Abstract
The green alga Haematococcus pluvialis produces large amounts of the pink carotenoid astaxanthin under high photon flux density (PFD) and other oxidative stress conditions. However, the regulation and physiological role of carotenogenesis leading to astaxanthin formation is not well understood. Comparative transcriptional expression of five carotenoid genes along with growth and pigment composition as a function of PFD was studied using a wild-type and an astaxanthin-overproduction mutant of H. pluvialis NIES144. The results indicate that astaxanthin biosynthesis was mainly under transcriptional control of the gene encoding carotenoid hydroxylase, and to a lesser extent, the genes encoding isopentenyl isomerase and phytoene desaturase, and to the least extent, the genes encoding phytoene synthase and carotenoid oxygenase. The expression of a plastid terminal oxidase (PTOX) gene ptox2 underwent transient up-regulation under elevated PFDs, suggesting that PTOX may be functionally coupled with phytoene desaturase through the plastoquinone pool and may play a role in reducing redox-potential-dependent and oxygen-concentration-dependent formation of reactive oxygen species in the chloroplast. Over-expression of both the carotenogenic and PTOX genes confers to the astaxanthin-overproduction mutant more effective photoprotective capability than that of the wild type under photooxidative stress.
Keywords: Astaxanthin, Haematococcus pluvialis, High light, mRNA expression, Oxidative stress
Introduction
Astaxanthin (3,3′-dihydroxy-β,β-carotene-4, 4′-dione) is a pink carotenoid synthesized and accumulated in the green alga Haematococcus pluvialis Flotow under photooxidative stress caused by excess photon flux density (PFD) for photosynthesis, or high PFDs in combination with other environmental stress conditions such as nutrient starvation and high salinity (Boussiba 2000; Kobayashi et al. 1993; Yong and Lee 1991). The physiological role of carotenogenesis in H. pluvialis in response to oxidative stress has been extensively investigated yet our understanding of this process is not complete. The mainstream hypothesis proposes that it is astaxanthin, the end product of carotenogenesis, that plays a major role in protecting the cell against photooxidative stress through shading of the photosynthetic machinery from exposure to excess PFDs (Hagen et al. 1994; Wang et al. 2003; Yong and Lee 1991). A possible role of astaxanthin as an antioxidant against stress-induced excess reactive oxygen species (ROS) from photooxidative stress has been proposed in H. pluvialis (Kobayashi 2000; Kobayashi et al. 1997). In contrast, the photoprotective role of astaxanthin in H. pluvialis was challenged by Fan et al. (1998) who reported that astaxanthin-rich cysts of H. pluvialis were susceptible to high light stress to the same extent or even higher than astaxanthin-free green vegetative cells upon exposure to high PFD. They proposed that astaxanthin is not itself the protective agent, but rather the end-result of the protection process through carotenogenesis, although the exact component(s) or step(s) in the carotenogenic pathway responsible for photoprotection was unknown (Fan et al. 1998).
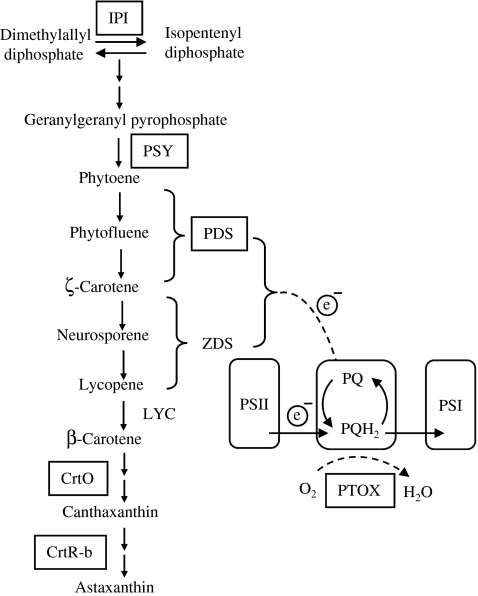
A number of carotenoid genes involved in astaxanthin biosynthesis have been cloned and partially characterized from H. pluvialis (Fig. 1). They include genes encoding isopentenyl-diphosphate δ-isomerase (ipi; Kajiwara et al. 1997; Sun et al. 1998), phytoene synthase (psy; Steinbrenner and Linden 2001), phytoene desaturase (pds; Grunewald et al. 2000), lycopene β cyclase gene (lyc; Steinbrenner and Linden 2003), β-carotenoid oxygenase (crtO; Huang et al. 2006; Kajiwara et al. 1995; Lotan and Hirschberg 1995), and β-carotenoid hydroxylase (crtR-b; Linden 1999). The transcripts of these genes were up-regulated, as indicated by Northern blotting or reverse-transcription PCR (RT-PCR), with concomitant formation and accumulation of astaxanthin under oxidative stress, suggesting the involvement of transcriptional control of astaxanthin biosynthesis in H. pluvialis. However, the previous carotenoid gene expression studies were conducted either under a fixed PFD (Huang et al. 2006; Steinbrenner and Linden 2001; Sun et al. 1998) or under various PFDs but analyzed only at a single point in time (Steinbrenner and Linden 2003). The relationship between PFD, maximum gene transcript level, and astaxanthin concentration was not established. Also, since the previous studies were performed at a gene level rather than a pathway level, the relative contribution of individual carotenoid genes at the transcriptional level to overall astaxanthin production is not known. Due to limited resolution and sensitivity of Northern blotting, expression of crtO transcripts was not detected in H. pluvialis in response to various PFDs (Steinbrenner and Linden 2003).
Fig. 1.
Overall pathway for astaxanthin biosynthesis in Haematococcus pluvialis (Cunningham and Gantt 1998; Fan et al. 1995; Fraser et al. 1998) and possible role of PTOX in response of Haematococcus pluvialis to oxidative stress. Enzymes are indicated by their gene assignment symbols: IPI isopentenyl-diphosphate δ-isomerase, PSY phytoene systhase, PDS phytoene desaturase, ZDS ζ-carotene desaturase, LYC lycopene cyclase, CrtO β-carotene ketolase, CrtR-b β-carotene 3,3′-hydroxylase, PTOX plastid terminal oxidase. The genes encoding boxed enzymes were studied in this work, and the dashed lines represent proposed chlororespiratory reactions
A thylakoid membrane-bound plastid terminal oxidase (PTOX) was isolated and identified from Arabidopsis thaliana, pepper, and tomato, which was suggested to be a co-factor in carotenoid desaturation (Barr et al. 2004; Carol et al. 1999; Josse et al. 2000; Wu et al. 1999). PTOX may also function as a stress protein by preventing the over-reduction of the PQ pool and providing a safety valve for excess electrons under stress conditions (Aluru and Rodermel 2004; Joet et al. 2002; Niyogi 2000; Rizhsky et al. 2002). Recently, the presence of PTOX in H. pluvialis has been identified (Wang et al. 2006) and potential coupling of PTOX and carotenogenesis has been proposed in this organism (Hu et al. 2008; Li et al. 2008). However, further experiments will be required to elucidate the relationship from both molecular and cellular perspectives.
In this study, the relationship between PFD, maximum transcriptional expression of carotenoid genes, and astaxanthin formation in H. pluvialis was quantitatively evaluated. The relative contribution of five carotenoid genes (i.e., ipi, psy, pds, crtO, and crtR-b) at the transcriptional level to astaxanthin production was assessed by comparative study of these genes in the wild type and an astaxanthin-overproduction mutant (MT 2877) that was recently characterized (Hu et al. 2008). Transcriptional expression of a newly isolated PTOX gene under high PFD provided new insight into multi-functional roles of the coupling of carotenogenesis with photosynthesis in response to photooxidative stress.
Materials and methods
Organism, growth medium, and culture conditions
Haematococcus pluvialis NIES144 was obtained from the National Institute for Environmental Studies in Tsukuba, Japan. Chemical mutagenesis of H. pluvialis was performed using the chemical mutagen, N-methyl-N-nitro-N-nitrosoguanidine (MNNG). Screening for pigment mutants was performed by examining cells from individual colonies by light microscopy and pigment analysis using reverse-phase HPLC. As a result, several putative pigment mutants were obtained, one of which (MT 2877) exhibited a high astaxanthin content and was chosen for further study.
A basal growth medium described by Kobayashi et al. (1991) was used. Algal cells were grown in 250-mL Erlenmeyer flasks containing 100 mL of growth medium. Cultures were incubated in a Percival growth chamber (1–35LLVL; Boone, IA, USA) at 22°C and 20 μmol photons m−2 s−1 of light (20 W white fluorescent lamps) under a 12 h:12 h light/dark cycle. Cultures were manually shaken daily. For induction of astaxanthin biosynthesis and red cyst formation, exponentially growing cultures (cell density of approximately 5 × 105 cells mL−1) were exposed to continuous illumination of 50, 125, 300, 600 μmol photons m−2 s−1. Cultures maintained at continuous, low light intensity of 20 μmol photons m−2 s−1 served as a control.
Cell counting, dry weight measurement, and pigment analysis
The cell number was determined using a hemacytometer under a light microscope. For cell dry weight measurement, a 10-mL aliquot of culture was filtered through pre-weighed Whatman GF/C filter paper. The filter paper was dried overnight in an oven at 80°C until a constant weight was reached. The difference between the final weight and the weight before filtration was the dry weight of the sample.
Pigment composition of algal cells was analyzed at 25°C by HPLC using a Beckman Ultrasphere C18 column (250 mm long, 4.6 mm i.d.; 5 μm; Beckman Instruments, USA). Pigments of freeze-dried algal cells were extracted in a solvent mixture of dichloromethane and methanol in a ratio of 25:75 (v/v). The mobile phase consisted of solvent A (dichloromethane:methanol:acetonitrile:water = 5.0:85.0:5.5:4.5 v/v) and solvent B (dichloromethane:methanol:acetonitrile:water = 25.0:28.0:42.5:4.5 v/v). The flow rate was 1.0 mL min−1. Chromatographic peaks were measured at a wavelength of 480 nm to facilitate the detection of astaxanthin species, canthaxanthin lutein, and β-carotene. Chlorophyll (chl) a and b were measured at 450 nm according to Yuan et al. (1997). The peaks were identified by comparing retention times and spectra against known standards or by comparing their spectra with published data.
RNA isolation and cDNA preparation
Algal cells collected by centrifugation at various time intervals were frozen and ground under liquid nitrogen using a mortar and pestle. RNA was isolated according to the miniprep RNA extraction procedure with minor changes: RNA extract was treated with DNase-I (Invitrogen, USA) at 37°C for 30 min, and then mixed thoroughly with equal volume of phenol:chloroform (1:1). After centrifugation at 8,000×g, for 10 min at 4°C, a 0.75 volume of 8 M LiCl was added into the supernatant for precipitation. RNA extracts were quantified by NanoDrop 3.0.0 (NanoDrop Technologies, Wilmington, USA).
For real-time RT-PCR analysis, first-strand cDNA synthesis was carried out using a Taqman Reverse Transcription system according to manufacturer’s instruction (Applied Biosystems, USA). In a 10-μL reaction system, 250 ng of total RNA was used. Controls used water instead of reverse transcriptase to assess any contamination of genomic DNA.
Primer sequence selection
Primer sequences of 18–25 nucleotides in length, unless otherwise stated, were designed using the Primer Express® Software Version 2.0 (Applied Biosystems) and designed to produce 50–160-bp PCR products with a melting temperature of about 60°C. Primer sequences for pds were from Grunewald et al. (2000). The primer sequences, concentrations, and gene bank accession numbers are summarized in Table 1.
Table 1.
Gene-specific oligonucleotide sequences used in this study
| Gene | Accession number | Forward primer (5′–3′) | Reverse primer (5′–3′) | Primer concentration (nm) |
|---|---|---|---|---|
| 18S | AF159369 | TGCCTAGTAAGCGCGAGTCA | CCCACCGCTAAAGTCAATCC | 100 |
| crtO | D45881 | ACGCCTACAAACCTCCAGCA | AAAACACTGCGGTCCAGGTG | 100 |
| crtR-b | AF162276 | GGGCTGAACTGGAGCAGTTG | GCGGAGTCATTGCTTCACAA | 50 |
| ipi | AB019034 | GGTACGTGACGCAGGAGGAG | TTCCCCAATCCTCGTGTTTG | 250 |
| psy | AF305430 | ACCAGACCTTCGACGAGCTG | TGCCCATGACAGGCATAGTC | 150 |
| pds | X86783 | TCGCATCGGCCTGCTGC | GGCCAGGTGCTTGACGCT | 250 |
| ptox2 | DQ485458 | GAGCTGCACCACCTGCAGAT | CTGCATGAAGTTGTACGCCACCTT | 250 |
Generation of gene-specific real-time PCR standards
Twenty-five nanograms cDNA was amplified with 100 nΜ of specific primer pairs by conventional PCR using the MasterTaq Kit (Eppendorf, USA). PCR fragments were run on a 3% agarose gel, excised, and eluted using the Geneclean Kit (Qbiogene, USA). A Shot®TOP chemically competent E. coli strain (from TACloning® Kit, Invitrogen) was used for PCR products cloning. DNA fragments containing the cDNA sequence of target genes were ligated to a pCR21 vector (Invitrogen) overnight at 14°C. Transformation of the ligates were carried out following instructions of the Invitrogen kit. Positive clones were screened on 50 mg mL−1 Kam plates by white/blue selection and confirmed by whole-cell PCR method with M13 primers. Plasmids were extracted and quantified by the standard methods described by Sambrook et al. (1989), and the plasmid DNA was used to prepare the templates for the standards. The molar concentrations were calculated on the basis of the mass concentration and the length in base pairs of each fragment. Equimolar quantities of the standards were tenfold serially diluted and used to generate standard curves. Specific amplification of the targeted cDNAs was confirmed by sequencing the PCR products using the dideoxy-nucleotide terminator method with an ABI Prism Dye-terminator system (PE Biosystems, USA).
Real-time quantitative PCR using SYBR Green
Real-time PCR was performed by an ABI Prism 7900 sequence detection system (Applied Biosystems). In each experiment, a standard dilution series of plasmids containing specific PCR fragments or 25 ng cDNA (total RNA equivalent) of unknown samples were amplified in a 20-μL reaction containing 1× SYBR Green PCR Master Mix (Applied Biosystems) and each primer. The primer concentrations shown in Table 1 were determined when specific amplification relative to primer-dimers was maximal in a positive versus negative control experiment. After heating at 50°C for 2 min and 95°C for 10 min, PCR reactions proceeded via 40 cycles of 15 s at 95°C and 15 s at 60°C. Finally, a dissociation stage of 15 s at 95°C, 15 s at 60°C, and 15 s at 95°C was used to detect any non-specific amplification products. The entire experiment (RNA isolation, cDNA synthesis followed by real-time PCR assay) was repeated three times, and the data were averaged.
Quantification and data analysis
Data were captured as amplification plots. Transcription level of a target gene was calculated from a threshold cycle by interpolation from the standard curve. To standardize the results, the relative abundance of 18S rRNA gene was determined and used as the internal standard. All calculations and statistical analyses were performed using ABI 7900 sequence detection system (User Bulletin 2, Applied Biosystems).
Results
Growth and cell viability at different photon flux densities
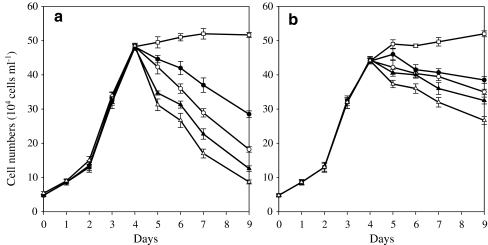
Growth kinetics was determined in cultures of the wild type (WT) and mutant (MT 2877) of H. pluvialis grown under various PFDs. Cultures were first maintained under a low PFD of 20-μmol photons m−2 s−1 and 22°C for 4 days. Little difference in growth was observed between the WT and MT 2877 cultures for the first 3 days. By day 4, a small reduction (ca. 10%) in growth was observed in the mutant culture relative to the WT.
On day 5, the cultures were subjected to continuous illumination of various PFDs (i.e., 50, 125, 300, and 600 μmol photons m−2 s−1). As a control, a set of cultures were maintained under continuous illumination of the low PFD of 20-μmol photons m−2 s−1 (LL) throughout the experiment. At LL, WT cell numbers increased for 2 to 3 days before cell numbers stabilized in the culture. The final cell density was ca. 51 × 104 cells mL−1 after a total of 9 days of cultivation (Fig. 2a). In contrast, MT 2877 cell numbers continued to increase, and by the end of day 9, the final population density of the mutant culture was comparable to that of the WT culture (Fig. 2b).
Fig. 2.
Growth of Haematococcus pluvialis WT (a) and MT 2877 (b) under various PFDs. During the first 4 days, algae grew under 20 μmol photons m−2 s−1 of light in basal growth medium described by Kobayashi et al. (1991) under a light/dark cycle of 12 h. Afterwards, the cultures were subjected to continuous illumination at different PFDs of 20, 50, 125, 300, and 600 μmol photons m−2 s−1 (open square 20 μmol photons m−2 s−1, filled circle 50 μmol photons m−2 s−1, open circle 125 μmol photons m−2 s−1, filled upright triangle 300 μmol photons m−2 s−1, empty upright triangle 600 μmol photons m−2 s−1)
When exposed to the higher PFDs, significant cell mortality occurred in cultures of both strains, with the higher the PFD the greater the cell mortality in the cultures. After 5 days of being illuminated at 50, 125, 300, or 600 μmol photons m−2 s−1, the WT cultures lost ca. 40%, 62%, 74%, and 82%, respectively, of the cells counted just before the high PFD treatments. MT 2877 cultures were more vital than the WT ones at higher PFDs, and the cell mortality rates were ca. 13%, 20%, 26%, and 40% at 50, 125, 300, and 600 μmol photons m−2 s−1, respectively, during the same culture period. In other words, the mutant cultures retained about 35.1%, 92.3%, 157.9%, and 206.9% more viable cells than that did the WT cultures after 5 days at 50, 125, 300, and 600 μmol photons m−2 s−1, respectively (Fig. 2a, b). At higher PFDs, those WT and mutant cells that survived high PDFs underwent transformation from green flagellates to red cysts. In contrast, the cells at LL were largely green non-motile cysts.
Astaxanthin profiles at different photon flux densities
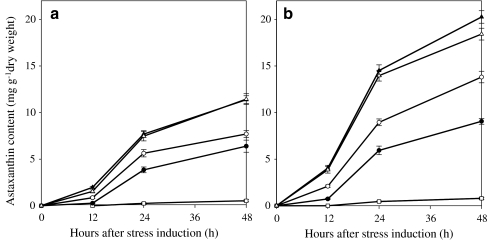
Astaxanthin was not detected in cultures maintained at 20 μmol photons m−2 s−1 until day 5, and ca. 0.53 and 0.79 mg astaxanthin g−1 dry weight were measured in the WT and MT 2877 cells, respectively, after 6 days of cultivation at LL. Large increases in astaxanthin were observed in both strains exposed to higher PFDs. The higher the PFD, the earlier the astaxanthin occurred and the larger the quantity of astaxanthin accumulated in the cells. Under a given PFD, MT 2877 cells accumulated significantly (P < 0.05) more astaxanthin than the WT (Fig. 3a, b). Over 100% increase in astaxanthin was obtained in mutant cells compared to the WT after exposed to a higher PFD for 4 days. However, no further increase in astaxanthin was observed in both strains exposed to 600 μmol photons m−2 s−1.
Fig. 3.
Astaxanthin content of Haematococcus pluvialis WT (a) and MT 2877 (b) under various PFDs during the first 48 h (open square 20 μmol photons m−2 s−1, filled circle 50 μmol photons m−2 s−1, open circle 125 μmol photons m−2 s−1, filled upright triangle 300 μmol photons m−2 s−1, empty upright triangle 600 μmol photons m−2 s−1)
Effect of photon flux density on expression of carotenoid genes
In order to determine the quantitative relationship between the gene expression and astaxanthin formation, five genes involved in astaxanthin formation were studied at the transcriptional level. They were ipi, psy, pds, crtO, and crtR-b encoding isopentenyl-diphosphate δ-isomerase (IPI), phytoene synthase (PSY), phytoene desaturase (PDS), β-carotene oxygenase (CrtO), and β-carotene hydroxylase (CrtR-b), respectively. The 18S rRNA gene was determined and used as the internal standard. The relative abundance of 18S rRNA in the cell was relatively constant throughout the cultivation period (SD within 10%). Previously, it was reported that transcripts of these genes underwent transient up-regulation under various environmental stress conditions, such as high light and salinity, and excess iron, with drastic changes occurring within 24 to 48 h (Li et al. 2008). In this study, mRNA expression kinetics was measured during the first 48 h of exposure of WT and MT 2877 to elevated PFDs using a real-time PCR method.
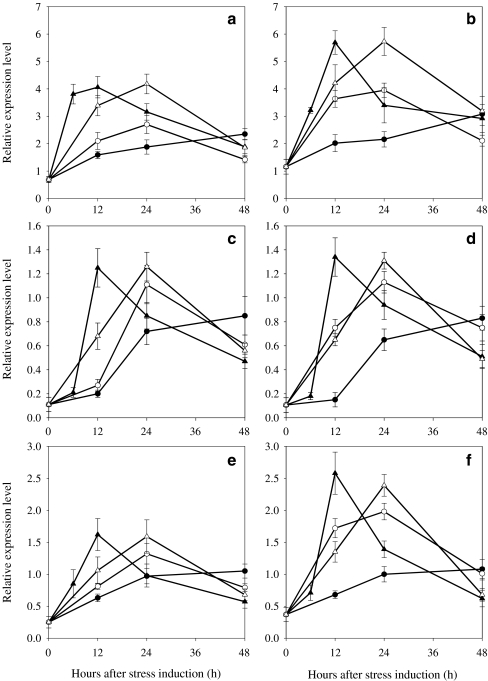
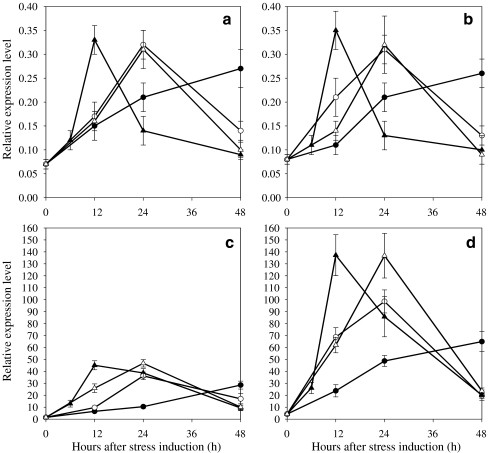
The ipi encoded a protein homologue of a 32.5-kDa isopentenyl-diphosphate δ-isomerase (IPI) reported by Sun et al. (1998) and was found to be induced during cyst formation and suggested to be responsible for ensuring the substrate supply for carotenogenesis (Kajiwara et al. 1997; Sun et al. 1998). The mRNA level of the ipi was transiently up-regulated in the WT and MT 2877 at higher PFDs. When PFD was increased from 20 to 50, 125, and 300 μmol photons m−2 s−1, the maximum transcript level of the ipi in the WT increased 3.4-, 3.9-, and 5.9-fold, respectively, and the time at which the maximum transcripts occurred was reduced from 48 to 24 to 12 h (Fig. 4a). As the PFD was increased to 600 μmol photons m−2 s−1, the occurrence of the maximum transcript level was delayed from 12 to 24 h with little increase in maximum transcript level observed. MT 2877 showed the same trend as the WT in mRNA expression under higher PFDs, but the mutant had higher maximum transcripts levels than the WT (ca. 31.1%, 46.3%, 40%, and 37.1% at 50, 125, 300, and 600 μmol photons m−2 s−1, respectively; Fig. 4b).
Fig. 4.
Relative expression profile of ipi, psy, pds of Haematococcus pluvialis WT (a, c, e) and MT 2877 (b, d, f) under different PFDs. Relative amounts were calculated and normalized with respect to one thousandth of 18S gene transcript levels. Data shown represent mean values obtained from three independent experiments (including RNA isolation, cDNA synthesis followed by real-time PCR assay), and the error bars indicate the SD of the mean. Note that different scales are used in graphs (a and bipi, c and dpsy, e and fpds, filled circle 50 μmol photons m−2 s−1, empty circle 125 μmol photons m−2 s−1, filled upright triangle 300 μmol photons m−2 s−1, empty upright triangle 600 μmol photons m−2 s−1)
The gene expression behavior of psy, pds, crtO, and crtR-b at the transcription level in the both strains was essentially the same as that of ipi: up to 300 μmol photons m−2 s−1, the higher the PFD the earlier and the greater the maximum transcripts of the gene occurred in the cells. However, a further increase in PFD to 600 μmol photons m−2 s−1 caused a delay in the occurrence and only a small increase in maximum transcript levels (Figs. 4 and 5). Compared to the WT, the maximum relative transcript levels of ipi and pds in MT 2877 were higher by ca. 60%, whereas that of crtR-b was increased by at least 127% at a higher PFD. On the other hand, little difference in relative expression of psy and crtO transcripts was observed in the two strains at the various PFDs tested.
Fig. 5.
Relative expression profile of crtO and crtR-b of Haematococcus pluvialis WT (a, c) and MT 2877 (b, d) under different PFDs. (a and bcrtO, c and dcrtR-b, filled circle 50 μmol photons m−2 s−1, empty circle 125 μmol photons m−2 s−1, filled upright triangle 300 μmol photons m−2 s−1, empty upright triangle 600 μmol photons m−2 s−1)
Among the five genes studied, crtR-b showed the most pronounced changes in transcripts in response to elevated PFDs. Exposure of WT cultures to PFD of 50, 125, 300, and 600 μmol photons m−2 s−1 resulted in 17.9-, 23-, 28.9-, and 29.9-fold increase in the maximum transcripts of crtR-b, respectively. Under the same conditions, the mutant exhibited ca. 127.6%, 172.1%, 204.3%, and 193% more transcripts of crtR-b relative to the WT. The maximum transcript level of crtR-b was at least threefold and ninefold higher in the WT and MT 2877 cells, respectively, than that of ipi, psy, pds, and crtO under a given PFD.
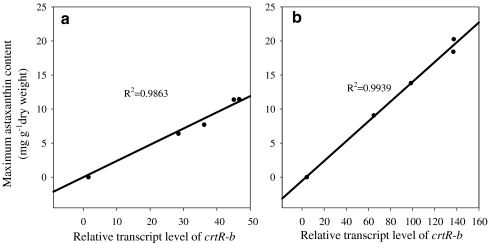
Linear relationship between the maximum mRNA level of crtR-b and astaxanthin concentration
A linear relationship (R2 ≥ 0.98) between maximum transcripts of crtR-b and astaxanthin concentration was observed when both WT and MT 2877 were maintained at high PFDs, indicating that crtR-b may play a regulative role in astaxanthin formation under stress (Fig. 6). A similar correlation was observed between the maximum mRNA level of ipi or pds and astaxanthin production in response to higher PFDs, though with a lower coefficient (R2 < 0.91). A poor correlation was observed between the maximum mRNA level of psy or crtO and astaxanthin production in response to higher PFDs, with an R2 of ca. 0.79 and 0.62, suggesting that these genes may be mainly controlled at other levels (e.g., at translational or post-translational level).
Fig. 6.
Correlation between maximum crtR-b transcript level and accumulation of astaxanthin after 48 h of light stress. a WT b MT 2877 (content of astaxanthin was obtained from Fig. 3, and maximum crtR-b transcript level was obtained from Fig. 5; the SDs were within 10%)
Expression of a plastid terminal oxidase gene at high photon flux density
A gene encoding PTOX (ptox2) was recently cloned from H. pluvialis. In this study, the transcription of ptox2 was measured in WT and MT 2877 cells grown at 300 μmol photons m−2 s−1. Like the carotenogenesis genes studied above, ptox2 underwent transient up-regulation after the culture was transferred from 20 to 300 μmol photons m−2 s−1. The maximum transcript level of ptox2 occurred at 12 h and was 53.9% higher in MT 2877 than in WT. At the same time, the maximum transcript level of pds occurred and was 59.3% higher in MT 2877 than in the WT (Table 2).
Table 2.
Transcript levels of plastid terminal oxidase and phytoene desaturase genes in MT 2877 and WT at 300 μmol photons m−2 s−1 of PFD
| pds | ptox2 | |
|---|---|---|
| WT 0 h | 0.25 ± 0.09 | 1.78 ± 0.39 |
| WT maximuma | 1.62 ± 0.25 | 4.64 ± 0.19 |
| MT 2877 0 h | 0.37 ± 0.11 | 2.94 ± 0.38 |
| MT 2877 maximuma | 2.58 ± 0.33 | 7.14 ± 0.79 |
Relative transcript level of the genes was calculated and normalized with respect to one thousandth of 18S gene transcript level. Data shown represent mean values obtained from three independent experiments, and the error bars indicate the SD of the mean
aSamples were collected from different growth conditions at 0, 6, 12, 24, 48, 72, and 96 h and the maximum pds and ptox2 gene transcripts were observed at 12 h in WT and MT 2877
Discussion
Considerable effort has been focused on developing an understanding of the physiological role of astaxanthin in H. pluvialis in response to high PFD and other oxidative stressors, whereas elucidating the possible molecular mechanisms underlying the protective role of astaxanthin in the stress response is just beginning. Previously, most of the investigations carried out used astaxanthin-free vegetative cells and astaxanthin-rich cysts (Hagen et al. 1993; 1994; Kobayashi et al. 1997; Tan et al. 1995; Wang et al. 2003). This experimental model was problematic because red cysts differ considerably from green vegetative cells by cell size and subcellular composition and structure, in addition to the presence or absence of astaxanthin. Any difference in response to oxidative stress between the two forms of cells cannot be attributed solely to astaxanthin. In this study, a stable astaxanthin-overproduction mutant (MT 2877) of H. pluvialis NIES144 has been employed that was identical to the WT with respect to cell morphology, pigment composition, and growth kinetics during the early vegetative stage of the life cycle (Hu et al. 2008). The comparative study of MT 2877 and the WT enabled us to obtain a quantitative relationship between PFD and oxidative cell death: the higher the PFD, the greater the magnitude and rate of cell death. However, MT 2877 was able to retain more than 50% of its population after experiencing oxidative cell damage at a high PFD compared to only 20% of its population in the WT, providing direct evidence of enhanced carotenogenesis augmenting cellular photoprotection under stress.
Astaxanthin has been suggested to be the major photoprotective agent acting as a ‘sunscreen’ to reducing excess light penetrating into the chloroplast (Hagen et al. 1994; Wang et al. 2003; Yong and Lee 1991) and also serving as an antioxidant against stress-induced excess ROS (Kobayashi 2000; Kobayashi et al. 1997) and carbon and energy storage under stress (Hagen et al. 1993). Considering that astaxanthin resides only in cytosolic lipid bodies located peripherally in the cell, whereas extremely short-lived ROS are mainly produced in the chloroplast, we postulate that an additional or perhaps more important role of enhanced carotenogenesis in stress response must be associated with certain intermediate steps of carotenogenesis taking place in the chloroplast, as originally proposed by Fan et al. (1998). Recently, it was proposed that consumption of oxygen by astaxanthin biosynthesis in the chloroplast can be a protective mechanism against oxidative stress in H. pluvialis (Li et al. 2008).
The transcriptional expression of ipi, psy, pds, crtO, and crtR-b, as well as lycopene β cyclase gene (lyc), in H. pluvialis under high PFDs has previously been studied by northern blotting and RT-PCR (Grunewald et al. 2000; Huang et al. 2006; Steinbrenner and Linden 2001; 2003; Sun et al. 1998). A general trend revealed from those studies was that individual carotenoid genes underwent transient up-regulation in response to increased light. In the present study, a more sensitive, quantitative real-time PCR method was introduced to investigate expression kinetics of ipi, psy, pds, crtO, and crtR-b simultaneously in response to various PFDs. While confirming the general trend of transient up-regulation of carotenoid genes in response to light, the present study uncovered several new aspects of carotenoid gene expression from a pathway level: (1) when PFD was 300 μmol photons m−2 s−1 or below, the maximum mRNA transcript level increased with increasing PFDs. No further increase in mRNA transcripts occurred when PFD reached 600 μmol photons m−2 s−1. (2) When PFD was 300 μmol photons m−2 s−1 or below, the maximum mRNA transcript level occurred earlier upon exposure to a higher PFD. As PFD reached 600 μmol photons m−2 s−1, the occurrence of maximum mRNA transcripts of individual genes was delayed. (3) Among the five genes studied, crtR-b underwent the most drastic transient up-regulation of mRNA transcripts relative to that measured right before light induction, and showed the highest relative transcript level. (4) A linear relationship between maximum mRNA transcripts and astaxanthin concentration was observed for crtR-b in both stains, indicating that astaxanthin biosynthesis is mainly under control of crtR-b at the transcription level. (5) In contrast, there was little difference in maximum mRNA transcripts of psy and crtO between the WT and the astaxanthin-overproduction mutant in response to light, and the correlation between their maximum mRNA transcripts and astaxanthin production was poor. (6) The relationship between the maximum mRNA level of ipi or pds and astaxanthin production in response to higher PFDs fell between the two cases above, suggesting that these genes may be under translational or post-translational regulation in addition to the transcription control. Accordingly, multiple regulatory mechanisms at transcriptional, translational, and post-translational levels co-exist among these genes controlling the overall carotenogenesis process in H. pluvialis.
Previously, Steinbrenner and Linden (2003) observed that light-induced carotenoid gene expression was saturated at a low PFD of 50 μmol photons m−2 s−1 based upon Northern blot analysis of H. pluvialis exposed to increasing PFDs from 10 to 250 μmol photons m−2 s−1 for 24 h and suggested that carotenoid gene expression was not dependent on increasing PFDs (Steinbrenner and Linden 2003). The failure to detect light-dependent expression of carotenogenesis genes above 50 μmol photons m−2 s−1 was perhaps due to insufficient numbers of sampling points under stress. As shown in our study, the transcription expression of all five selected genes achieved their maximum transcript levels earlier and declined in their steady-state mRNA transcripts after 24 h at 300 μmol photons m−2 s−1.
It has been proposed that light regulation of carotenogenesis and astaxanthin formation in H. pluvialis is under photosynthetic redox control (Steinbrenner and Linden 2001; 2003). Accordingly, excess light for photosynthesis may result in over-reduction of the plastoquinone (PQ) pool and other components of the photosynthetic electron transport. The PQ pool serves as a redox sensor which may in turn trigger transcriptional activation of carotenoid genes resulting in astaxanthin formation (Steinbrenner and Linden 2003). On the other hand, PDS and PTOX have a close association with the PQ pool, thereby reducing the PQ pool using electrons derived from carotenoid desaturation steps (Carol et al. 1999; Wu et al. 1999). In this study, transient up-regulation of ptox2 in concert with that of pds under increased PFD suggests that PTOX in H. pluvialis may have a function identical to that observed in higher plants (Aluru and Rodermel 2004; Carol et al. 1999; Casano et al. 2000; Joet et al. 2002; Wu et al. 1999), which reoxidizes the PQ pool and reduces molecular oxygen to water.
As depicted in Fig. 1, a role of carotenogenesis is to ensure the flow of electrons out of the photosynthetic electron transport chain to reduce redox-potential-dependent formation of ROS under stress, and to use the released electrons to reduce molecular oxygen to water catalyzed by PTOX, thereby reducing oxygen-concentration-dependent formation of ROS in the chloroplast. Such protective mechanisms can be significant in this alga since about 10% of photosynthetically generated oxygen in H. pluvialis can be consumed during carotenogenesis (Li et al. 2008). Over-expression of both the carotenogenic and PTOX genes confers the astaxanthin-overproduction mutant with a more effective photoprotective capability than that of the wild type under photooxidative stress.
Acknowledgments
This work was partially supported by Science Foundation Arizona Small Business Catalytic Program, the Research Grants Council of Hong Kong, and the University of Hong Kong Outstanding Young Researcher Award and Outstanding Research Student Supervisor Award.
Abbreviations
- CRTO
carotenoid ketolase
- CRTR-B
carotenoid hydroxylase
- IPI
isopentenyl-diphosphate δ-isomerase
- PDS
phytoene desaturase
- PFD
photon flux density
- PQ
plastoquinone
- PSY
phytoene synthase
- PTOX
plastid terminal oxidase
- ROS
reactive oxygen species
Contributor Information
Feng Chen, Email: sfchen@hkusua.hku.hk.
Qiang Hu, Phone: +1-480-7271484, FAX: +1-480-7271236, Email: huqiang@asu.edu.
References
- Aluru MR, Rodermel SR. Control of chloroplast redox by the IMMUTANS terminal oxidase. Physiol Plant. 2004;120:4–11. doi: 10.1111/j.0031-9317.2004.0217.x. [DOI] [PubMed] [Google Scholar]
- Barr J, White WS, Chen L, et al. The GHOST terminal oxidase regulates developmental programming in tomato fruit. Plant Cell Environ. 2004;27:840–852. doi: 10.1111/j.1365-3040.2004.01190.x. [DOI] [Google Scholar]
- Boussiba S. Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiol Plant. 2000;108:111–117. doi: 10.1034/j.1399-3054.2000.108002111.x. [DOI] [Google Scholar]
- Carol P, Stevenson D, Bisanz C, et al. Mutations in the Arabidopsis gene immutans cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell. 1999;11:57–68. doi: 10.1105/tpc.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casano LM, Zapata JM, Martin M, et al. Chlororespiration and poising of cyclic electron transport - Plastoquinone as electron transporter between thylakoid NADH dehydrogenase and peroxidase. J Biol Chem. 2000;275:942–948. doi: 10.1074/jbc.275.2.942. [DOI] [PubMed] [Google Scholar]
- Cunningham FX, Gantt E. Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:557–583. doi: 10.1146/annurev.arplant.49.1.557. [DOI] [PubMed] [Google Scholar]
- Fan L, Vonshak A, Gabbay R, et al. The biosynthetic pathway of astaxanthin in a green alga Haematococcus pluvialis as indicated by inhibition with diphenylamine. Plant Cell Physiol. 1995;36:1519–1524. [Google Scholar]
- Fan L, Vonshak A, Zarka A, et al. Does astaxanthin protect Haematococcus against light damage? Z Naturforsch. 1998;53(C):93–100. doi: 10.1515/znc-1998-1-217. [DOI] [PubMed] [Google Scholar]
- Fraser PD, Shimada H, Misawa N. Enzymic confirmation of reactions involved in routes to astaxanthin formation, elucidated using a direct substrate in vitro assay. Eur J Biochem. 1998;252:229–236. doi: 10.1046/j.1432-1327.1998.2520229.x. [DOI] [PubMed] [Google Scholar]
- Grunewald K, Eckert M, Hirschberg J, et al. Phytoene desaturase is localized exclusively in the chloroplast and up-regulated at the mRNA level during accumulation of secondary carotenoids in Haematococcus pluvialis (Volvocales, Chlorophyceae) Plant Physiol. 2000;122:1261–1268. doi: 10.1104/pp.122.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen C, Braune W, Birckner E, et al. Functional aspects of secondary carotenoids in Haematococcus lacustris (Girod) Rostafinski (Volvocales).I. The accumulation period as an active metabolic process. New Phytol. 1993;125:625–633. doi: 10.1111/j.1469-8137.1993.tb03912.x. [DOI] [PubMed] [Google Scholar]
- Hagen C, Braune W, Bjorn LO. Functional aspects of secondary carotenoids in Haematococcus lacustris (Volvocales).III. Action as a sunshade. J Phycol. 1994;30:241–248. doi: 10.1111/j.0022-3646.1994.00241.x. [DOI] [Google Scholar]
- Hu Z, Li Y, Sommerfeld M, et al. Enhanced protection against oxidative stress in an astaxanthin-overproduction Haematococcus mutant (Chlorophyceae) Eur J Phycol. 2008;43:365–376. doi: 10.1080/09670260802227736. [DOI] [Google Scholar]
- Huang JC, Chen F, Sandmann G. Stress-related differential expression of multiple beta-carotene ketolase genes in the unicellular green alga Haematococcus pluvialis. J Biotechnol. 2006;122:176–185. doi: 10.1016/j.jbiotec.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Joet T, Genty B, Josse EM, et al. Involvement of a plastid terminal oxidase in plastoquinone oxidation as evidenced by expression of the Arabidopsis thaliana enzyme in tobacco. J Biol Chem. 2002;277:31623–31630. doi: 10.1074/jbc.M203538200. [DOI] [PubMed] [Google Scholar]
- Josse EM, Simkin AJ, Gaffe J, et al. A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol. 2000;123:1427–1436. doi: 10.1104/pp.123.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara S, Kakizono T, Saito T, et al. Isolation and functional identification of a novel cDNA for astaxanthin biosynthesis from Haematococcus pluvialis, and astaxanthin synthesis in Escherichia coli. Plant Mol Biol. 1995;29:343–352. doi: 10.1007/BF00043657. [DOI] [PubMed] [Google Scholar]
- Kajiwara S, Fraser PD, Kondo K, et al. Expression of an exogenous isopentenyl diphosphate isomerase gene enhances isoprenoid biosynthesis in Escherichia coli. Biochem J. 1997;324:421–426. doi: 10.1042/bj3240421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M. In vivo antioxidant role of astaxanthin under oxidative stress in the green alga Haematococcus pluvialis. Appl Microbiol Biotechnol. 2000;54:550–555. doi: 10.1007/s002530000416. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Kakizono T, Nagai S. Astaxanthin production by a green alga, Haematococcus pluvialis accompanied with morphological changes in acetate media. J Ferment Bioeng. 1991;71:335–339. doi: 10.1016/0922-338X(91)90346-I. [DOI] [Google Scholar]
- Kobayashi M, Kakizono T, Nagai S. Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol. 1993;59:867–873. doi: 10.1128/aem.59.3.867-873.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Kakizono T, Nishio N, et al. Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl Microbiol Biotechnol. 1997;48:351–356. doi: 10.1007/s002530051061. [DOI] [PubMed] [Google Scholar]
- Li Y, Sommerfeld M, Chen F, et al. Consumption of oxygen by astaxanthin biosynthesis: a protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae) J Plant Physiol. 2008;165:1783–1797. doi: 10.1016/j.jplph.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Linden H. Carotenoid hydroxylase from Haematococcus pluvialis: cDNA sequence, regulation and functional complementation. Biochim Biophys Acta-Gene Struct Expression. 1999;1446:203–212. doi: 10.1016/S0167-4781(99)00088-3. [DOI] [PubMed] [Google Scholar]
- Lotan T, Hirschberg J. Cloning and expression in Escherichia coli of the gene encoding beta-C-4-oxygenase, that converts beta-carotene to the ketocarotenoid canthaxanthin in Haematococcus pluvialis. FEBS Lett. 1995;364:125–128. doi: 10.1016/0014-5793(95)00368-J. [DOI] [PubMed] [Google Scholar]
- Niyogi KK. Safety valves for photosynthesis. Curr Opin Plant Biol. 2000;3:455–460. doi: 10.1016/S1369-5266(00)00113-8. [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Hallak-Herr E, Breusegem F, et al. Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J. 2002;32:329–342. doi: 10.1046/j.1365-313X.2002.01427.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Steinbrenner J, Linden H. Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced astaxanthin formation in the green alga Haematococcus pluvialis. Plant Physiol. 2001;125:810–817. doi: 10.1104/pp.125.2.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrenner J, Linden H. Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: regulation by photosynthetic redox control. Plant Mol Biol. 2003;52:343–356. doi: 10.1023/A:1023948929665. [DOI] [PubMed] [Google Scholar]
- Sun Z, Cunningham FX, Gantt E. Differential expression of two isopentenyl pyrophosphate isomerases and enhanced carotenoid accumulation in a unicellular chlorophyte. Proc Natl Acad Sci USA. 1998;95:11482–11488. doi: 10.1073/pnas.95.19.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Cunningham FX, Youmans M, et al. Cytochrome f loss in astaxanthin-accumulating red cells of Haematococcus pluvialis (Chlorophyceae): Comparison of photosynthetic activity, photosynthetic enzymes, and thylakoid membrane polypeptides in red and green cells. J Phycol. 1995;31:897–905. doi: 10.1111/j.0022-3646.1995.00897.x. [DOI] [Google Scholar]
- Wang B, Zarka A, Trebst A, et al. Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process under high irradiance. J Phycol. 2003;39:1116–1124. doi: 10.1111/j.0022-3646.2003.03-043.x. [DOI] [Google Scholar]
- Wang J, Sommerfeld M, Hu Q (2006) Genes encoding plastid terminal oxidase in Haematococcus pluvialis: cDNA cloning, characterization, and expression under oxidative stress. The Phycological Society of America 60th Annual Meeting. Juneau, AL, the conference proceedings, pp 51-52
- Wu DY, Wright DA, Wetzel C, et al. The immutans variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell. 1999;11:43–55. doi: 10.1105/tpc.11.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong YYR, Lee YK. Do carotenoids play a photoprotective role in the cytoplasm of Haematococcus lacustris (Chlorophyta) Phycologia. 1991;30:257–261. [Google Scholar]
- Yuan JP, Gong XD, Chen F. Separation and analysis of carotenoids and chlorophylls in Haematococcus lacustris by high-performance liquid chromatography photodiode array detection. J Agric Food Chem. 1997;45:1952–1956. doi: 10.1021/jf970002b. [DOI] [Google Scholar]