Abstract
Purpose
Most children today with bone sarcomas undergo limb-sparing surgery. When treating children younger than 12 years of age, the result is significant limb length discrepancy (LLD). One of the solutions is the use of an expandable endoprosthesis.
Methods
A retrospective analysis of 38 skeletally immature patients with bone sarcoma of the lower limb in whom different types of expandable endoprostheses were used from January 1988 to December 2005 were included. All patients were under the age of 14 years. There were 26 osteosarcoma and 12 Ewing’s sarcomas. The data collected included the tumor characteristics, the surgical and other treatment modalities, complications and their treatment, and the final LLD and functional results.
Results
Fifty-five percent of the patients survived and had a mean follow-up of 113 months. All survivors reached skeletal maturity at the time of last follow-up. Seventy-one percent of the survivors had satisfactory function and 29% had a poor result. There were three secondary amputations due to local recurrence. Complications were documented in 58% of patients; the most common was infection that was diagnosed 56 times (primary 16% and secondary 84%). A significant correlation was found between function and final LLD (greater than 5 cm = inferior function), the number of complications, and the number of surgical procedures performed other than prosthesis elongation. The younger the patient was at definitive surgery, the shorter the time it took for the prosthesis to fail.
Conclusion
In order to improve results, the number of operations must be reduced. This can be achieved by the use of novel non-invasive expandable endoprostheses or biological reconstruction.
Keywords: Bone sarcoma, Expandable endoprosthesis, Limb-sparing surgery (LSS), Limb length discrepancy (LLD)
Introduction
Bone sarcomas are rare malignancies which mainly affect children and young adults (5–25 years old). In the past, the only treatment option was amputation surgery [1]. Survivorship was 0–15%, and most patients died without their limbs. The cause of death was usually suffocating lung metastases (95% of sarcomas metastases are to the lungs) [1]. The past 30 years have witnessed many advances in various disciplines that contributed to the emergence of the modern concept of limb-sparing surgery (LSS), the main one being effective systemic chemotherapy.
Today, 85% of the procedures for bone and soft tissue sarcomas are by LSS compared to 15% for amputation, and the overall 5-year survival is around 60% [1]. LSS in young children is challenging because of the expected future longitudinal and radial growth of the remaining limb, which leads to limb length discrepancy (LLD) that profoundly affects function [2–4]. For example, resection of the distal femur in children younger than 10 years of age leads to the loss of up to 1.6 cm every year, whereupon the LLD can reach 10–20 cm at the time of skeletal maturity [2, 3].
There are several surgical approaches for dealing with LLD:
Amputation and rehabilitation with external prosthesis.
A rotationplasty procedure for tumors at the distal femur, in which the remaining leg is rotated 180° so that the ankle functions as the knee joint, allowing flexion and extension, while the foot is supported with a relatively short and stable external prosthesis, giving good function but a bizarre appearance.
Osteoarticular allograft, which have no growth ability and are associated with complications, including non-union, fractures, and infections.
Combining standard non-expanding endoprostheses with epiphysiodesis of the contralateral growth plate, thereby, eliminating LLD but causing short stature.
Arthrodesis techniques using a combination of allograft and autografts (vascularized or not), followed by a conventional elongating technique, such as an Ilizarov external fixator.
Each of these solutions has profound disadvantages [4–6]. The optimal surgical solution is different for each age group. LSS is almost impossible for children under 5 years of age, leaving amputation as the only reasonable option for most cases. LSS for the 5–14 years age group is possible if LLD is managed (e.g., by an expandable endoprosthesis). A standard non-expanding tumor endoprosthesis is usually preferable for patients older than 14 years of age, when most of the growth has usually taken place.
Expandable endoprostheses with different expansion mechanisms have been used since the late 1970s. They are usually made of two main parts; a stem and an articulation. The elongating system is usually located within the stem part.
The first models (Figs. 1 and 2) had to be fully exposed during surgery, with the patient under anesthesia in order to carry out the elongation, and expansion was achieved by introducing a spacer, such as a ball bearing or serial sleeves.
Fig. 1.
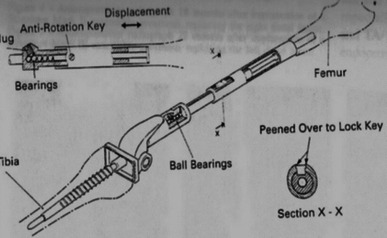
Elongating system via ball bearings
Fig. 2.
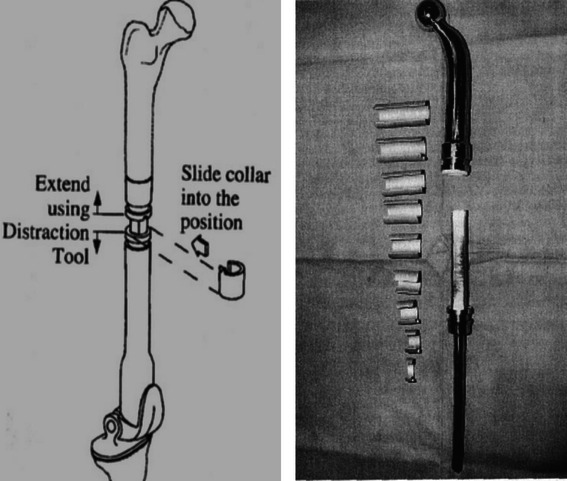
Elongating system via serial sliding collars
The second generation of devices (Fig. 3) was minimally invasive and had an elongating screw mechanism that was rotated via a small incision and guided with a C-ARM intensifier, but it too required the operating room and general anesthesia [7].
Fig. 3.
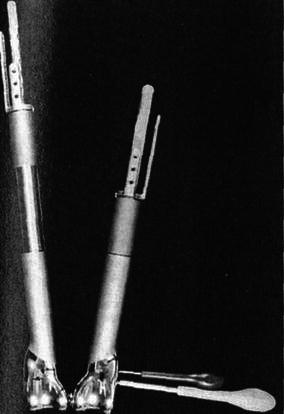
Minimally invasive expandable endoprosthesis using a screw and locking device in its closed and elongated positions
The third generation of expandable endoprostheses (Figs. 4 and 5) is the non-invasive type, in which the elongating stem is manipulated by an external force consisting of either a rotating external magnet or an electromagnetic field. The latter have been used for only the past 5–10 years, and so, follow-up has been relatively short term. All of the systems bear a high rate of complications that are often treated by numerous surgical procedures and revisions until the patient reaches maturity [7–9].
Fig. 4.
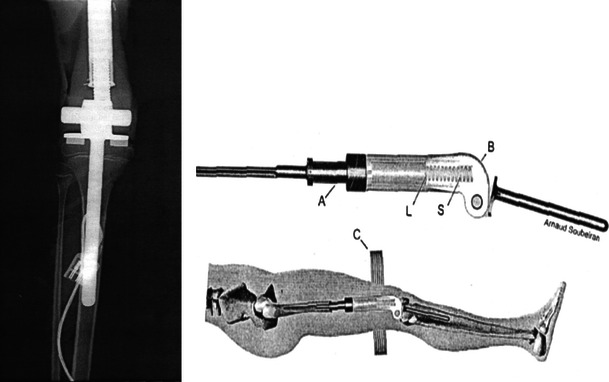
Non-invasive expansion mechanism using a pretensioned spring manipulated by an external magnetic device
Fig. 5.
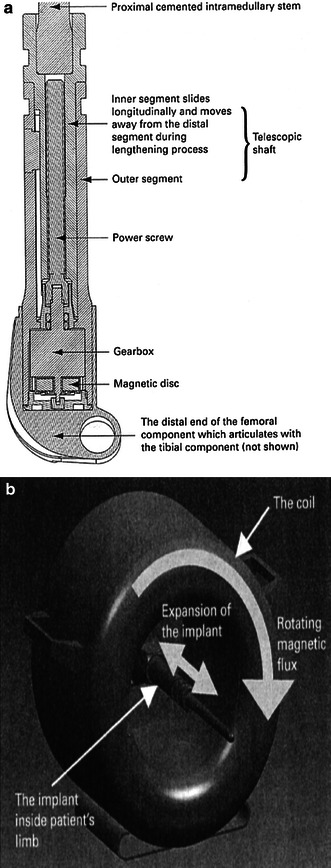
A novel system including an expanding internal device (a) moved via a rotating external magnet (b)
The current paper is a retrospective analysis of the long-term follow-up data on 38 skeletally immature patients with bone sarcoma of the lower limb that had been treated with expandable endoprostheses by a single team between 1988 to 2005.
The focus of this work is complications associated with surgery and final function at maturity.
Materials and methods
Prostheses
The surgical team used several types of expandable prostheses during the study period. The first model, which was elongated with sleeves in two cases, then, at the beginning of the 1990s, a Lewis expandable adjustable prosthesis (LEAP) endoprosthesis was used in five cases, followed by a Kotz (minimally invasive) endoprosthesis that was used in 29 cases. A novel non-invasive prosthesis was introduced in 2003 and the data on the two patients who underwent surgery with this prosthesis are included. The clinical indication for extendable endoprosthesis was the age of the patient less than 12–14 years (depending on their height and their parents’ height as well—factors which represent remaining growth potential). The elongation procedure was done usually when LLD had become greater than 2 cm and this determined its frequency and intervals. Elongation was done in the operating theater with the patient under general anesthesia. The surgeon made a small incision and used a C-ARM intensifier. No more than 2 cm elongation at one time was done. This was followed by hospitalization for effective analgesic care and supervised physiotherapy for a few days, which included the relief of muscle tension and joint mobilization. In cemented implants, weight bearing was allowed after a few days and in uncemented cases, it was postponed for up to 6 weeks. It should be mentioned that always during the first surgery (introduction of the implant), we try to over-elongate the limb by 2 cm in order to gain growth time until needing the first elongation.
Methods
The medical and surgical records and the imaging files of all consecutive patients who underwent LSS with expandable endoprostheses for bone sarcomas in the National Unit of Orthopedic Oncology between January 1988 to December 2005 were evaluated. The inclusion criteria were age at diagnosis between 0 and 14 years, diagnosis of high-grade bone sarcoma located in the long bones of the lower limb (femur or tibia), and treatment by LSS using an expandable prosthesis.
We extracted data on non-surgical therapy, surgical procedures, complications and their treatment, and the final functional status. Complications were divided into infections (deep and superficial), soft tissue problems (dehiscence, nerve damage, vascular problems, and contractures), periprosthetic fractures, and mechanical problems of the prosthesis. An “early” complication was defined as one that occurred up to 1 month after the definitive surgery and a “late” complication as one that occurred more than 1 month after surgery.
Functional evaluation was done according to the AMSTS Functional Scoring System at the last follow-up [10, 11]. Prosthetic survival was defined as the time between implantation to removal/revision.
Statistical methods
Prosthetic survival was calculated by log-rank statistics and plotted by the Kaplan–Meier method (P < 0.05) [12]. All of the retrieved data were entered into an Excel table and the statistical analysis was performed with STATA 8 software.
Results
A total of 38 patients (24 males and 14 females) were included, of whom 21 were under the age of 10 years (Table 1). The average age at diagnosis was 10.5 years (range 6–14 years). The diagnosis was osteosarcoma in 26 and Ewing’s sarcoma in 12. The location of the tumor was at the distal femur in 23, proximal femur in ten, and proximal tibia in five. All patients had local staging with plain X-ray, computed tomography (CT), and magnetic resonance imaging (MRI) of the affected limb. Systemic staging was carried out with a bone scan and chest CT. According to the Enneking surgical staging system, at the time of diagnosis, 33 patients were IIB and 5 were IIIA or B (metastatic) [13]. All 38 patients underwent an open incision biopsy followed by neoadjuvant systemic chemotherapy and LSS. Adjuvant therapy was given after analyzing the effect of neoadjuvant chemotherapy on the specimen by the percentage of tumor necrosis [14–16]. Patients with Ewing’s sarcoma were treated with radiotherapy before and/or after definitive surgery.
Table 1.
Patient study cohort
| Patient no. | Age (years) | Diagnosis | Location | Follow-up (months) | No. of elongation procedures | Total no. of operations | Revision | Infection | Mechanical failure | Other complications | Survived | Function | LLD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | os | df | 168 | 3 | 8 | 2 | 8 | 0 | 2 | Yes | Fair | 60 |
| 2 | 14 | os | df | 12 | 0 | 0 | 0 | 0 | 0 | 1 | No | 15 | |
| 3 | 14 | os | df | 108 | 1 | 5 | 1 | 4 | 0 | 3 | Yes | Fair | 50 |
| 4 | 9 | os | df | 120 | 3 | 1 | 0 | 0 | 0 | 1 | Yes | Good | 20 |
| 5 | 6 | es | pf | 144 | 12 | 12 | 3 | 5 | 1 | 5 | Yes | Good | 20 |
| 6 | 9 | os | df | 108 | 3 | 0 | 0 | 1 | 0 | 2 | Yes | Excellent | 15 |
| 7 | 9 | os | df | 84 | 2 | 3 | 1 | 3 | 0 | 3 | Yes | Good | 40 |
| 8 | 9 | os | df | 168 | 7 | 6 | 3 | 7 | 0 | 6 | Yes | Poor | 80 |
| 9 | 8 | es | pf | 112 | 3 | 5 | 3 | 0 | 0 | 4 | Yes | Good | 90 |
| 10 | 9 | os | pt | 84 | 3 | 6 | 2 | 6 | 1 | 4 | Yes | Good | 50 |
| 11 | 10 | os | df | 192 | 5 | 12 | 4 | 5 | 1 | 6 | Yes | Fair | 60 |
| 12 | 13 | os | df | 36 | 0 | 0 | 0 | 1 | 0 | 2 | Yes | Excellent | 0 |
| 13 | 14 | es | pf | 24 | 0 | 0 | 0 | 0 | 0 | 0 | No | 50 | |
| 14 | 12 | os | pf | 36 | 0 | 0 | 0 | 0 | 0 | 0 | Yes | Excellent | 0 |
| 15 | 9 | Es | pt | 60 | 1 | 1 | 0 | 0 | 0 | 3 | Yes | Good | 35 |
| 16 | 7 | os | df | 24 | 0 | 0 | 0 | 0 | 0 | 1 | No | 15 | |
| 17 | 7 | os | pt | 120 | 4 | 1 | 1 | 1 | 0 | 1 | Yes | Fair | 30 |
| 18 | 10 | os | df | 38 | 0 | 3 | 0 | 2 | 0 | 2 | No | 0 | |
| 19 | 11 | es | df | 72 | 1 | 1 | 0 | 1 | 0 | 2 | Yes | Good | 20 |
| 20 | 12 | os | df | 72 | 3 | 0 | 0 | 0 | 0 | 0 | Yes | Excellent | 0 |
| 21 | 12 | os | df | 84 | 3 | 0 | 0 | 0 | 0 | 0 | Yes | Good | 30 |
| 22 | 14 | os | df | 12 | 0 | 0 | 0 | 0 | 0 | 0 | No | 0 | |
| 23 | 11 | es | pf | 120 | 0 | 2 | 1 | 1 | 0 | 2 | Yes | Excellent | 30 |
| 24 | 13 | os | df | 12 | 0 | 1 | 0 | 0 | 0 | 0 | No | 20 | |
| 25 | 8 | os | pt | 12 | 0 | 0 | 0 | 0 | 0 | 0 | No | 0 | |
| 26 | 12 | os | df | 12 | 0 | 1 | 0 | 0 | 0 | 1 | No | amp | |
| 27 | 10 | os | df | 36 | 0 | 1 | 0 | 1 | 0 | 0 | No | 0 | |
| 28 | 11 | os | pf | 132 | 4 | 6 | 3 | 1 | 0 | 4 | Yes | Good | 10 |
| 29 | 11 | es | pf | 41 | 2 | 4 | 1 | 4 | 0 | 1 | No | 15 | |
| 30 | 10 | os | df | 24 | 3 | 2 | 0 | 0 | 0 | 2 | No | amp | |
| 31 | 9 | os | pf | 12 | 0 | 0 | 0 | 0 | 0 | 1 | No | 0 | |
| 32 | 14 | es | pf | 12 | 0 | 2 | 0 | 0 | 0 | 2 | No | 0 | |
| 33 | 13 | os | df | 18 | 2 | 1 | 0 | 0 | 0 | 1 | No | 20 | |
| 34 | 9 | es | df | 12 | 0 | 0 | 0 | 0 | 0 | 1 | No | 0 | |
| 35 | 10 | es | pf | 18 | 0 | 3 | 0 | 0 | 0 | 2 | No | 20 | |
| 36 | 7 | es | pt | 204 | 9 | 4 | 3 | 1 | 1 | 3 | Yes | Good | 40 |
| 37 | 13 | es | pf | 24 | 1 | 2 | 0 | 2 | 0 | 0 | No | amp | |
| 38 | 10 | os | df | 156 | 0 | 10 | 1 | 3 | 0 | 6 | Yes | Poor | 150 |
Twenty-one of the 38 patients survived (55%) and all of them reached skeletal maturity during the follow-up period. Their average follow-up time was 10 years (113 months, range 36–204 months) and none of them had evidence of local or systemic disease. There were three local recurrences that underwent amputation and these patients did not survive. All of the 17 deceased patients had metastatic lung disease.
Elongation
The definitive surgery in 35 patients included an expandable prosthesis and it was inserted at a second stage after a temporary spacer was removed in three patients. Twenty-one patients had an elongation procedure (1–12 per patient, yielding a total of 75). The 21 survivors had an average of three elongations per patient. One patient had contralateral epiphysiodesis at the distal femur. At the last follow-up, the average LLD was 37 mm (range 0–150 mm).
Function (AMSTS functional scoring system)
The functional score was excellent in five patients, good in ten, fair in four, and poor in two. There was no significant correlation between function and age at diagnosis or location of the tumor.
Overall complications
The complications were divided into early and late and were conservatively treated or operated (Table 2). Twenty-two patients of the 38 had at least one complication. Among the 21 survivors, 19 had at least one complication and 16 had at least two complications. In total, there were 136 complications, yielding a mean of 3.5 per patient. There was a significant correlation between the number of surgical procedures performed for complications and the final LLD and functional result (P = 0.02).
Table 2.
Complications
| No. | Superficial infection | Deep infection | Nerve damage | Dislocation | Peri-prosthetic fractures | Contractures | Mechanical failure | Other | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | S | C | S | C | S | C | S | C | S | C | S | C | S | C | S | |
| 1 | 1, 1 | 1 | 5 | 1 | 1 | |||||||||||
| 2 | 1 | |||||||||||||||
| 3 | 1 | 3 | 1 | 1 | 1 WHD | |||||||||||
| 4 | 1 WHD | |||||||||||||||
| 5 | 1 | 1 | 3 | 1 | 1 | 2 | 2 | 1 WHD | ||||||||
| 6 | 1 | 2 | ||||||||||||||
| 7 | 2 | 1 | 1 | 1 | 1 | WHD | ||||||||||
| 8 | 1, 2 | 3 | 1 | 3 | 1 | 1 | 1 | 1 NU | ||||||||
| 9 | 2, 1 | 1 AL | ||||||||||||||
| 10 | 3 | 2, 1 | 1 | 1 | 1 | 2 WHD | ||||||||||
| 11 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 2 AL | ||||||||
| 12 | 1 | 1 | 1 | AL | ||||||||||||
| 13 | ||||||||||||||||
| 14 | ||||||||||||||||
| 15 | 2 | 1 | ||||||||||||||
| 16 | ||||||||||||||||
| 17 | 1 | 1 AL | ||||||||||||||
| 18 | 2 | 1 | 1 | |||||||||||||
| 19 | 1 | 1 | 1 | |||||||||||||
| 20 | ||||||||||||||||
| 21 | ||||||||||||||||
| 22 | ||||||||||||||||
| 23 | 1 | 1 | 1 AL | |||||||||||||
| 24 | ||||||||||||||||
| 25 | ||||||||||||||||
| 26 | ||||||||||||||||
| 27 | ||||||||||||||||
| 28 | 1 | 1 | 1 | 1 | 1 NU | |||||||||||
| 29 | 1 | 1, 2 | 1 | |||||||||||||
| 30 | ||||||||||||||||
| 31 | ||||||||||||||||
| 32 | ||||||||||||||||
| 33 | ||||||||||||||||
| 34 | ||||||||||||||||
| 35 | ||||||||||||||||
| 36 | 1 | 2 | 1 | 1 | 2 AL | |||||||||||
| 37 | 1, 1 | |||||||||||||||
| 38 | 3 | 1 | 1, 4 WHD, NU | |||||||||||||
Numbers in bold indicate early complications, whereas normal type indicate late complications
C conservative treatment, S surgical treatment, AL aseptic loosening, NU non-union, WHD wound healing dehiscence
Infection
Infection was the most prevalent complication and it was diagnosed 58 times (47% of the total 38 patients). There were 13 superficial wound infections, of which three required surgical treatment. Deep wound infection was diagnosed 43 times: five (11.6%) were early and 38 were late. Thirty of the deep infections required surgery (32% of surgical procedures other than elongation and the most common indication for revision). All of the revisions that were carried out for infection (n = 19) were comprised of two-stage procedures using a temporary antibiotic-embedded cement spacer. The bacteria cultured in order of frequency were Staphylococcus aureus, Pseudomonas, Enterococcus, and Escherichia coli.
Non-surgical treatment with antibiotics for deep infection was sufficient in only one case (case no. 23 of Table 1). No amputation was needed due to infection.
The end result in four cases was chronic osteomyelitis with a poor functional result and a large LLD. There was a significant negative correlation between function and septic complications (P = 0.02).
Peri-prosthetic fractures and dislocations
There were ten cases of fractures (an incidence of 15.7%), nine of which were late and one was early and all were caused by minor trauma. Nine were treated conservatively with plaster of paris cast and non-weight bearing with excellent results and one patient underwent a revision with a poor outcome (case 38 of Table 1).
There were six cases of subluxation/dislocation, of which three were early and three were late. All dislocations were at the hip joint. One case was treated conservatively (closed reduction plus immobilization) and the rest surgically (open reduction plus acetabular osteotomies), with good results and a stable hip joint at the last follow-up.
Soft tissue problems
Wound healing problems
There were ten cases of wound dehiscence (non-infectious), three of them early and seven late. All were related to primary surgery or revisions, not to elongations. Nine of them were treated by local revisions and soft tissue flaps and the tenth by conservative means.
Contractures
Twenty-one soft tissue contractures were recorded in 13 patients, 19 in the knee area and two at the hip. Forty-five percent of the cases had a surgical release with good functional range of motion at the last follow-up.
Nerve injury
There were eight cases of nerve injury (all early), of which seven were neuropraxia that involved the common peroneal nerve and only one occurred during an elongation procedure. They fully recovered with splints and rest. One case of painful entrapment of the lateral coetaneous nerve of the thigh in a surgical scar was released with a good result (case 11 of Table 1).
Mechanical failure of the prosthesis and aseptic loosening
There were five cases of mechanical failure, which included fatigue fracture of the prosthesis, disassembly of parts, and collapsed/broken elongation mechanism. They were all late complications, with no connection to the elongation procedure and were treated with revision surgery. The six cases of aseptic loosening were all late complications and were treated with revision surgery. In one case (no. 11 of Table 1), the loosening was due to a proved allergic reaction to the titanium in the implant.
Discussion
Many young patients with bone sarcoma survive with a potentially functioning limb. Their demands and expectations from the limb are as high as those of any young active child. This poses many challenges to the surgical and rehabilitation team since children still in their skeletal growing years will develop LLD, one of the main factors influencing the quality of life and satisfaction of LSS. Using an expandable endoprosthesis is one of the possibilities for addressing these issues and it has gained considerable popularity during the past 20 years. However, this solution is not without problems and disadvantages, even when considering the technical and metallurgical advances that have been made.
The overall survival rate in this group was 55%, which is slightly less than the 60–70% reported in other publications [9, 17]. One explanation for this discrepancy is that 12 patients who had Ewing’s sarcoma and five patients at stage III (metastatic at presentation) were included, both being groups with less favorable survival rates.
When the data of these 17 patients were removed from the analysis, the overall survival was the similar to the other reports [18].
Among the survivors, every patient underwent 8.7 surgeries during his/her growth period, which is also similar to other reports [18].
Seventeen of the survivors had elongations of up to 20 mm each time (an average of three for each patient). At the final follow-up, the average LLD was 37 mm. This LLD was well tolerated, in most cases compensated by elevations of shoes, and the AMSTS function score was satisfactory (excellent and good) in 17 patients.
A multivariate analysis revealed that the only significant difference between the group of the survivors with satisfactory results and the group of the survivors with unsatisfactory results (fair and poor) was LLD > 50 mm in the latter group (P = 0.005) (Fig 6).
Fig. 6.
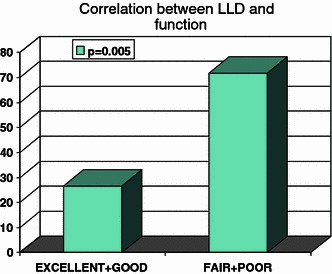
Correlation between functional result and the final limb length discrepancy
The most common complication was infection. The rate of deep wound infection was 38%, a number similar to the report by Schiller et al. [19] and slightly higher than the 27% reported by Futani et al. [9]. Most of the deep infections were a late event and were the main indication for more than half of the revisions. They are indicative of the vulnerability of these patients due to their basic immunocompromised status and the long period of exposure to the risk of a hematogenous infection. The patients with a deep infection needed more operations, their final LLD was significantly larger, and their final function score was reduced. None of our patients underwent an amputation because of infection, even after the team recommended it following a failed two-stage revision and several episodes of massive debridements.
Our patients preferred to live with chronic infections, long-term suppressive antibiotic therapy, and flare-ups.
The contracture of soft tissues, especially around the knee, was the second most common complication affecting half of all our patients. Each case first underwent intensive physiotherapy, usually when hospitalized and under the supervision of the rehabilitation team. This approach failed in 45% of cases, and those children underwent corrective surgery with good results. Contracture of soft tissues is described by others; Neel and Letson [20] reported that, after each elongation, the 37 patients which they followed had 1 week of intensive in-hospital physiotherapy and continued therapy at home thereafter. Eckardt et al. reported a rate of 9% contractures and a 25% rate of soft tissue problems [21] and Schiller et al. [19] reported that all six of their patients had difficulty reaching a good range of motion after each elongation.
Aseptic loosening (which occurred in 28% of our 21 survivors), implant mechanical failure (23%), and dislocations of the hip joint (10.5%) are major problems influencing the survival of the prosthesis. They were also reported in other publications and in similar numbers [8, 9, 22, 23]. They were mostly treated by revision surgery and represent 35% of the indications for the 29 revisions. The survivorship of the endoprosthesis are represented in a Kaplan–Meier curve (Fig. 7) showing the chances for failure of an endoprosthesis against the time since the implantation was done.
Fig. 7.
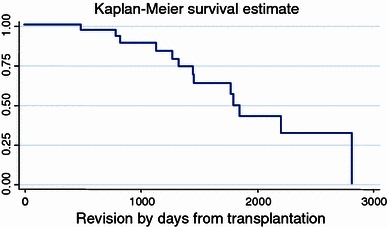
Survivorship over time from implantation to revision
One of the assumptions which we made was that there is a difference in survivorship of the endoprosthesis between patients younger than 10 years and those who were older. We analyzed the difference between these groups using a log-rank test represented by the curves in Fig. 8 and found that there was a difference, but it did not reach a level of significance (log-rank > 0.05). We found no significant correlation between the age groups and several other factors, such as the number of infections, the number of non-infectious complications, and the number of revisions performed. We also did not find a significant correlation between the anatomical location of the tumor and these factors. In spite of the many problems with which these young patients have to cope, 71% had a satisfactory outcome at the last follow-up. Three patients underwent amputations, all for local recurrence after LSS, a figure similar to that reported by others [24].
Fig. 8.
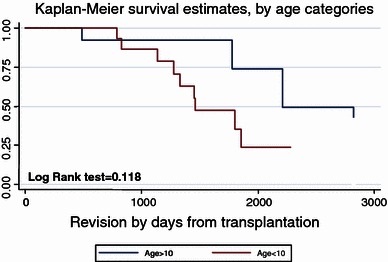
Survivorship of implant by age
The limitations of our study are the relatively small number of patients and the fact that there was no single prosthetic device used over the years but, rather, several types. Another limitation is the diversity in diagnosis and differences in the location of the tumor in the limb. It seems that our study is more an observational one, since we deal with 38 complicated and different case studies, rather than with a simple consecutive series.
In conclusion, LSS using expandable endoprostheses for skeletally immature patients is difficult to manage and bears many complications, but is worthwhile and beneficial for most patients.
Careful patient and family selection is imperative and only those who are highly motivated and willing to withstand the long and difficult process should be considered for this surgery. The prevention of deep infection is critical for success, and possible ways for doing so include silver coating of the prostheses, which has proved to be beneficial in other studies [25] and the use of non-invasive endoprostheses (the ultimate solution).
A final LLD of about 3 cm allows good function and is easily treated by special footwear and insoles. It might sometimes be preferable to have this much of an LLD than to cause more complications by trying to achieve limb length equality.
Finally, if possible, one revision at the end of skeletal growth and replacement by a non-expanding endoprosthesis might be beneficial.
Future expectations are that non-invasive elongating mechanisms or a biological approach will be able to answer the special needs of this population.
References
- 1.DiCaprio MR, Friedlaender GE. Malignant bone tumors: limb sparing versus amputation. J Am Acad Orthop Surg. 2003;11:25–37. [PubMed] [Google Scholar]
- 2.Dominkus M, Krepler P, Schwameis E, Windhager R, Kotz R. Growth prediction in extendable tumor prostheses in children. Clin Orthop Relat Res. 2001;390:212–220. doi: 10.1097/00003086-200109000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Cool WP, Carter SR, Grimer RJ, Tillman RM, Walker PS. Growth after extendible endoprosthetic replacement of the distal femur. J Bone Joint Surg Br. 1997;79:938–942. doi: 10.1302/0301-620X.79B6.7868. [DOI] [PubMed] [Google Scholar]
- 4.Lewis VO. Limb salvage in the skeletally immature patient. Curr Oncol Rep. 2005;7:285–292. doi: 10.1007/s11912-005-0052-7. [DOI] [PubMed] [Google Scholar]
- 5.Malawer M. Surgical technique and results of limb sparing surgery for high grade bone sarcomas of the knee and shoulder. Orthopedics. 1985;8:597–607. doi: 10.3928/0147-7447-19850501-14. [DOI] [PubMed] [Google Scholar]
- 6.Grimer RJ. Surgical options for children with osteosarcoma. Lancet Oncol. 2005;6:85–92. doi: 10.1016/S1470-2045(05)01734-1. [DOI] [PubMed] [Google Scholar]
- 7.Unwin PS, Walker PS. Extendible endoprostheses for the skeletally immature. Clin Orthop Relat Res. 1996;322:179–193. [PubMed] [Google Scholar]
- 8.Letson GD, D’Amato G, Windham TC, Muro-Cacho CA. Extendable prostheses for the treatment of malignant bone tumors in growing children. Curr Opin Orthop. 2003;14:413–418. doi: 10.1097/00001433-200312000-00010. [DOI] [Google Scholar]
- 9.Futani H, Minamizaki T, Nishimoto Y, Abe S, Yabe H, Ueda T. Long-term follow-up after limb salvage in skeletally immature children with a primary malignant tumor of the distal end of the femur. J Bone Joint Surg Am. 2006;88:595–603. doi: 10.2106/JBJS.C.01686. [DOI] [PubMed] [Google Scholar]
- 10.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 11.Frieden RA, Ryniker D, Kenan S, Lewis MM. Assessment of patient function after limb-sparing surgery. Arch Phys Med Rehabil. 1993;74:38–43. [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1953;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 13.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 14.Bacci G, Ferrari S, Mercuri M, Longhi A, Capanna R, Tienghi A, Brach del Prever A, Comandone A, Cesari M, Bernini G, Picci P. Neoadjuvant chemotherapy for extremity osteosarcoma—preliminary results of the Rizzoli’s 4th study. Acta Oncol. 1998;37:41–48. doi: 10.1080/028418698423168. [DOI] [PubMed] [Google Scholar]
- 15.Keohan ML, Taub RN. Chemotherapy for advanced sarcoma: therapeutic decisions and modalities. Semin Oncol. 1997;24:572–579. [PubMed] [Google Scholar]
- 16.Bramwell VHC. The role of chemotherapy in the management of non-metastatic operable extremity osteosarcoma. Semin Oncol. 1997;24:561–571. [PubMed] [Google Scholar]
- 17.Schindler OS, Cannon SR, Briggs TW, Blunn GW. Stanmore custom-made extendible distal femoral replacements. Clinical experience in children with primary malignant bone tumours. J Bone Joint Surg Br. 1997;79:927–937. doi: 10.1302/0301-620X.79B6.7164. [DOI] [PubMed] [Google Scholar]
- 18.Belthur MV, Grimer RJ, Suneja R, Carter SR, Tillman RM. Extensible endoprostheses for bone tumors of the proximal femur in children. J Pediatr Orthop. 2003;23:230–235. [PubMed] [Google Scholar]
- 19.Schiller C, Windhager R, Fellinger EJ, Salzer-Kuntschik M, Kaider A, Kotz R. Extendable tumour endoprostheses for the leg in children. J Bone Joint Surg Br. 1995;77:608–614. [PubMed] [Google Scholar]
- 20.Neel MD, Letson GD. Modular endoprostheses for children with malignant bone tumors. Cancer Control. 2001;8:344–348. doi: 10.1177/107327480100800406. [DOI] [PubMed] [Google Scholar]
- 21.Eckardt JJ, Kabo JM, Kelley CM, Ward WG, Sr, Asavamongkolkul A, Wirganowicz PZ, Yang RS, Eilber FR. Expandable endoprosthesis reconstruction in skeletally immature patients with tumors. Clin Orthop Relat Res. 2000;373:51–61. doi: 10.1097/00003086-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Baumgart R, Hinterwimmer S, Krammer M, Muensterer O, Mutschler W. The bioexpandable prosthesis: a new perspective after resection of malignant bone tumors in children. J Pediatr Hematol Oncol. 2005;27:452–455. doi: 10.1097/01.mph.0000178268.07830.d5. [DOI] [PubMed] [Google Scholar]
- 23.Tillman RM, Grimer RJ, Carter SR, Cool WP, Sneath RS. Growing endoprostheses for primary malignant bone tumors. Semin Surg Oncol. 1997;13:41–48. doi: 10.1002/(SICI)1098-2388(199701/02)13:1<41::AID-SSU7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68:1331–1337. [PubMed] [Google Scholar]
- 25.Ewald A, Glückermann SK, Thull R, Gbureck U. Antimicrobial titanium/silver PVD coatings on titanium. Biomed Eng Online. 2006;5:22. doi: 10.1186/1475-925X-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]


