- 16.Preliminary criteria for the classification of systemic sclerosis (scleroderma). subcommittee for scleroderma criteria of the american rheumatism association diagnostic and therapeutic criteria committee. Arthritis Rheum. 1980 May;23(5):581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 17.Medsger TA, Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, et al. A disease severity scale for systemic sclerosis: Development and testing. J Rheumatol. 1999 Oct;26(10):2159–2167. [PubMed] [Google Scholar]
- 18.Clements PJ, Lachenbruch PA, Seibold JR, Zee B, Steen VD, Brennan P, et al. Skin thickness score in systemic sclerosis: An assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993 Nov;20(11):1892–1896. [PubMed] [Google Scholar]
- 19.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J Rheumatol. 1988 Feb;15(2):202–205. [PubMed] [Google Scholar]
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999 Jan;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 21.Knudson RJ, Kaltenborn WT, Knudson DE, Burrows B. The single-breath carbon monoxide diffusing capacity. reference equations derived from a healthy nonsmoking population and effects of hematocrit. Am Rev Respir Dis. 1987 Apr;135(4):805–811. doi: 10.1164/arrd.1987.135.4.805. [DOI] [PubMed] [Google Scholar]
- 22.Mukerjee D, St George D, Knight C, Davar J, Wells AU, Du Bois RM, et al. Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology (Oxford) 2004 Apr;43(4):461–466. doi: 10.1093/rheumatology/keh067. [DOI] [PubMed] [Google Scholar]
- 23.Gelber AC, Pillemer SR, Baum BJ, Wigley FM, Hummers LK, Morris S, et al. Distinct recognition of antibodies to centromere proteins in primary sjogren's syndrome compared with limited scleroderma. Ann Rheum Dis. 2006 Aug;65(8):1028–1032. doi: 10.1136/ard.2005.046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zantos D, Zhang Y, Felson D. The overall and temporal association of cancer with polymyositis and dermatomyositis. J Rheumatol. 1994 Oct;21(10):1855–1859. [PubMed] [Google Scholar]
- 25.Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med. 2001 Jun 19;134(12):1087–1095. doi: 10.7326/0003-4819-134-12-200106190-00008. [DOI] [PubMed] [Google Scholar]
- 26.Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: A population-based study. Lancet. 2001 Jan 13;357(9250):96–100. doi: 10.1016/S0140-6736(00)03540-6. [DOI] [PubMed] [Google Scholar]
- 27.Bernatsky S, Boivin JF, Joseph L, Rajan R, Zoma A, Manzi S, et al. An international cohort study of cancer in systemic lupus erythematosus. Arthritis Rheum. 2005 May;52(5):1481–1490. doi: 10.1002/art.21029. [DOI] [PubMed] [Google Scholar]
- 28.Yeh S, Karne NK, Kerkar SP, Heller CK, Palmer DC, Johnson LA, et al. Ocular and systemic autoimmunity after successful tumor-infiltrating lymphocyte immunotherapy for recurrent, metastatic melanoma. Ophthalmology. 2009 May;116(5):981.e1–989.e1. doi: 10.1016/j.ophtha.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caspi RR. Immunotherapy of autoimmunity and cancer: The penalty for success. Nat Rev Immunol. 2008 Dec;8(12):970–976. doi: 10.1038/nri2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer DC, Chan CC, Gattinoni L, Wrzesinski C, Paulos CM, Hinrichs CS, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Correale P, Fioravanti A, Bertoldi I, Montagnani F, Miracco C, Francini G. Occurrence of autoimmunity in a long-term survivor with metastatic colon carcinoma treated with a new chemo-immunotherapy regimen. J Chemother. 2008 Apr;20(2):278–281. doi: 10.1179/joc.2008.20.2.278. [DOI] [PubMed] [Google Scholar]
- 32.Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006 Feb 16;354(7):709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 33.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002 Oct 25;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husain I, Mohler JL, Seigler HF, Besterman JM. Elevation of topoisomerase I messenger RNA, protein, and catalytic activity in human tumors: Demonstration of tumor-type specificity and implications for cancer chemotherapy. Cancer Res. 1994 Jan 15;54(2):539–546. [PubMed] [Google Scholar]
- 35.Giaccone G, van Ark-Otte J, Scagliotti G, Capranico G, van der Valk P, Rubio G, et al. Differential expression of DNA topoisomerases in non-small cell lung cancer and normal lung. Biochim Biophys Acta. 1995 Dec 27;1264(3):337–346. doi: 10.1016/0167-4781(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 36.Lynch BJ, Bronstein IB, Holden JA. Elevations of DNA topoisomerase I in invasive carcinoma of the breast. Breast J. 2001 May–Jun;7(3):176–180. doi: 10.1046/j.1524-4741.2001.007003176.x. [DOI] [PubMed] [Google Scholar]
- 37.Kuwana M, Fujii T, Mimori T, Kaburaki J. Enhancement of anti-DNA topoisomerase I autoantibody response after lung cancer in patients with systemic sclerosis. A report of two cases. Arthritis Rheum. 1996 Apr;39(4):686–691. doi: 10.1002/art.1780390423. [DOI] [PubMed] [Google Scholar]
- 38.Cohen IS, Mosher MB, O'Keefe EJ, Klaus SN, De Conti RC. Cutaneous toxicity of bleomycin therapy. Arch Dermatol. 1973 Apr;107(4):553–555. [PubMed] [Google Scholar]
- 39.Finch WR, Rodnan GP, Buckingham RB, Prince RK, Winkelstein A. Bleomycin-induced scleroderma. J Rheumatol. 1980 Sep–Oct;7(5):651–659. [PubMed] [Google Scholar]
- 40.Berger CC, Bokemeyer C, Schneider M, Kuczyk MA, Schmoll HJ. Secondary raynaud's phenomenon and other late vascular complications following chemotherapy for testicular cancer. Eur J Cancer. 1995 Dec;31A(13–14):2229–2238. doi: 10.1016/0959-8049(95)00460-2. [DOI] [PubMed] [Google Scholar]
- 41.Bessis D, Guillot B, Legouffe E, Guilhou JJ. Gemcitabine-associated scleroderma-like changes of the lower extremities. J Am Acad Dermatol. 2004 Aug;51(2 Suppl):S73–S76. doi: 10.1016/j.jaad.2001.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Clowse ME, Wigley FM. Digital necrosis related to carboplatin and gemcitabine therapy in systemic sclerosis. J Rheumatol. 2003 Jun;30(6):1341–1343. [PubMed] [Google Scholar]
- 43.Abu-Shakra M, Lee P. Exaggerated fibrosis in patients with systemic sclerosis (scleroderma) following radiation therapy. J Rheumatol. 1993 Sep;20(9):1601–1603. [PubMed] [Google Scholar]
- 44.Colver GB, Rodger A, Mortimer PS, Savin JA, Neill SM, Hunter JA. Post-irradiation morphoea. Br J Dermatol. 1989 Jun;120(6):831–835. doi: 10.1111/j.1365-2133.1989.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 45.Anderson NE, Rosenblum MK, Posner JB. Paraneoplastic cerebellar degeneration: Clinical-immunological correlations. Ann Neurol. 1988 Oct;24(4):559–567. doi: 10.1002/ana.410240413. [DOI] [PubMed] [Google Scholar]
- 46.Lorusso L, Hart IK, Giometto B, Pezzani R, Broome JC, Gritti D, et al. Immunological features of neurological paraneoplastic syndromes. Int J Immunopathol Pharmacol. 2004 May–Aug;17(2):135–144. doi: 10.1177/039463200401700205. [DOI] [PubMed] [Google Scholar]
- 47.Vitaliani R, Zoccarato M, Giometto B. Diagnosis and treatment of paraneoplastic neurological syndromes. Curr Clin Pharmacol. 2008 Jan;3(1):46–50. doi: 10.2174/157488408783329922. [DOI] [PubMed] [Google Scholar]