Abstract
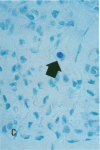
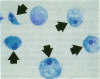
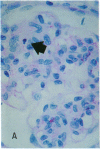
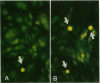
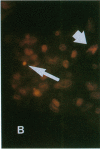
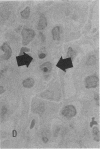
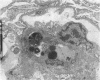
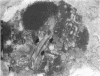
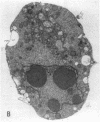
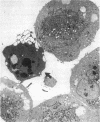
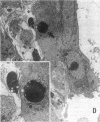
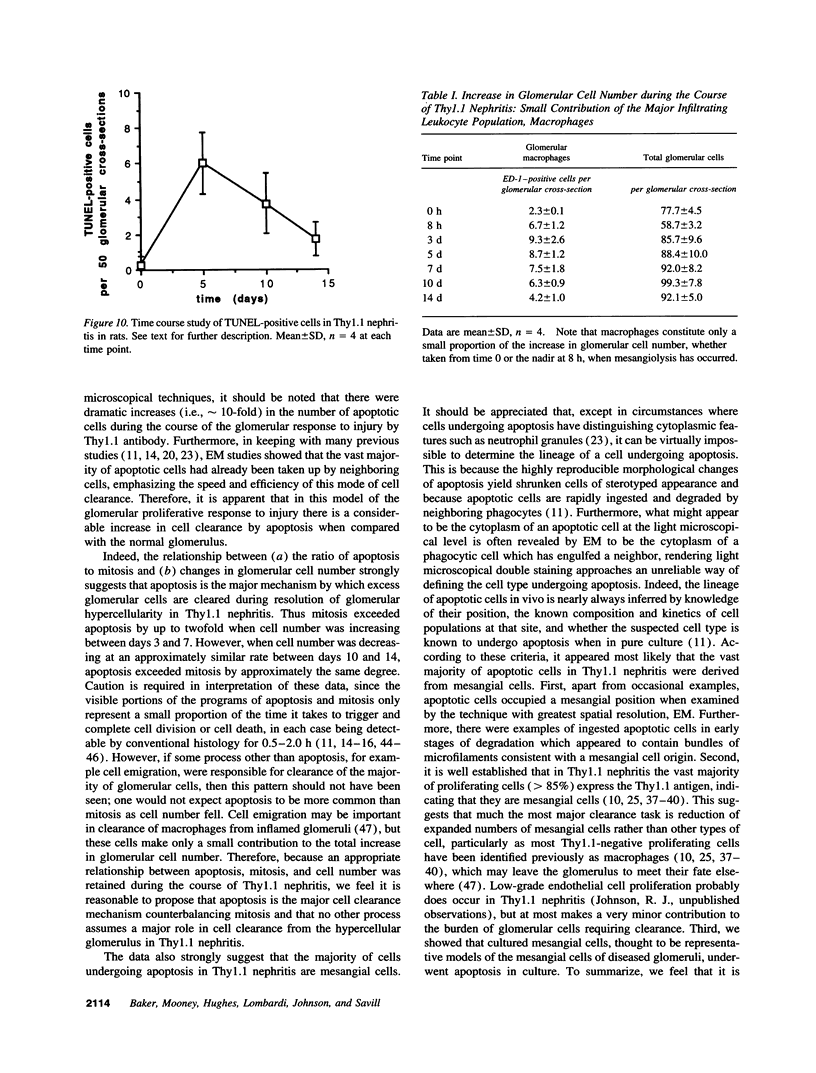
Increases in mesangial cell number may herald glomerular scarring, but they are not irreversible. This study sought mechanisms by which surplus glomerular mesangial cells can be cleared. A small proportion of cultured mesangial cells exhibited typical morphological features of apoptosis (programmed cell death), which was increased by growth factor deprivation or exposure to cycloheximide, stimuli known to increase apoptosis in other cell types. Apoptosis was confirmed by typical internucleosomal chromatin cleavage. In vivo, clear morphological evidence of mesangial apoptosis leading to phagocytosis by neighboring mesangial cells was obtained in self-limited mesangial proliferation induced in rats by Thy1.1 antibody, apoptosis occurring approximately 10-fold more frequently than in the healthy rat glomerulus. Indeed, changes in glomerular cell number in Thy1.1 nephritis strongly suggested that apoptosis is the major cell clearance mechanism counterbalancing cell division, thereby mediating resolution of glomerular hypercellularity in experimental mesangial proliferation.
Full text
PDF


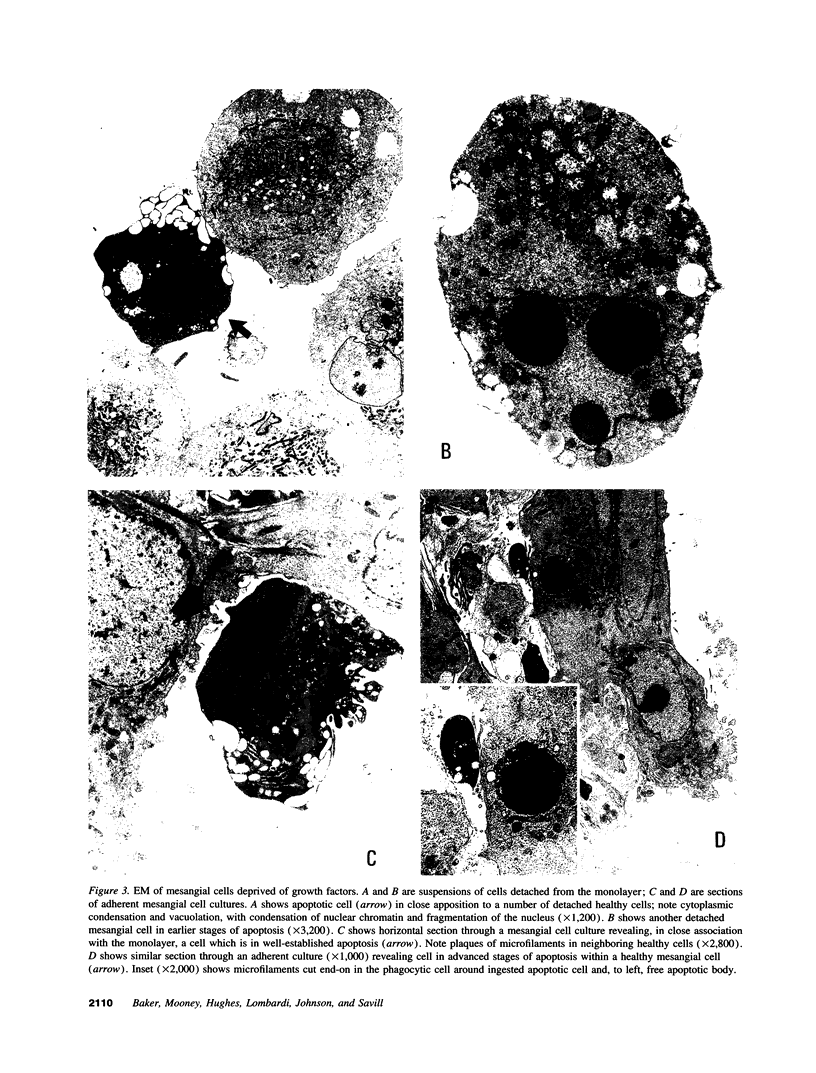
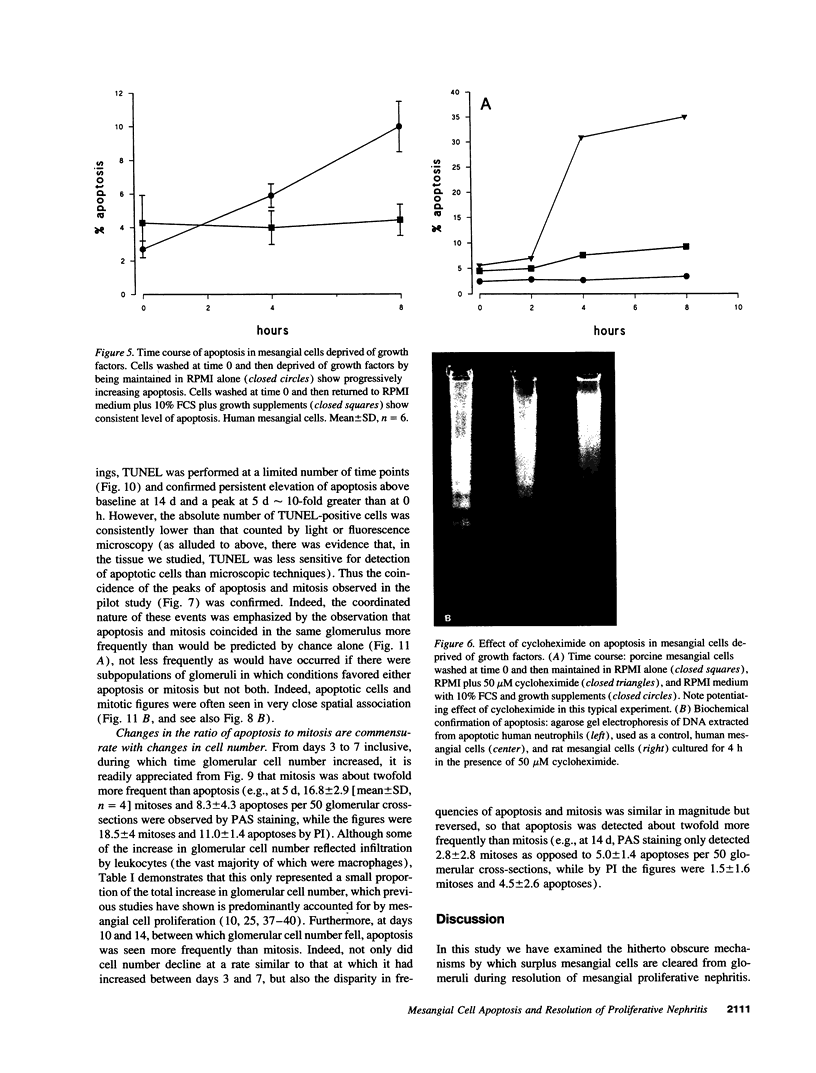
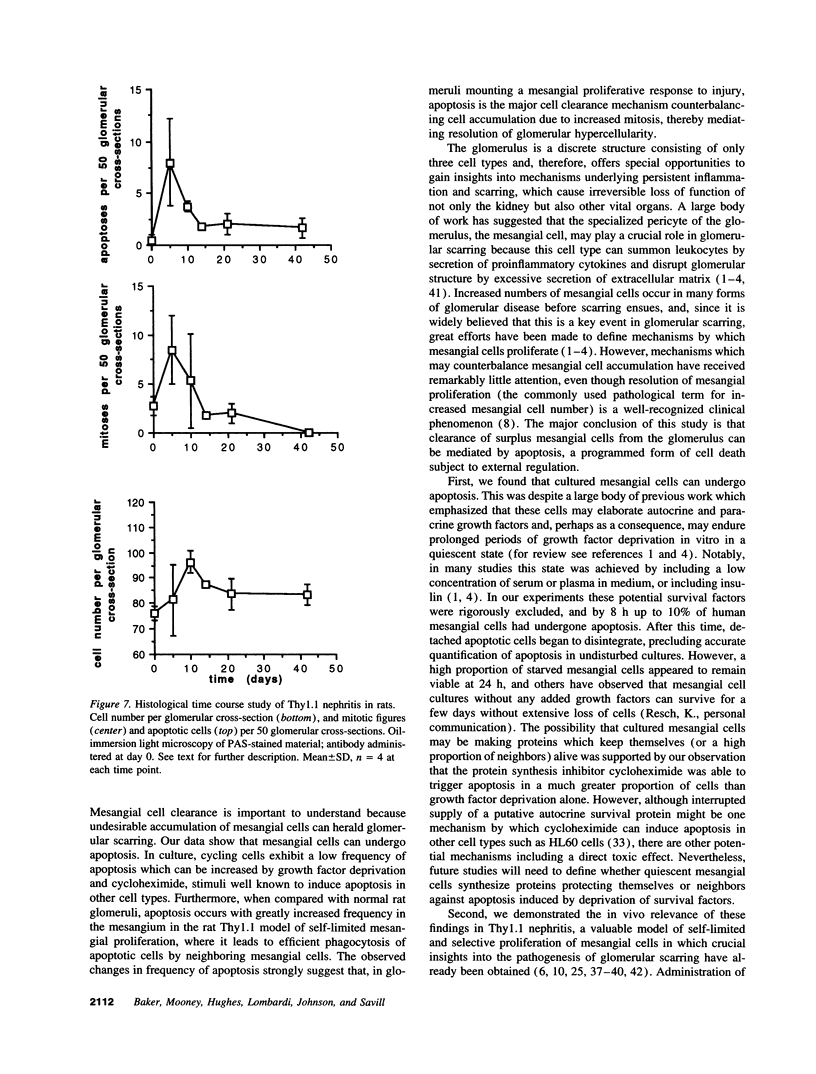
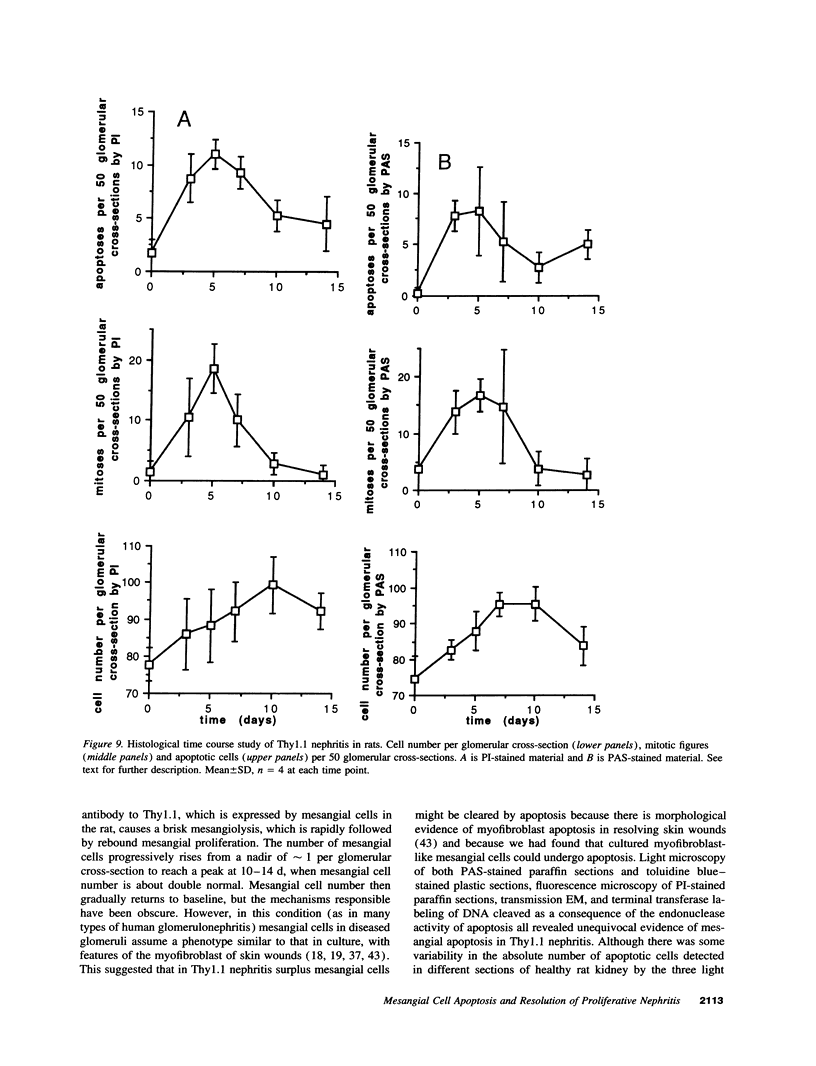


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott F., Ryan J. J., Ceska M., Matsushima K., Sarraf C. E., Rees A. J. Interleukin-1 beta stimulates human mesangial cells to synthesize and release interleukins-6 and -8. Kidney Int. 1991 Oct;40(4):597–605. doi: 10.1038/ki.1991.250. [DOI] [PubMed] [Google Scholar]
- Abboud H. E. Growth factors in glomerulonephritis. Kidney Int. 1993 Jan;43(1):252–267. doi: 10.1038/ki.1993.39. [DOI] [PubMed] [Google Scholar]
- Alpers C. E., Hudkins K. L., Gown A. M., Johnson R. J. Enhanced expression of "muscle-specific" actin in glomerulonephritis. Kidney Int. 1992 May;41(5):1134–1142. doi: 10.1038/ki.1992.173. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Bagchus W. M., Hoedemaeker P. J., Rozing J., Bakker W. W. Glomerulonephritis induced by monoclonal anti-Thy 1.1 antibodies. A sequential histological and ultrastructural study in the rat. Lab Invest. 1986 Dec;55(6):680–687. [PubMed] [Google Scholar]
- Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992 Jul 10;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Border W. A., Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992 Jul;90(1):1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- Coles H. S., Burne J. F., Raff M. C. Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development. 1993 Jul;118(3):777–784. doi: 10.1242/dev.118.3.777. [DOI] [PubMed] [Google Scholar]
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–29. [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Duke R. C., Cohen J. J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986 Fall;5(4):289–299. [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Floege J., Eng E., Young B. A., Couser W. G., Johnson R. J. Heparin suppresses mesangial cell proliferation and matrix expansion in experimental mesangioproliferative glomerulonephritis. Kidney Int. 1993 Feb;43(2):369–380. doi: 10.1038/ki.1993.55. [DOI] [PubMed] [Google Scholar]
- Floege J., Johnson R. J., Gordon K., Iida H., Pritzl P., Yoshimura A., Campbell C., Alpers C. E., Couser W. G. Increased synthesis of extracellular matrix in mesangial proliferative nephritis. Kidney Int. 1991 Sep;40(3):477–488. doi: 10.1038/ki.1991.235. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. J. Cell death in the diseased glomerulus. Histopathology. 1988 Jun;12(6):679–683. doi: 10.1111/j.1365-2559.1988.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Iida H., Seifert R., Alpers C. E., Gronwald R. G., Phillips P. E., Pritzl P., Gordon K., Gown A. M., Ross R., Bowen-Pope D. F. Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6560–6564. doi: 10.1073/pnas.88.15.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaka Y., Fujiwara Y., Ueda N., Kaneda Y., Kamada T., Imai E. Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest. 1993 Dec;92(6):2597–2601. doi: 10.1172/JCI116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Floege J., Yoshimura A., Iida H., Couser W. G., Alpers C. E. The activated mesangial cell: a glomerular "myofibroblast"? J Am Soc Nephrol. 1992 Apr;2(10 Suppl):S190–S197. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Garcia R. L., Pritzl P., Alpers C. E. Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am J Pathol. 1990 Feb;136(2):369–374. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Iida H., Alpers C. E., Majesky M. W., Schwartz S. M., Pritzi P., Gordon K., Gown A. M. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991 Mar;87(3):847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Pritzl P., Iida H., Alpers C. E. Platelet-complement interactions in mesangial proliferative nephritis in the rat. Am J Pathol. 1991 Feb;138(2):313–321. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Raines E. W., Floege J., Yoshimura A., Pritzl P., Alpers C., Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992 May 1;175(5):1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990 Apr 20;248(4953):378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- Lan H. Y., Nikolic-Paterson D. J., Atkins R. C. Trafficking of inflammatory macrophages from the kidney to draining lymph nodes during experimental glomerulonephritis. Clin Exp Immunol. 1993 May;92(2):336–341. doi: 10.1111/j.1365-2249.1993.tb03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda-Columbano G. M., Columbano A., Coni P., Faa G., Pani P. Cell deletion by apoptosis during regression of renal hyperplasia. Am J Pathol. 1989 Oct;135(4):657–662. [PMC free article] [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B. Cell culture approaches to the analysis of glomerular inflammation. Kidney Int. 1986 Aug;30(2):246–254. doi: 10.1038/ki.1986.176. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Lennon S. V., Bonham A. M., Cotter T. G. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990 Sep 15;145(6):1859–1867. [PubMed] [Google Scholar]
- Mené P., Simonson M. S., Dunn M. J. Physiology of the mesangial cell. Physiol Rev. 1989 Oct;69(4):1347–1424. doi: 10.1152/physrev.1989.69.4.1347. [DOI] [PubMed] [Google Scholar]
- Pabst R., Sterzel R. B. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983 Nov;24(5):626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Henderson Z., Linden R. Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. J Comp Neurol. 1983 Sep 20;219(3):356–368. doi: 10.1002/cne.902190309. [DOI] [PubMed] [Google Scholar]
- Polunovsky V. A., Chen B., Henke C., Snover D., Wendt C., Ingbar D. H., Bitterman P. B. Role of mesenchymal cell death in lung remodeling after injury. J Clin Invest. 1993 Jul;92(1):388–397. doi: 10.1172/JCI116578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990 Jan 11;343(6254):170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Savill J., Hogg N., Ren Y., Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992 Oct;90(4):1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Smith J., Sarraf C., Ren Y., Abbott F., Rees A. Glomerular mesangial cells and inflammatory macrophages ingest neutrophils undergoing apoptosis. Kidney Int. 1992 Oct;42(4):924–936. doi: 10.1038/ki.1992.369. [DOI] [PubMed] [Google Scholar]
- Schumer M., Colombel M. C., Sawczuk I. S., Gobé G., Connor J., O'Toole K. M., Olsson C. A., Wise G. J., Buttyan R. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 1992 Apr;140(4):831–838. [PMC free article] [PubMed] [Google Scholar]
- Sendtner M., Kreutzberg G. W., Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990 May 31;345(6274):440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- Shultz P. J., DiCorleto P. E., Silver B. J., Abboud H. E. Mesangial cells express PDGF mRNAs and proliferate in response to PDGF. Am J Physiol. 1988 Oct;255(4 Pt 2):F674–F684. doi: 10.1152/ajprenal.1988.255.4.F674. [DOI] [PubMed] [Google Scholar]
- Striker L. J., Peten E. P., Elliot S. J., Doi T., Striker G. E. Mesangial cell turnover: effect of heparin and peptide growth factors. Lab Invest. 1991 Apr;64(4):446–456. [PubMed] [Google Scholar]
- Whyte M. K., Hardwick S. J., Meagher L. C., Savill J. S., Haslett C. Transient elevations of cytosolic free calcium retard subsequent apoptosis in neutrophils in vitro. J Clin Invest. 1993 Jul;92(1):446–455. doi: 10.1172/JCI116587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]