Abstract
Background
Pericapsular acetabuloplasty procedures have been widely used as an integral component of combined surgery to treat developmental hip dislocation after walking age. The stability of the acetabuloplasty and the maintenance of the acetabular correction will depend on the structural integrity of the iliac crest autograft, which, traditionally, has been inserted as the interposition material. Problems related to the use of an autograft have been encountered by various surgeons—including the authors—namely, graft displacement and resorption, which may necessitate internal fixation or result in revision surgery. To overcome autograft failure, the use of an allograft as the interposition material has been introduced by some surgeons. This study describes the radiologic results of 147 hips treated for developmental hip dislocation by means of a standard protocol of open hip reduction and pericapsular acetabuloplasty with a contoured iliac crest allograft as the interposition material.
Methods
This retrospective study reviewed the radiographs of 147 hips presenting with late developmental dislocation which were treated by open reduction and a concomitant pericapsular acetabuloplasty using a contoured iliac crest allograft as the interposition material. The minimum follow up period was 2 years. Measurement of the acetabular index (AI) was the main variable. The efficacy of the interposed iliac crest allograft as the main stabiliser of the acetabuloplasty was reflected by the maintenance of the corrected AI during the follow up period. Loss of acetabular correction, graft extrusion or resorption, the need for osteotomy internal fixation, delayed or non union, infection, hip redislocation and avascular necrosis (AVN) as possible complications were documented.
Results
The treatment protocol of a combined open reduction of the hip and pericapsular acetabuloplasty, inserting a contoured iliac crest allograft as the interposition material, resulted in concentrically reduced and stable hips in 96.6% of our cases. The redislocation rate was 3.4%. All of the allografts were completely incorporated at 6 months post-surgery with no graft-related infections. In only two hips was the acetabular correction not maintained. None of the osteotomies required internal fixation for stability, even in older children.
Conclusion
We believe that a contoured iliac crest allograft as the pericapsular acetabuloplasty interposition material renders excellent osteotomy stability that eliminates the need for internal fixation and—in the short-term—maintains the correction of the acetabulum achieved intra-operatively.
Keywords: Hip dysplasia, Pericapsular osteotomy, Allograft
Introduction
Developmental dysplasia of the hip (DDH) is a condition with incidence ranging from low (0–0.1 per 1,000) to high (5–195 per 1,000) [1]. The incidence of DDH in the southern region of Saudi Arabia has been reported to be moderate (3.5 per 1,000). Most cases of DDH in Saudi Arabia, however, are late presenting, with a mean age at diagnosis of 14.5 months [2].
The treatment of late presenting DDH at walking age is operative. The surgery comprises an open relocation of the hip in conjunction with a pelvic osteotomy to correct the acetabular dysplasia and afford immediate hip stability. In older children, a femoral shortening osteotomy may be required as an additional procedure to facilitate relocation of the hip. Combined surgical procedures to treat DDH in this age group have become the norm.
Pericapsular osteotomies have widely been used as an important part of the combined surgery strategy to treat developmental hip dislocation after walking age. The stability and the maintenance of this osteotomy are very much dependent on the strength of the iliac crest autograft interposition, which was used in the original description of this procedure. The authors have, over the years, not been satisfied that iliac crest autograft as the interposition material for the osteotomy is structurally sound and sufficiently stable. Problems such as graft extrusion, rotation and absorption, leading to loss of acetabular correction, were often noted in cases previously treated at the authors’ institution. In cases of bilateral dislocation, the iliac crest autograft was found to be profusely weak at the time of the second hip surgery due to spica immobilisation after first hip surgery. Autograft concern is further supported by authors who suggest the routine use of internal fixation, especially in older children [3, 4]. Graft displacement resulting in redislocation of the hip has been reported in the literature [5, 6]. We believe that these complications can be attributed to the paucity of the autograft. Concerns regarding the integrity of the autograft prompted the authors to use a more stable and structurally sound interposition material which could be contoured to the shape of the osteotomy site. The use of allograft in paediatric orthopaedics has gained acceptance and is regarded to be safe.
Over the past 7 years at King Faisal Specialist Hospital and Research Center (KFSH&RC) in Riyadh, we have treated late presenting developmental dislocated hips by means of an open reduction and a routine concomitant pericapsular acetabuloplasty adaptation utilising contoured iliac crest allograft as the interposition material.
This retrospective study reviewed the radiographic results of this treatment protocol in 147 developmental dislocated hips which met our inclusion criteria. The efficacy of this method to achieve and maintain a well covered and stable hip was the main objective of the study. Possible complications such as loss of acetabular correction, hip redislocation, graft extrusion, the need for osteotomy internal fixation, delayed or non union, infection or avascular necrosis (AVN) were documented in this series.
Methods
We reviewed the antero posterior (AP) pelvis X-rays of children who were surgically treated for late presenting developmental dislocation of the hip by means of an open reduction and a routine concomitant pericapsular acetabuloplasty adaptation, using a contoured iliac crest allograft as the osteotomy interposition material.
The main criteria for inclusion in the study were an untreated, delayed developmental dislocation of the hip, Tonnis [7] type 3 or 4 (Table 1). The exclusion criteria were acetabular dysplasia without dislocation, revision surgery for DDH and dislocated hips associated with neuromuscular disease or teratologic conditions.
Table 1.
Tonnis classification of hip dysplasia
| Grade | Criteria |
|---|---|
| 1 | Capital femoral epiphysis medial to Perkin’s line |
| 2 | Capital femoral epiphysis lateral to Perkin’s line but below the level of the superior acetabular rim |
| 3 | Capital femoral epiphysis at the level of the superior acetabular rim |
| 4 | Capital femoral epiphysis above the level of the superior acetabular rim |
The study comprised 147 dislocated hips in 118 patients treated over the 5-year period from 2003 to 2007. The age at index surgery, sex and bilateral involvement of the dislocated hips were documented. The minimum post-surgery follow up was 2 years.
The acetabular index (AI) was measured as the main variable to evaluate the correction of the acetabular dysplasia and the subsequent maintenance thereof. The AI was measured pre-operatively, day one post-operatively and at 3, 6 months, 1 and 2 years. The X-rays were reviewed by a single observer to eliminate inter-observer error.
The allograft was considered to have been stable if the correction of the dysplastic acetabulum was maintained during the 2-year post-operative period and non extrusion of the graft had occurred from the osteotomy site. Allograft incorporation into the ilium was considered to have taken place if complete union at the osteotomy site had occurred and confluence of the graft to the ilium was evident on the X-rays.
The status of reduction of the femur head was measured by means of the Tonnis classification and was documented throughout the follow up period.
Avascular necrosis (AVN) of the femur head was documented and classified according to the system of Kalamchi and MacEwen [8].
The statistical correlation of all variables was done by means of the SPSS software package.
Surgical technique
A conventional open reduction of the hip is done through an anterior approach with routine soft tissue releases. Reduction under tension in older children necessitates a femoral shortening osteotomy. Stability of the reduced hip is assessed intra-operatively. Should instability be present with the hip in extension, the acetabular deficiency is located mainly anterior, and if instability is present in adduction, the deficiency is more lateral [9, 10]. This information helps to determine the amount and direction of the acetabulum roof displacement (acetabuloplasty) to render the required coverage of the femur head.
The osteotomy is done through the outer iliac table only, obviating the necessity for inner iliac table dissection. The joint capsule is mobilised off the false acetabulum, taking care to protect the acetabular cartilage rim. The outer iliac table is exposed from the anterior inferior iliac spine (AIIS) to the greater sciatic notch to allow adequate visualisation. The level and direction of the osteotomy is determined and guided with the aid of fluoroscopy. It is marked 1 cm proximal to and parallel with the curved bony roof of the true acetabulum. The osteotomy cut runs curvilinear from the level of the AIIS to the triradiate cartilage of the ischium (Fig. 1). The outer cortex is breached in a perpendicular plane with a straight quarter inch osteotome, taking care to maintain the 1 cm bone distance from the joint. Distal slippage of the osteotome on the oblique false acetabulum cortex should be avoided, as it could compromise the thickness of the acetabulum roof, rendering it vulnerable to an intra-articular fracture during osteotomy displacement. A quarter inch curved osteotome is now used to complete the osteotomy, which should converge towards the triradiate cartilage of the ischium (Fig. 2). The thinner anterior two-thirds portion of the ilium is breached completely through the inner table by directing the osteotome medially and caudally. The iliacus muscle protects the intra-pelvic structures in this area, but controlled penetration of the inner table is essential. The osteotomy breaches the outer table of the posterior third of the ilium, ending at the triradiate cartilage. The inner cortex and the posterior column are left intact (Fig. 4).
Fig. 1.
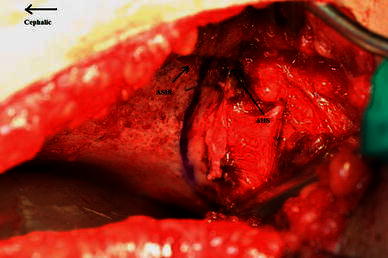
The level of osteotomy is marked 1 cm proximal to the acetabulum
Fig. 2.
The direction of the curved osteotomy towards the triradiate cartilage
Fig. 4.
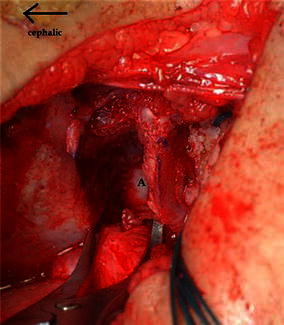
The lamina spreader is inserted posteriorly in the osteotomy site. A shows the intact posterior third of the medial cortex
Inserting a broader half inch curved osteotome allows controlled leverage to open the osteotomy site (Fig. 3). A small curved lamina spreader is now inserted into the posterior corner of the osteotomy site (Fig. 4). The device opens the osteotomy to the required extent and toggling of the spreader can adjust the distal fragment in either an anterior or a lateral direction, whichever is required to cover the femur head sufficiently. Posterior placement of the instrument in the apex of the osteotomy also increases the power of correction and allows comfortable placement of the allograft in the middle third of the osteotomy.
Fig. 3.
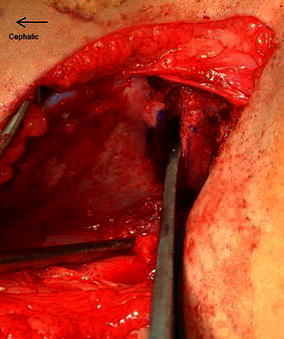
The broad half inch osteotome is used to open the osteotomy
The extent of osteotomy displacement and the direction thereof is determined by clinical assessment of hip stability and intra-operative arthrography. The objective is to obtain a bony AI of ~20° (Fig. 5).
Fig. 5.
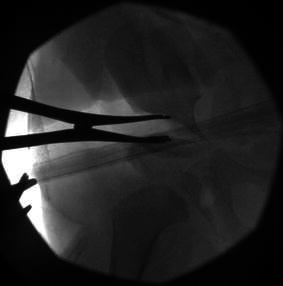
An intra-operative image of the right hip demonstrates the opening of the pericapsular osteotomy by the lamina spreader to ~20° acetabular index (AI)
The extent of osteotomy displacement varies in patients and depends upon the degree of acetabular dysplasia which needs to be corrected. It is also age dependant and, generally, older children would require greater osteotomy displacement to correct the dysplasia.
The configuration of the osteotomy opening is a contoured triangular wedge. The height and length of the iliac crest allograft can now be determined by measuring the opening and depth at the mid osteotomy level.
The iliac crest allografts come in different sizes, varying from 1 to 1.5 cm base and 2.5 to 3 cm length (Fig. 6). The selected size is contoured to fill most of the osteotomy opening and is impacted posteriorly and medially into the middle third.
Fig. 6.
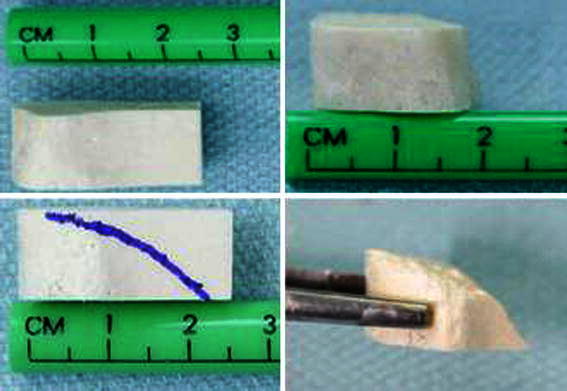
The dehydrated iliac crest allograft dimensions before and after contouring
The strong base of the allograft should ideally be flush with or medial to the superior edge of the osteotomy in the outer table to ensure stability and prevent possible extrusion (Fig. 7).
Fig. 7.
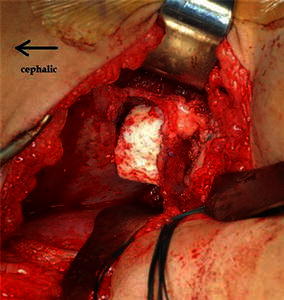
The iliac crest allograft is impacted into the osteotomy site
In older children, the osteotomy opening is occasionally too large to be adequately filled by the available sizes of allograft. In such cases, we contour the largest available allograft based upon a trigonometric ratio.
For example, if the AI before the surgery is 40° and the desired AI after the surgery is 20°, this necessitates the osteotomy to be distracted to 20° at its apex and the allograft needs to be contoured in height and length to maintain the correction. If the largest available allograft measures 30 mm in length and 15 mm in height, the graft should be contoured according to the following formula:
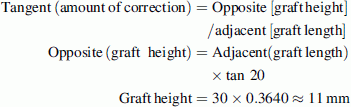 |
The graft dimensions after contouring should be 30 × 11 mm. This relatively small but sound graft is then impacted to reach the apex of the osteotomy to maintain the desired correction (Fig. 8).
Fig. 8.
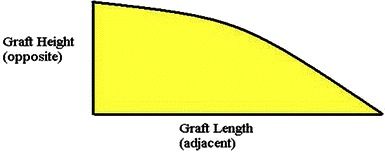
The allograft contour by which trigonometric ratios between the height and length are maintained to produce the desired angle at the apex of the osteotomy
When the lamina spreader is removed, the inherent elasticity of the distal fragment wedges the allograft against the ilium, rendering stability and preventing graft extrusion. This obviates the need for internal fixation. The capsulorrhaphy is now completed (Fig. 9).
Fig. 9.
Post-operative picture showing the desired graft placement
Stability of the hip is now reconfirmed clinically by a medial ballottement test across the greater trochanter. Should the hip be unstable, a K-wire is passed percutaneously through the greater trochanter apophysis, passing extra articularly into the ilium above the acetabulum.
The anterior and lateral aspect of the osteotomy site is filled with the remainder of the iliac crest allograft to increase the superior acetabular bone stock. The wound is closed in layers and a 1 and a half hip spica is applied with the hip in ~30° of flexion, 30° of abduction and neutral rotation.
The post-operative hospital stay is 48 h. The spica cast remains in place for 6 weeks and is converted to a broom-stick cast for a further 6 weeks.
Results
In this study, 147 hips in 118 patients met the inclusion criteria and were treated by means of an open reduction of the hip and a concomitant pericapsular acetabuloplasty. Ninety-five (80%) were female and 23 patients (20%) were male. Sixty-eight patients had unilateral hip involvement, of which 38 were isolated left hip involvement (33.3%) and 30 hips were isolated right hip involvement (26.3%). The remaining patients had bilateral involvement (40.4%). The minimum follow up period was 2 years. The age of the patients at surgery varied from 1.5 to 11.2 years, with a mean of 2.9 years. The patients’ data are shown in Table 2. Five hips were reoperated for subluxation or redislocation. The results of their revision surgeries were not included.
Table 2.
Patient data
| Variable | N | Median | Mean | SD | Min. | Max. | Range |
|---|---|---|---|---|---|---|---|
| Age | 147 | 2.3 | 2.9 | 1.8 | 1.5 | 11.2 | 9.7 |
| AI 0 | 147 | 43.3 | 43.2 | 6.9 | 22.1 | 65.3 | 43.2 |
| AI 1 | 147 | 24.4 | 24.3 | 8.8 | 0 | 48.1 | 48.1 |
| AI 3 m | 143 | 21.2 | 21.1 | 8.4 | 0 | 42.9 | 42.9 |
| AI 6 m | 143 | 19.7 | 19.9 | 8.6 | −9.4 | 43.6 | 53 |
| AI 12 m | 141 | 19 | 18.4 | 6.8 | 4.3 | 36.4 | 32.1 |
| AI 24 m | 142 | 17 | 16.9 | 6.7 | −4.2 | 37.2 | 41.4 |
| Power of correction | 147 | 18.4 | 18.9 | 9.4 | 2 | 58.5 | 56.5 |
AI 0 acetabular index before surgery, AI 1 acetabular index after surgery, AI 3 m acetabular index at 3 months, AI 6 m acetabular index at 6 months, AI 12 m acetabular index at 12 months, AI 24 m acetabular index at 24 months, Power of correction acetabular index before surgery − acetabular index after surgery
The acetabular indices were studied throughout the whole series. The average pre-operative AI was 43.2°, which was corrected to an average of 24.3° post-operatively. The average AI correction was 18.9°. The AI was maintained in the post-operative period and improved spontaneously over the ensuing 6 month period to a mean of 19.9° and 16.9° at 2 years follow up (Table 2). We ascribe the maintenance of the AI to the structural integrity of the allograft and the improvement of the AI to the concentric reduction and stability of the relocated hip.
Only 2 (1.4%) of the 142 hips which completed 2 years follow up lost acetabular correction. One was ascribed to surgical technique error. The other had a post-operative AI of 28°, which deteriorated to 40° at 6 months post-surgery. A possible explanation for this rapid deterioration could be graft resorption.
All allografts were fully incorporated at 6 months post-surgery, with the average incorporation time being 3 months.
All of the osteotomies were intra-operatively deemed to be stable after allograft placement, and none required additional internal fixation (Fig. 10).
Fig. 10.
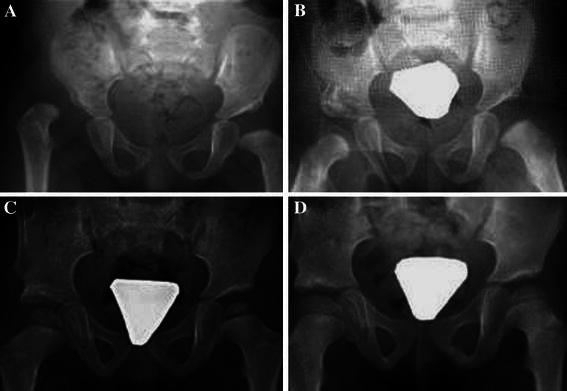
Serial radiographs of a child with right hip developmental dislocation: a pre-operative, b post-operative, c 1 year post-operative, d 2 years after the surgery
Forty-four hips (30%) required a femoral shortening osteotomy and 8 hips (5.4%) needed additional K-wire fixation to manage residual hip instability. The probability of residual hip instability increased with advancing age at the time of surgery. This correlation was estimated by a T-test and the P value was found to be 0.05. The residual hip instability was also found to correlate with inadequate acetabular correction. Hips that needed K-wire stabilisation were found to have a mean of only 10° of AI correction in comparison to the stable hips, where an average of 19° of acetabular correction was attained at the index procedure. This finding is significant, with a P value of 0.01.
At 6 months follow up, 142 hips (96.6%) were found to be concentrically reduced (Tonnis type 1), 3 hips (2%) had subluxated (Tonnis type 2) and 2 hips (1.4%) had redislocated (Tonnis type 3). Upon diagnosis, the subluxated and redislocated hips underwent revision surgical relocation (Table 3).
Table 3.
Subluxated and redislocated hips
| Age at surgery | Sex | Bilateral | Surgery type | Surgery site | AI 0 | AI 1 | K-wire | Time of displacement | Type of displacement |
|---|---|---|---|---|---|---|---|---|---|
| 5.17 | M | Y | P. osteotomy | R | 35 | 24 | N | 6/12 | Tonnis 2 |
| 5.25 | F | N | P. osteotomy | L | 50 | 26.8 | N | 6/12 | Tonnis 2 |
| 5.5 | F | N | P. osteotomy | R | 45.6 | 38.5 | Y | D 1 | Tonnis 2 |
| 2.1 | M | Y | P. osteotomy | R | 34.2 | 31.6 | N | 6/52 | Tonnis 3 |
| 2.1 | M | Y | P. osteotomy | R | 48.3 | 40.5 | N | 6/12 | Tonnis 3 |
P pericapsular
Although redislocation occurred more frequently in male patients, it was not of statistical significance. Redislocation also failed to correlate with other factors such as age at surgery, bilaterality, AI before surgery, AI after surgery or power of correction.
The presence of AVN and the type according to Kalamchi and MacEwen [8] was observed to be present in 21 of 142 hips. Fifteen hips (10.6%) developed type 1 AVN, 3 hips (2.1%) type 2 AVN, 3 (2.1%) type 3 AVN and no type 4 AVN was seen. There was no correlation between the age at surgery or post-operative AI and the incidence of AVN. The mean post-operative AI was 24° in both the AVN and non AVN groups. However, using the analysis of variance (ANOVA) test, there was a significant correlation between the pre-operative AI and the presence of AVN. Hips which developed AVN tended to have a lower pre-operative AI (mean of 32.3° in patients with type 3 AVN compared to 43.9° in the non AVN group), with a P value of <0.01. The AI correction was lower in the AVN group (mean 15°) compared to the non AVN group (mean 20°), with a P value of 0.03.
There were no graft-related infections in this series. The blood loss from this procedure is acceptable, with a maximum of 2 g/dl drop in the haemoglobin level post-operatively. Post-operative blood transfusion was seldom required.
Discussion
Simultaneous open reduction of the hip and a pelvic osteotomy to correct the dysplastic acetabulum has been proposed by many authors, irrespective of the type of pelvic osteotomy which they perform [11–13]. This simultaneous approach renders immediate stability to the surgically reduced hip and minimises the need for secondary procedures to correct residual acetabular dysplasia [14, 15]. Surgical relocation of the hip in isolation renders adequate stability in only 2–37% of cases over 12 months of age [9, 10]. The complication rate with combined treatment modalities compares favourably to that of staged surgical procedures [11–13].
Pelvic osteotomies were initially introduced for the treatment of dysplastic hips in children by Robert Salter in 1961 [16]. The procedure he described entails a complete innominate osteotomy with redirection of the acetabulum. Internal fixation for this procedure is required to ensure stability of the osteotomy. This necessitates a secondary procedure for removal of the internal fixation.
Paul Pemberton in 1965 [5] described an osteotomy to correct acetabular dysplasia which he labelled as a pericapsular osteotomy. This is an incomplete osteotomy of the ilium which permits downward and lateral displacement of the roof of the acetabulum to cover the reduced femoral head. The osteotomy is done curvilinear 1 cm proximal to the socket through the outer table of the ilium, penetrates the anterior two-thirds of the inner table and stops at the posterior limb of the triradiate cartilage of the ischium. It is postulated that the osteotomy hinges at this point due to the inherent plasticity of the triradiate cartilage. It has also been suggested that the displacement and reorientation of the superior acetabulum either in a more anterior or lateral direction can be subtly influenced by minor variations in the sagittal placement of the osteotomy cuts. This procedure has been reported to have good results, even in older age groups [3, 4]. The original surgical technique describes both tables of the ilium to be exposed, which increases the amount of intra-operative bleeding [5].
Several modifications of the classical Pemberton osteotomy have been proposed. The variations differ mainly in regards to the endpoint of the posterior osteotomy cut and, therefore, the site of the hinge. Perlik et al. [17] described an osteotomy extending distal to the posterior limb of the triradiate cartilage into the ischium. Tavares [18] described a posterior osteotomy line being more horizontal and ending anterior to the greater sciatic notch, relying on a green stick fracture as pivot at that level. Both iliac tables were exposed to perform these osteotomies.
Dega in 1969 developed a similar pelvic osteotomy to correct acetabular dysplasia [19]. This procedure was not thoroughly described until Grudziak and Ward published their experience in 2001 [20]. This procedure is similar, in principle, to the classic Pemberton procedure. The osteotomy is, however, done only through the outer iliac table, requiring fluoroscopy to monitor the direction of instrumentation through the unexposed inner iliac table. Mubarak et al. [21] described a variation of the Dega osteotomy to suitably render hip stability in cerebral palsy, where the acetabular dysplasia is located posteriorly. This osteotomy differs from our technique in that it breaches the outer and inner iliac tables at the most anterior and posterior aspects whilst cutting the outer table only in the middle section.
We choose to refer to the osteotomy we perform as a pericapsular acetabuloplasty to eliminate the confusion that could result from calling it either a Pemberton or Dega osteotomy. The osteotomy hinges through the posterior limb of the triradiate cartilage as does the Pemberton osteotomy, but as with the Dega osteotomy, requires less surgical exposure. We believe that both osteotomies (Pemberton and Dega) follow similar principles and target the same goal.
All pericapsular osteotomies have features which could be regarded as advantages. Firstly, the acetabular volume increases by almost 60% [22–24]. Secondly, the power of correction of acetabular dysplasia is quite significant. Thirdly, they are considered to be relatively stable osteotomies with little need for internal fixation, especially in younger children.
Iliac crest autograft has traditionally been used as the interposition material in the osteotomy site but the lack of sufficient graft thickness and density required for stability has been a concern for many surgeons. In older children, the need for autograft fixation has been reported to be as high as 70% [3, 4]. Internal fixation of the pericapsular osteotomy necessitates additional surgical dissection, longer operation time, higher incidence of joint penetration and second surgery for the removal of hardware. These co morbidities can be avoided if the osteotomy interposition material could provide sufficient stability. Autograft extrusion and resorption with loss of acetabular correction has frequently been reported [5, 6]. The lack of graft integrity might lead to residual acetabular dysplasia and even redislocation of the hip, which may require revision surgery. The stability of the osteotomy depends very much on the structural integrity of the interposed bone grafting, which needs to withstand the inherent recoil plasticity forces across the displaced osteotomy site. In order to demonstrate such deficiencies in a paediatric iliac crest, we reviewed a number of cases at our institution that had pelvic computed tomography (CT) scans done for unrelated orthopaedic problems. We found that the maximum iliac crest thickness at the tubercle level measures 4 mm at the age of 1 year and 9 mm at the age of 7 years (Fig. 11).
Fig. 11.
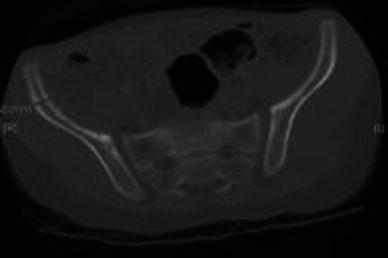
Computed tomography (CT) scan of the pelvis in a 2-year-old
Allografts have commonly been used in orthopaedic practice and, in particular, in paediatric orthopaedic surgery with good safety and efficacy [25–27]. Interest gradually developed in the utilisation of allografts as the interposition material for pericapsular osteotomies. Grudziak and Ward [20] referred to the use of a fibula allograft to render stability in a Dega osteotomy for revision surgery.
Kessler et al. [28] reported their experience with patella allografts in 26 Pemberton osteotomies and concluded that the technique yielded a good correction of the acetabular dysplasia with immediate graft stability. Their series included only one case of developmental hip dislocation. They used absorbable pins for internal fixation in their series, which could have possibly contributed to the stability of the allografts.
As of 2003, we routinely treat developmental dislocated hips with an open reduction and a concomitant pericapsular acetabuloplasty using a contoured iliac crest allograft as the interposition material to avoid the complications related to autograft use. All of the 147 hips reported on in this study were treated with this standardised protocol.
The tricortical iliac crest allografts that were used in this treatment protocol were imported from bone banks that adhere to the standards of the American Association of Tissue Banks (AATB). These grafts are of a processed type (freeze-dried) which have been proven to be safe [29].
The adult tricortical iliac crest allograft we use comes in various sizes related to thickness, height and length. The allografts are rectangular in shape, with the height and length varying from 10 × 25 mm to 15 × 30 mm. The graft thickness varies from 10 to 20 mm. The crest of the ilium forms the base of the allograft, which is broad with a thick cortex and structurally sound. This structural integrity readily withstands impaction forces during insertion in the osteotomy site (Fig. 6).
The iliac crest allograft is contoured to conform to the configuration of the middle third of the osteotomy site. This parabola-like triangular configuration of the contoured allograft and the substantial surface area thereof due to its width contributes to the stability of the graft, which is further augmented by the inherent recoil plasticity of the acetabulum roof. Once the lamina spreader is removed, this stability is evident intra-operatively by the graft not being able to be translated or rotated. This exceptional graft stability eliminates the need for routine internal fixation of the osteotomy, even in older children. Graft extrusion or displacement was not encountered in our series.
Another advantage of the iliac crest allograft is that the larger contact surface area acts as a tamponade on the osteotomy surfaces, reducing intra-operative and immediate post-operative bleeding. This was encountered in our study, where the drop in haemoglobin was minimised to 2 g/dl and obviated the need for blood transfusion, which, in turn, reduces the potential for disease transmission.
The allografts were all incorporated into the ilium and the osteotomies were fully healed by 6 months post-surgery. This 6-month time frame was deemed to be sufficient to effectively assess the biologic response of the allograft within the ilium.
The cost of a single allograft is ~US $530. It is difficult to determine the cost-effectiveness of the allograft. The absence for the need of routine internal fixation in all of the 147 osteotomies could justify its routine use, especially in older children.
This surgical protocol yielded concentrically reduced and stable hips in 96.6% of hips treated.
We measured acetabular correction by the bony AI, which is considered as a valid method for the radiographic assessment of acetabular development [30, 31]. We elected not to use the central-edge angle of Weiberg (CE angle), as the femoral heads in the majority of hips were partially ossified.
The average correction of the dysplastic acetabulae was 18.9°. The AI was maintained in the post-operative period and improved spontaneously over the ensuing 6-month period to a mean of 19.9° and 16.9° at 2 years follow up. We ascribe the maintenance of the AI to the structural integrity of the allograft and the improvement of the AI to the concentric reduction and continued stability of the relocated hip.
A post-operative AI exceeding 25° was regarded as residual acetabular dysplasia and attributed to under correction at the time of surgery. At 6 months post-surgery, this was present in 34 of the 142 hips (24%) and in only 11 hips at 2 years follow up (7.8%), which is indicative of continuous acetabular remodelling.
Before data analysis for this series, correction of the AI below 20° was generally avoided in order to not increase the pressure over the femoral head, which could possibly lead to hip instability with redislocation or even AVN of the femur head. There was no correlation with post-operative AI over correction and redislocation of the hip or AVN. The study suggests that the ideal amount of AI correction to be achieved intra-operatively is ~20°.
Intra-operative instability of the hip following the combined procedure was present in 5.4% of hips. Stability was provided by passing a percutaneous K-wire from the greater trochanter to the ilium, avoiding hip joint penetration. Intra-operative instability can be attributed to a number of causes, of which an important reason is probably under correction of the dysplastic acetabulum. In all of the unstable hips, the AI had been corrected by an average of only 10°. Most long-standing dislocated hips have morphologic changes of both the acetabulum and femur head, which result in a measure of incongruity and, therefore, some instability, despite the acetabuloplasty, a fact which explains why this phenomenon was more prevalent in the older children in this study. Such hips are often stable only in wide abduction, which may jeopardise the blood supply to the femoral head, leading to AVN. In these cases, we elected to immobilise the hip in a safe position and augment the hip stability by K-wire fixation.
Post-operatively, we immobilise all hips in a spica cast for 6 weeks and a broom-stick cast for a further 6 weeks. This traditional regimen is followed to allow sufficient soft tissue healing. It is difficult to determine to what extent the spica cast contributed to the allograft stability. We feel that the structural integrity of the allograft is probably the main factor in maintaining acetabular correction.
AVN (other than type 1) at 2 years follow up was present in only 4% of hips. There was no significant correlation between AVN and age at surgical relocation. This aspect has been supported in the literature [32–34]. The only significant correlation [P value of <0.001] to the development of severe AVN is the lower pre-operative AI (average 32.3°) as compared to the non AVN group (average 43.3°). This aspect has not previously been reported and we do not have an explanation for it. The post-operative AI was not found to have any correlation with the development of AVN. Mild over correction in pericapsular acetabuloplasty is, therefore, unlikely to result in AVN.
The main objective of the study was to radiologically assess the efficacy of the contoured iliac crest allografts to maintain correction of the dysplastic acetabulum.
This study reports on a substantial number of late developmental dislocated hips meeting strict inclusion criteria for the index surgical procedure. Neuromuscular diseases and revision surgery which could influence the results were excluded. We did not attempt any direct comparison with other series, as there are too many variables regarding patient selection, operative techniques and length of follow up.
This retrospective study looked only at the radiologic outcome of pericapsular acetabuloplasty using a contoured iliac crest allograft and does not report on the clinical outcome.
Conclusion
Considering the number of hips that were included in the study, we conclude that the short-term results of contoured iliac crest allograft interposition in pericapsular acetabuloplasty to treat late presenting developmental hip dislocation is an effective method. This study achieved concentrically reduced and stable hips with maintained acetabular correction in 95.2% of cases. Under correction of the acetabular index (AI) and advanced age at the time of surgery were the main causes for residual hip instability. The contoured iliac crest allografts were structurally sound and provided and maintained excellent stability to the pericapsular osteotomies. We recommend the use of contoured iliac crest allograft as the interposition material for pericapsular acetabuloplasty procedures, especially in older children, as it obviates the need for internal fixation. We would also recommend achieving acetabular correction of 20° to afford the desired hip stability.
References
- 1.Mitchell PD, Redfern RC. The prevalence of dislocation in developmental dysplasia of the hip in Britain over the past thousand years. J Pediatr Orthop. 2007;27(8):890–892. doi: 10.1097/bpo.0b013e31815a6091. [DOI] [PubMed] [Google Scholar]
- 2.Mirdad T. Incidence and pattern of congenital dislocation of the hip in Aseer region of Saudi Arabia. West Afr J Med. 2002;21(3):218–222. doi: 10.4314/wajm.v21i3.28034. [DOI] [PubMed] [Google Scholar]
- 3.Vedantam R, Capelli AM, Schoenecker PL. Pemberton osteotomy for the treatment of developmental dysplasia of the hip in older children. J Pediatr Orthop. 1998;18(2):254–258. [PubMed] [Google Scholar]
- 4.Wada A, Fujii T, Takamura K, Yanagida H, Taketa M, Nakamura T. Pemberton osteotomy for developmental dysplasia of the hip in older children. J Pediatr Orthop. 2003;23(4):508–513. [PubMed] [Google Scholar]
- 5.Pemberton PA. Pericapsular osteotomy of the Ilium for treatment of congenital subluxation and dislocation of the hip. J Bone Joint Surg Am. 1965;47:65–86. [PubMed] [Google Scholar]
- 6.Szepesi K, Rigó J, Bíró B, Fazekas K, Póti L. Pemberton’s pericapsular osteotomy for the treatment of acetabular dysplasia. J Pediatr Orthop B. 1996;5(4):252–258. doi: 10.1097/01202412-199605040-00007. [DOI] [PubMed] [Google Scholar]
- 7.Tonnis D. Congenital dysplasia and dislocation of the hip in children and adults. Berlin Heidelberg New York: Springer; 1987. [Google Scholar]
- 8.Kalamchi A, MacEwen GD. Avascular necrosis following treatment of congenital dislocation of the hip. J Bone Joint Surg Am. 1980;62(6):876–888. [PubMed] [Google Scholar]
- 9.Zadeh HG, Catterall A, Hashemi-Nejad A, Perry RE. Test of stability as an aid to decide the need for osteotomy in association with open reduction in developmental dysplasia of the hip. J Bone Joint Surg Br. 2000;82(1):17–27. doi: 10.1302/0301-620X.82B1.9618. [DOI] [PubMed] [Google Scholar]
- 10.Lin CJ, Lin YT, Lai KA. Intraoperative instability for developmental dysplasia of the hip in children 12 to 18 months of age as a guide to Salter osteotomy. J Pediatr Orthop. 2000;20(5):575–578. doi: 10.1097/01241398-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Haidar RK, Jones RS, Vergroesen DA, Evans GA. Simultaneous open reduction and Salter innominate osteotomy for developmental dysplasia of the hip. J Bone Joint Surg Br. 1996;78(3):471–476. [PubMed] [Google Scholar]
- 12.Macnicol MF, Bertol P. The Salter innominate osteotomy: should it be combined with concurrent open reduction? J Pediatr Orthop B. 2005;14(6):415–421. doi: 10.1097/01202412-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Ruszkowski K, Pucher A. Simultaneous open reduction and Dega transiliac osteotomy for developmental dislocation of the hip in children under 24 months of age. J Pediatr Orthop. 2005;25(5):695–701. doi: 10.1097/01.bpo.0000164877.97949.22. [DOI] [PubMed] [Google Scholar]
- 14.Albinana J, Dolan LA, Spratt KF, Morcuende J, Meyer MD, Weinstein SL. Acetabular dysplasia after treatment for developmental dysplasia of the hip. Implications for secondary procedures. J Bone Joint Surg Br. 2004;86(6):876–886. doi: 10.1302/0301-620X.86B6.14441. [DOI] [PubMed] [Google Scholar]
- 15.Lindstrom JR, Ponseti IV, Wenger DR. Acetabular development after reduction in congenital dislocation of the hip. J Bone Joint Surg Am. 1979;61(1):112–118. [PubMed] [Google Scholar]
- 16.Salter RB. Innominate osteotomy in the treatment of congenital dislocation and subluxation of the hip. J Bone Joint Surg Br. 1961;43(3):518–539. [PubMed] [Google Scholar]
- 17.Perlik PC, Westin GW, Marafioti RL. A combination pelvic osteotomy for acetabular dysplasia in children. J Bone Joint Surg Am. 1985;67(6):842–850. [PubMed] [Google Scholar]
- 18.Tavares JO. Modified Pemberton acetabuloplasty for the treatment of congenital hip dysplasia. J Pediatr Orthop. 2004;24(5):501–507. doi: 10.1097/01241398-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Dega W. Selection of surgical methods in the treatment of congenital dislocation of the hip in children. Chir Narzadow Ruchu Ortop Pol. 1969;34:357–366. [PubMed] [Google Scholar]
- 20.Grudziak JS, Ward WT. Dega osteotomy for the treatment of congenital dysplasia of the hip. J Bone Joint Surg Am. 2001;83(6):845–854. doi: 10.2106/00004623-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Mubarak SJ, Valencia FG, Wenger DR. One-stage correction of the spastic dislocated hip. Use of pericapsular acetabuloplasty to improve coverage. J Bone Joint Surg Am. 1992;74(9):1347–1357. [PubMed] [Google Scholar]
- 22.Slomczykowski M, Mackenzie WG, Stern G, Keeler KA, Glutting J. Acetabular volume. J Pediatr Orthop. 1998;18(5):657–661. doi: 10.1097/01241398-199809000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Chung CY, Choi IH, Cho TJ, Woo WJ, Lee SH, Park MS. Morphometric changes in the acetabulum after Dega osteotomy in patients with cerebral palsy. J Bone Joint Surg Br. 2008;90(1):88–91. doi: 10.1302/0301-620X.90B1.19674. [DOI] [PubMed] [Google Scholar]
- 24.Ozgur AF, Aksoy MC, Kandemir U, Karcaaltncaba M, Aydingoz U, Yazici M, Surat A. Does Dega osteotomy increase acetabular volume in developmental dysplasia of the hip? J Pediatr Orthop B. 2006;15(2):83–86. doi: 10.1097/01.bpb.0000191870.15893.d1. [DOI] [PubMed] [Google Scholar]
- 25.Mankin HJ, Doppelt S, Tomford W. Clinical experience with allograft implantation. The first ten years. Clinic Orthop Relat Res. 1983;174:69–86. [PubMed] [Google Scholar]
- 26.Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Cinic Orthop Relat Res. 1996;324:86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Tavares JO, Molinero K. Elevation of medial tibial condyle for severe tibia vara. J Pediatr Orthop B. 2006;15(5):362–369. doi: 10.1097/01202412-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Kessler JI, Stevens PM, Smith JT, Carroll KL. Use of allografts in Pemberton osteotomies. J Pediatr Orthop. 2001;21(4):468–473. [PubMed] [Google Scholar]
- 29.Tomford WW. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am. 1995;77(11):1742–1754. doi: 10.2106/00004623-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Pirpiris M, Payman KR, Otsuka NY. The assessment of acetabular index: is there still a place for plain radiography? J Pediatr Orthop. 2006;26(3):310–315. doi: 10.1097/01.bpo.0000214920.54619.c7. [DOI] [PubMed] [Google Scholar]
- 31.Kay RM, Watts HG, Dorey FJ. Variability in the assessment of acetabular index. J Pediatr Orthop. 1997;17(2):170–173. doi: 10.1097/01241398-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Berkeley ME, Dickson JH, Cain TE, Donovan MM. Surgical therapy for congenital dislocation of the hip in patients who are twelve to thirty-six months old. J Bone Joint Surg Am. 1984;66(3):412–420. [PubMed] [Google Scholar]
- 33.Brougham DI, Broughton NS, Cole WG, Menelaus MB. Avascular necrosis following closed reduction of congenital dislocation of the hip. Review of influencing factors and long-term follow-up. J Bone Join Surg Br. 1990;72(4):557–562. doi: 10.1302/0301-620X.72B4.2380203. [DOI] [PubMed] [Google Scholar]
- 34.Thomas IH, Dunin AJ, Cole WG, Menelaus MB. Avascular necrosis after open reduction for congenital dislocation of the hip: analysis of causative factors and natural history. J Pediat Orthop. 1989;9(5):525–531. doi: 10.1097/01241398-198909010-00005. [DOI] [PubMed] [Google Scholar]


