Abstract
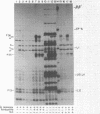
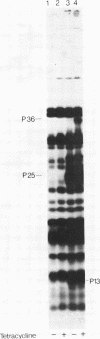
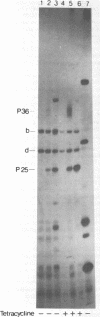
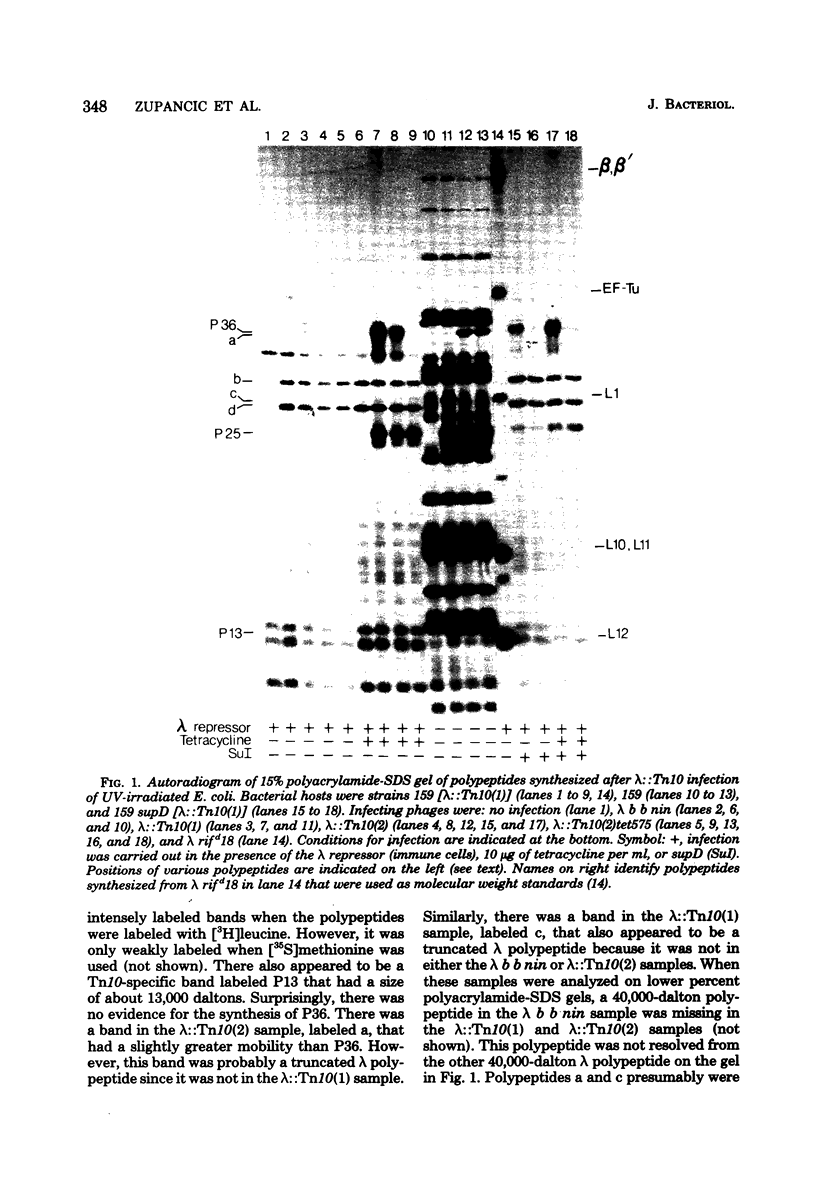
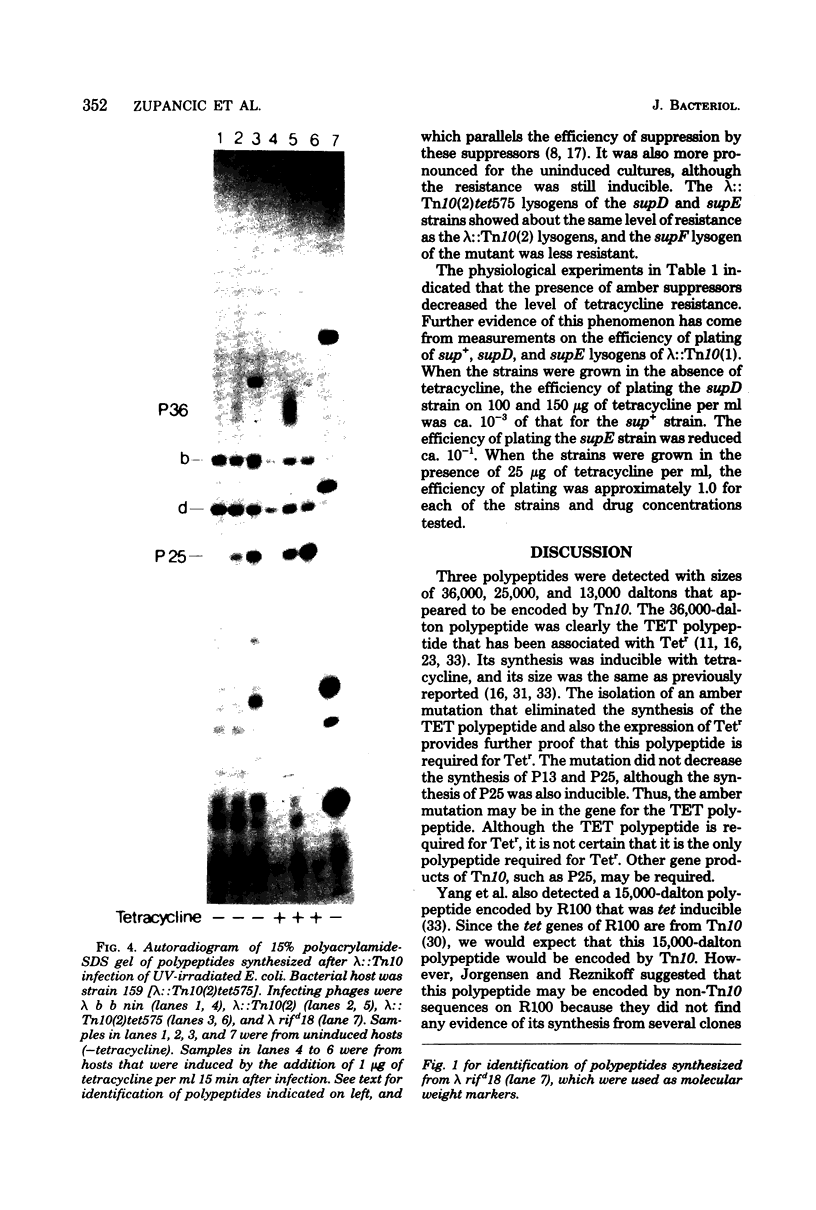
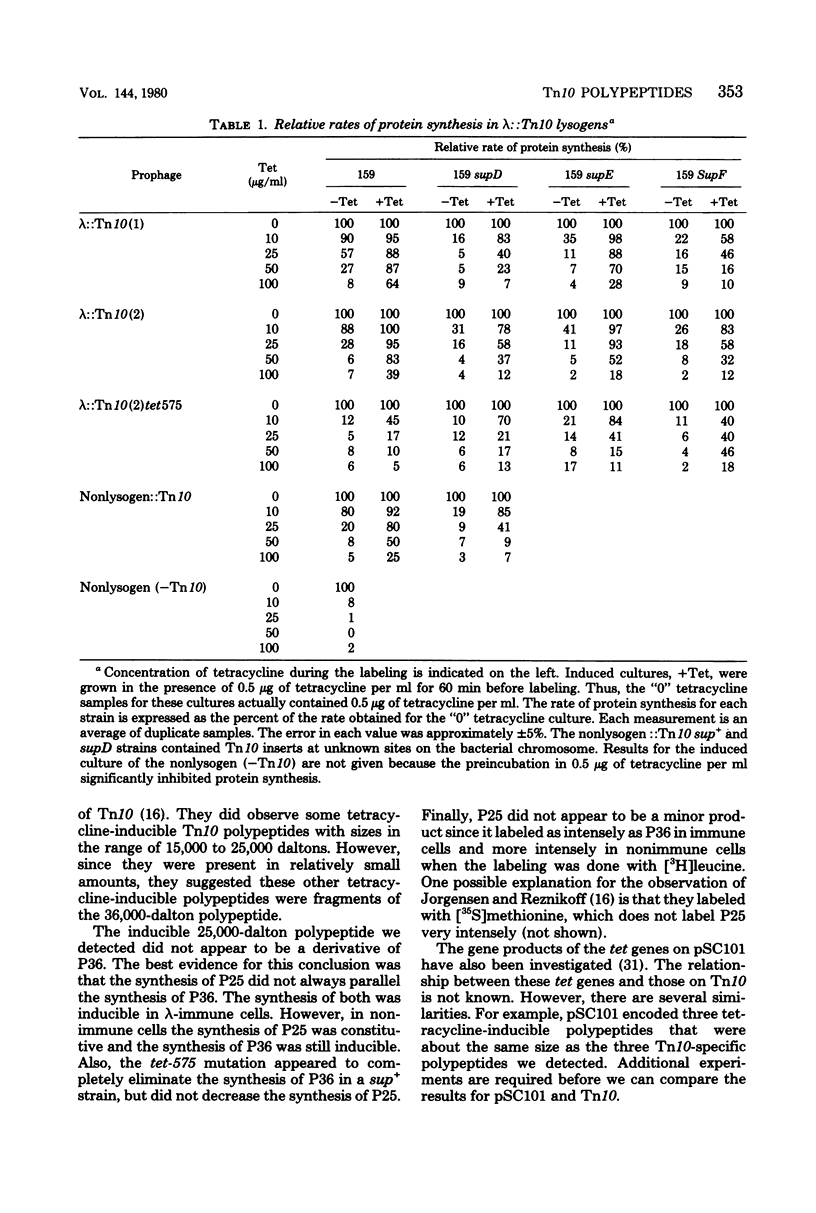
Three Tn10 polypeptides were detected by analyzing the proteins synthesized in ultraviolet light-irradiated Escherichia coli cells after infection with lambda::Tn10. One of these polypeptides was the previously identified 36,000-dalton TET polypeptide. The other two had approximate sizes of 25,000 and 13,000 daltons. The syntheses of both the TET polypeptide and the 25,000-dalton polypeptide were inducible by tetracycline in lambda-immune hosts. Similarly, the synthesis of the TET polypeptide was inducible in nonimmune hosts. However, the synthesis of the 25,000-dalton polypeptide was constitutive in nonimmune hosts. An amber mutation in a gene required for tetracycline resistance on lambda::Tn10 was isolated that eliminated the synthesis of the TET polypeptide in sup+ hosts but not the synthesis of the 25,000-dalton or the 13,000-dalton polypeptides. The expression of tetracycline resistance from wild-type Tn10 was found to be anomalous in E. coli strains carrying the amber suppressors supD, supE, and supF. In general, strains containing these nonsense suppressors were less resistant to tetracycline.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIMA K., IZAKI K. ACCUMULATION OF OXYTETRACYCLINE RELEVANT TO ITS BACTERICIDAL ACTION IN THE CELLS OF ESCHERICHIA COLI. Nature. 1963 Oct 12;200:192–193. doi: 10.1038/200192a0. [DOI] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw J. R. Accumulation of tetracyclines by Escherichia coli. J Bacteriol. 1968 Feb;95(2):498–506. doi: 10.1128/jb.95.2.498-506.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J. Resistance of Escherichia coli to tetracyclines. Changes in permeability to tetracyclines in Escherichia coli bearing transferable resistance factors. Biochem J. 1967 Oct;105(1):371–378. doi: 10.1042/bj1050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garen A. Sense and nonsense in the genetic code. Three exceptional triplets can serve as both chain-terminating signals and amino acid codons. Science. 1968 Apr 12;160(3824):149–159. doi: 10.1126/science.160.3824.149. [DOI] [PubMed] [Google Scholar]
- Gill R. E., Heffron F., Falkow S. Identification of the protein encoded by the transposable element Tn3 which is required for its transposition. Nature. 1979 Dec 20;282(5741):797–801. doi: 10.1038/282797a0. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Kiuchi K., Arima K. Specificity and mechanism of tetracycline resistance in a multiple drug resistant strain of Escherichia coli. J Bacteriol. 1966 Feb;91(2):628–633. doi: 10.1128/jb.91.2.628-633.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Specialized transducing phages for ribosomal protein genes of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jan;72(1):6–10. doi: 10.1073/pnas.72.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Berg D. E., Allet B., Reznikoff W. S. Restriction enzyme cleavage map of Tn10, a transposon which encodes tetracycline resistance. J Bacteriol. 1979 Jan;137(1):681–685. doi: 10.1128/jb.137.1.681-685.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Reznikoff W. S. Organization of structural and regulatory genes that mediate tetracycline resistance in transposon Tn10. J Bacteriol. 1979 Jun;138(3):705–714. doi: 10.1128/jb.138.3.705-714.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S., Stretton A. O., Brenner S. Amber suppressors: efficiency of chain propagation and suppressor specific amino acids. J Mol Biol. 1965 Dec;14(2):528–533. doi: 10.1016/s0022-2836(65)80202-9. [DOI] [PubMed] [Google Scholar]
- Kirschbaum J. B., Konrad E. B. Isolation of a specialized lambda transducing bacteriophage carrying the beta subunit gene for Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1973 Nov;116(2):517–526. doi: 10.1128/jb.116.2.517-526.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn L. J., Yanofsky C. Polarity suppressors defective in transcription termination at the attenuator of the tryptophan operon of Escherichia coli have altered rho factor. J Mol Biol. 1976 Sep 15;106(2):231–241. doi: 10.1016/0022-2836(76)90082-6. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Yanofsky C. Polarity suppressors increase expression of the wild-type tryptophan operon of Escherichia coli. J Mol Biol. 1976 May 15;103(2):395–409. doi: 10.1016/0022-2836(76)90319-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Plasmid-determined tetracycline resistance involves new transport systems for tetracycline. Nature. 1978 Nov 2;276(5683):90–92. doi: 10.1038/276090a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Privitera G., Sebald M., Fayolle F. Common regulatory mechanism of expression and conjugative ability of a tetracycline resistance plasmid in Bacteroides fragilis. Nature. 1979 Apr 12;278(5705):657–659. doi: 10.1038/278657a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Boyer H. W. On the nature of tetracycline resistance controlled by the plasmid pSC101. Cell. 1978 Jan;13(1):73–81. doi: 10.1016/0092-8674(78)90139-3. [DOI] [PubMed] [Google Scholar]
- Unowsky J., Rachmeler M. Mechanisms of antibiotic resistance determined by resistance-transfer factors. J Bacteriol. 1966 Aug;92(2):358–365. doi: 10.1128/jb.92.2.358-365.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Levy S. B. Synthesis of an R plasmid protein associated with tetracycline resistance is negatively regulated. Proc Natl Acad Sci U S A. 1976 May;73(5):1509–1512. doi: 10.1073/pnas.73.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]