Abstract
Mitochondrial dysfunction is involved in the pathogenesis of neurodegenerative diseases, including Parkinson’s disease (PD). Uncoupling proteins (UCPs) delink ATP production from biofuel oxidation in mitochondria to reduce oxidative stress. UCP2 is expressed in brain, and has neuroprotective effects under various toxic insults. We observed induction of UCP2 expression by leptin in neuronal cultures, and hypothesize that leptin may preserve neuronal survival via UCP2. We showed that leptin preserved cell survival in neuronal SH-SY5Y cells against MPP+ toxicity (widely used in experimental Parkinsonian models) by maintaining ATP levels and mitochondrial membrane potential (MMP); these effects were accompanied by increased UCP2 expression. Leptin had no effect in modulating reactive oxygen species levels. Stable knockdown of UCP2 expression reduced ATP levels, and abolished leptin protection against MPP+-induced mitochondrial depolarization, ATP deficiency, and cell death, indicating that UCP2 is critical in mediating these neuroprotective effects of leptin against MPP+ toxicity. Interestingly, UCP2 knockdown increased UCP4 expression, but not of UCP5. Our findings show that leptin preserves cell survival by maintaining MMP and ATP levels mediated through UCP2 in MPP+-induced toxicity.
Keywords: Uncoupling protein, UCP2, Leptin, Mitochondrial dysfunction, MPP+, Parkinson’s disease
Introduction
Mitochondrial uncoupling proteins (UCPs) are solute carriers which can uncouple biofuel oxidation from ATP synthesis by modulating proton gradient across the inner mitochondrial membrane (Andrews et al. 2005; Krauss et al. 2005). Partial uncoupling by UCPs may be a vital link between ATP and reactive oxygen species (ROS) production (Arsenijevic et al. 2000; Krauss et al. 2002), and UCPs are critical in controlling oxidative stress produced by the mitochondrial electron transport chain (Casteilla et al. 2001). Of five UCP homologues, UCP2 is ubiquitously expressed (Fleury et al. 1997), whereas UCP4 and UCP5 are specifically expressed in brain (Mao et al. 1999; Sanchis et al. 1998). UCP2 has protective properties against oxidative damage (Diano et al. 2003; Mattiasson et al. 2003), by modulating mitochondrial membrane potential (MMP), mitochondrial proliferation, ATP and ROS levels. UCP2 also has neuroprotective effects in different parkinsonian models (Conti et al. 2005; Andrews et al. 2006). UCP2 knockout mice resulted in increased mitochondrial ROS production (Arsenijevic et al. 2000).
Leptin is a circulating, 16 kDa hormone secreted by adipocytes. It is a four-helical cytokine encoded by the obese (ob) gene (Halaas et al. 1995). It regulates basal metabolism and energy homeostasis via its peripheral tissue receptors and by modulating release of neuropeptides in the ventro-medial hypothalamus (Friedman and Halaas 1998; Spiegelman and Flier 2001). Leptin receptors are widely expressed throughout brain, including substantia nigra (Couce et al. 1997; Elmquist et al. 1998). Although leptin receptors are localized in nigral dopaminergic neurons, their role in these neurons remains unclear. Studies of leptin in brain have mainly focused on its effects on hypothalamus as a peripheral signal in regulating energy balance and body weight (Friedman and Halaas 1998; Spiegelman and Flier 2001). Efflux of leptin from brain supports its direct action on the central nervous system (Esler et al. 1998). Apart from being an energy-regulating hormone, leptin has divergent effects on different brain regions (Maratos-Flier 2008). Nevertheless, little is known on whether leptin has any effect on mitochondria, the main provider of cellular energy.
We reported previously that MPP+ toxicity (widely used in experimental parkinsonian models) induced expression of UCP2, 4, and 5 in neuronal cultures in a dose-dependent manner, such that higher-toxicity levels induced greater levels of their expression (Ho et al. 2005). Knockdown of UCP5 expression increased MPP+-induced neuronal cell death (Ho et al. 2006). We have also reported that stable overexpression of UCP4 in SH-SY5Y cells protected against MPP+ and dopamine toxicity by reducing oxidative stress, preserving MMP and ATP levels (Chu et al. 2009). The protective effects of leptin have been reported in different tissues, including neurons, under a variety of insults, including MPP+ (Russo et al. 2004; Guo et al. 2008; Weng et al. 2007; Zhang et al. 2007). We noted that leptin can also modulate UCP expression in nonneuronal tissues (Ceddia et al. 2000; Ricquier and Bouillaud 2000). It is not known whether leptin can affect mitochondrial function, and if so, whether this effect is mediated via UCP2 expression. We observed that leptin-induced UCP2 expression in neuronal cultures, and hypothesize that leptin, via UCP2, may preserve neurons in MPP+ toxicity. In this study, we explored the effects of leptin in MPP+-induced mitochondrial dysfunction and oxidative stress in SH-SY5Y cells, with and without knocking down UCP2 expression.
Materials and Methods
Materials
Human neuroblastoma SH-SY5Y (CRL-2266) cells were obtained from American Type Culture Collection (ATCC); Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12), fetal bovine serum (FBS) from GibcoBRL (Gaithersburg, MD, USA); human recombinant leptin and MPP+ iodide from Sigma–Aldrich; BLOCK-iT Pol II miR RNAi expression vector kits from Invitrogen; ATP determination kit from Perkin-Elmer (MA, USA); 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl carbocyanine iodide (JC-1), tetramethylrhodamine methyl ester (TMRM), and dihydroethidium (DHE) from Molecular Probes (Eugene, OR); Taqman™ EZ RT-PCR reagent kit, probes, and StepOne™ Real-time PCR system from Applied Biosystems (ABI); cell lysis buffer for protein extraction was from Cell Signaling Technology (MA, USA); protease inhibitor cocktail was from Roche (Germany); polyclonal antibodies against human UCP2 from Alpha Diagnostic International, Inc. (San Antonio, TX); polyclonal anti-COX-IV antibody, monoclonal anti-actin from Santa Cruz Biotechnology (Santa Cruz, CA); horseradish peroxidase-conjugated secondary antibodies from DAKO (Carpinteria, CA); ECL western blotting detection system from Amersham Biosciences (Buckinghamshire, UK); GeneTools™ Analysis Software version 3.02 from Syngene (UK); and CellQuest™ flow cytometry software from BD Biosciences (CA, USA).
Cell Culture and Treatment
Human SH-SY5Y cells were cultured in DMEM/F12 supplemented with 10% FBS, 2 mM l-glutamine, and 100 μg/ml penicillin–streptomycin at 37°C in humidified 5% CO2 atmosphere. Cells seeded in 24-well plates at 70% confluence. Cells were treated with 0.5 mM MPP+ (freshly prepared in PBS) with or without leptin (100 nM) for 24 h in DMEM/F12 medium supplemented with 10% charcoal-stripped bovine serum. Cytotoxicity levels after treatment were determined by radioactive [3H]thymidine uptake assay and lactate dehydrogenase (LDH) release assay. Total RNA was extracted by Trizol™ for real-time quantitative RT-PCR. In parallel, live cells after treatment were collected for measurements of MMP and oxidative stress (ROS) by flow cytometry.
Stable Knockdown of UCP2 Expression
Four pre-designed artificial microRNA (miRNA) oligo-duplexes encoding sequences against human UCP2 were cloned separately into a mammalian miRNA expression vector (pcDNA6.2-GW/EmGFP-miR; Invitrogen). Sequences of the cloning oligos are summarized in Table 1. These plasmids were co-transfected into SH-SY5Y cells using Lipofectamine2000™ at a ratio of 2:1 to the amount of plasmid DNA. Twenty-four hours after transfection, cells were selected using blasticidin (4 μg/ml) to obtain single resistant colonies which stably expressed miRNA to trigger gene silencing. The transfection efficiency of target plasmids into SH-SY5Y cells was monitored by expression of GFP under fluorescence microscopy. Knockdown of UCP2 in each clone was confirmed by western blotting.
Table 1.
DNA sequences of UCP2 miRNA oligos
| Oligo name | Sequence |
|---|---|
| Hmi418044-UCP2-sense | 5′-TGCTGTTTAGCAGTATCCAGAGGAAAGTTTTGGCCACTGACTGACTTTCCTCTATACTGCTAAA-3′ |
| Hmi418044-UCP2-antisense | 5′-CCTGTTTAGCAGTATAGAGGAAAGTCAGTCAGTGGCCAAAACTTTCCTCTGGATACTGCTAAAC-3′ |
| Hmi418045-UCP2-sense | 5′-TGCTGTTTGACAGAATCATACAGGCCGTTTTGGCCACTGACTGACGGCCTGTAATTCTGTCAAA-3′ |
| Hmi418045-UCP2-antisense | 5′-CCTGTTTGACAGAATTACAGGCCGTCAGTCAGTGGCCAAAACGGCCTGTATGATTCTGTCAAAC-3′ |
| Hmi418046-UCP2-sense | 5′-TGCTGAATGCTGGCATGCTCAGAGCCGTTTTGGCCACTGACTGACGGCTCTGAATGCCAGCATT-3′ |
| Hmi418046-UCP2-antisense | 5′-CCTGAATGCTGGCATTCAGAGCCGTCAGTCAGTGGCCAAAACGGCTCTGAGCATGCCAGCATTC-3′ |
| Hmi418047-UCP2-sense | 5′-TGCTGATTACGAGCAACATTGGGAGAGTTTTGGCCACTGACTGACTCTCCCAATTGCTCGTAAT-3′ |
| Hmi418047-UCP2-antisense | 5′-CCTGATTACGAGCAATTGGGAGAGTCAGTCAGTGGCCAAAACTCTCCCAATGTTGCTCGTAATC-3′ |
Cell Proliferation and Viability
Cell viability in nondifferentiated cells was measured as the rate of cell proliferation by [3H]thymidine uptake assay as previously described (Chu et al. 2009). Cells were seeded at a density of 1 × 105 cells/ml in 96-well plates and treated with 0.5 mM MPP+ with or without leptin (100 nM) for 24 h. Six hours before the end of the treatment, cells were incubated with 3H-thymidine (1 μCi/ml). Cells were lysed and total DNA was harvested using an automated harvester (Filtermate Harvester Packard Bioscience Co.). The rate of cell proliferation was expressed as the percentage changes in the radioactivity of the samples counted in a liquid scintillation counter (Topcount, NXT Packard Bioscience Co.) compared with untreated controls.
Cell viability in fully differentiated cells was also estimated using LDH release assay as described (Ho et al. 2005). Cells were differentiated by trans-retinoic acid (10 μM) for 7 days, and then treated with 0.5 mM MPP+ with or without leptin (100 nM) for 48 h. The level of neuronal cell death was estimated by measuring the activity of released LDH in culture medium from each treatment group. Cytotoxicity was defined as the percentage increase in LDH release compared with untreated controls after normalization by total protein extractable from the culture.
Detection of Intracellular ROS
Intracellular oxidant levels in cells were measured based on oxidation of superoxide-sensitive DHE as previously described (Chu et al. 2009). Briefly, after treatment, the cells were incubated in DHE (10 μM) for 30 min at 37°C in the dark. The cells were washed with PBS and collected by trypsinization. Red fluorescence from the oxidation of DHE was monitored using a 585-nm filter (FL2-channel). The oxidant level was defined as the relative fluorescent intensity compared with that of vector controls.
Measurement of MMP
Changes in MMP in normal SH-SY5Y cells were measured by flow cytometry based on MMP-sensitive ratiometric fluorescent dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl carbocyanine iodide (JC-1) staining as previously described (Chu et al. 2009). Briefly, cells (1 × 106) were trypsinized in 1 ml of medium and incubated with 10 μg/ml of JC-1 for 20 min at 37°C in the dark. After incubation, the cells were washed and resuspended in PBS in a total volume of 0.5 ml. The intensity of both red and green colors was determined by flow cytometry. A minimum of 10,000 events per sample were acquired per analysis. Relative MMP was defined as the ratio between green and red signals.
Since the UCP2 knockdown cells expressed green fluorescent protein (EGFP), which may interfere the ratiometric measurement of MMP by JC-1, changes in MMP between UCP2 knockdown and vector control cells were estimated by flow cytometry after staining with another MMP-sensitive fluorescent dye, TMRM (Distelmaier et al. 2008; Scaduto and Grotyohann 1999). Briefly, cells (1 × 106) were trypsinized in 1 ml of medium and incubated with 200 nM of TMRM for 15 min at 37°C in the dark. After incubation, the cells were washed and resuspended in medium in a total volume of 1 ml. The relative MMP was estimated by the level of red fluorescence (FL-2) as measured by flow cytometry.
Measurement of Intracellular ATP
Intracellular ATP levels were assayed by utilizing luciferin-luciferase bioluminescene assay as previously described (Chu et al. 2009). Briefly, cells were washed with PBS and harvested 24 h after treatments. Total ATP was extracted in lysis buffer containing: 100 mM potassium phosphate buffer (pH 7.8), 2 mM EDTA, 1 mM DTT, and 1% Triton X-100. Samples were immediately frozen after extraction at −80°C until use. ATP concentrations were determined using a calibration curve of serial ATP dilutions provided in the kit and compared with normal vector controls. Aliquots from each sample extract were used to measure total protein for normalization.
Real-Time Quantitative RT-PCR of Steady-State mRNA Levels
UCPs mRNA expression was determined by quantitative RT-PCR. Total RNA was isolated and digested with DNase I as previously described (Ho et al. 2005). The OD260/280 ratio of extracted RNA was kept above 1.95 to ensure purity and integrity. The thermal cycling conditions were as follows: 2 min at 50°C; 30 min at 60°C and 5 min at 95°C followed by 40 cycles of 95°C for 20 s and 62°C for 1 min in Taqman™ EZ RT-PCR reaction mixtures. Each RNA sample was run in triplicate and repeated in at least three independent treatments. 18S-rRNA expression was used to normalize for mRNA loading. All reactions were performed using StepOne™ Real-time PCR System (ABI).
Western Blotting
Total cell lysate was extracted in 1× cell lysis buffer (Cell Signaling Technology, Inc.), supplemented with 1 mM phenylmethylsulfonyl fluoride. Equal amounts of protein (30 μg) were electrophoresed on 15% SDS-PAGE and transferred onto PVDF-membrane. Resulting blots were blocked with 5% nonfat skimmed milk and probed with the following: anti-UCP2 (1:1000), anti-COXIV (1:10000), or anti-actin (1:500). Blots were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000) followed by ECL substrate detection. Immunoblots were quantified by computerized scanning densitometry.
Statistics
Data are expressed as mean ± standard error mean (SEM) from at least four independent experiments. GraphPad PRISM software (Graph Pad Inc., San Diego, CA) was used to calculate statistical significance P values among groups using one-way ANOVA followed by the post hoc Tukey’s multiple comparison tests. Differences were considered significant at P < 0.05.
Results
Leptin Preserved Cell Survival Against MPP+-Induced Toxicity
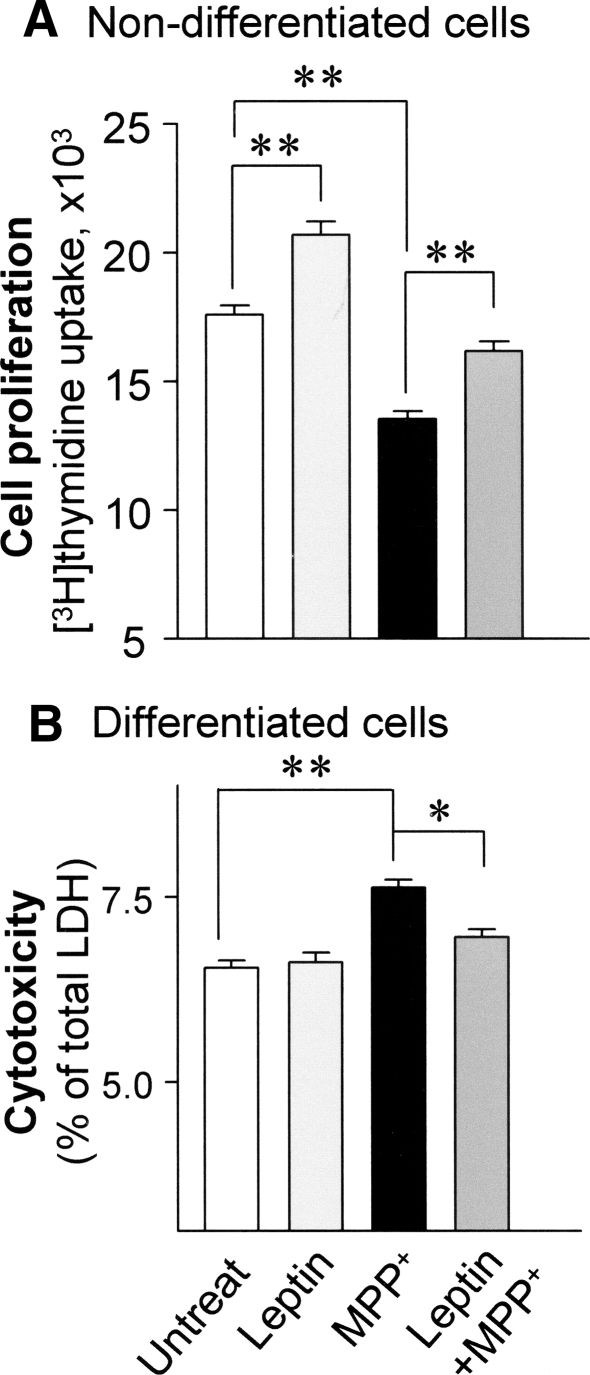
To determine whether leptin could protect against MPP+ toxicity, both differentiated and nondifferentiated SH-SY5Y cells were exposed to MPP+, with or without leptin treatment in charcoal-stripped medium. At 24 h, human recombinant leptin (100 nM) alone increased cell proliferation in nondifferentiated cells (by 18%) compared with untreated controls (P < 0.01) (Fig. 1a). At 24 h, MPP+ (0.5 mM) significantly decreased cell proliferation by 23% compared with untreated controls (P < 0.01). Combined treatment with leptin and MPP+ maintained cell proliferation at a rate similar to that of untreated cells and at a significantly greater rate (by 20%) compared with cells exposed to MPP+ only (P < 0.01) (Fig. 1a).
Fig. 1.
Leptin protects against MPP+-induced cell death in SH-SY5Y cells. a Cell proliferation was measured by [3H]thymidine uptake assay in nondifferentiated cells after MPP+ (0.5 mM) exposure for 24 h. b The level of cell death was measured by LDH release assay in differentiated nonreplicating cells after MPP+ (0.5 mM) exposure for 48 h. Results are mean ± SEM of four separate experiments (N = 4). Statistical significance at * P < 0.05; ** P < 0.01, compared to untreated vector controls
Leptin treatment alone did not affect cell viability as measured by LDH release in differentiated cells (Fig. 1b). Exposure to MPP+ (0.5 mM) for 48 h significantly increased cell death (by 17%) compared with untreated controls (P < 0.01). Leptin with MPP+ significantly preserved cell survival (by 9%) compared with MPP+ only (P < 0.05; Fig. 1b). These findings indicate significant protective effects of leptin against MPP+ toxicity in SH-SY5Y cells.
Leptin Preserved MMP but had no Effect on Oxidative Stress Induced by MPP+
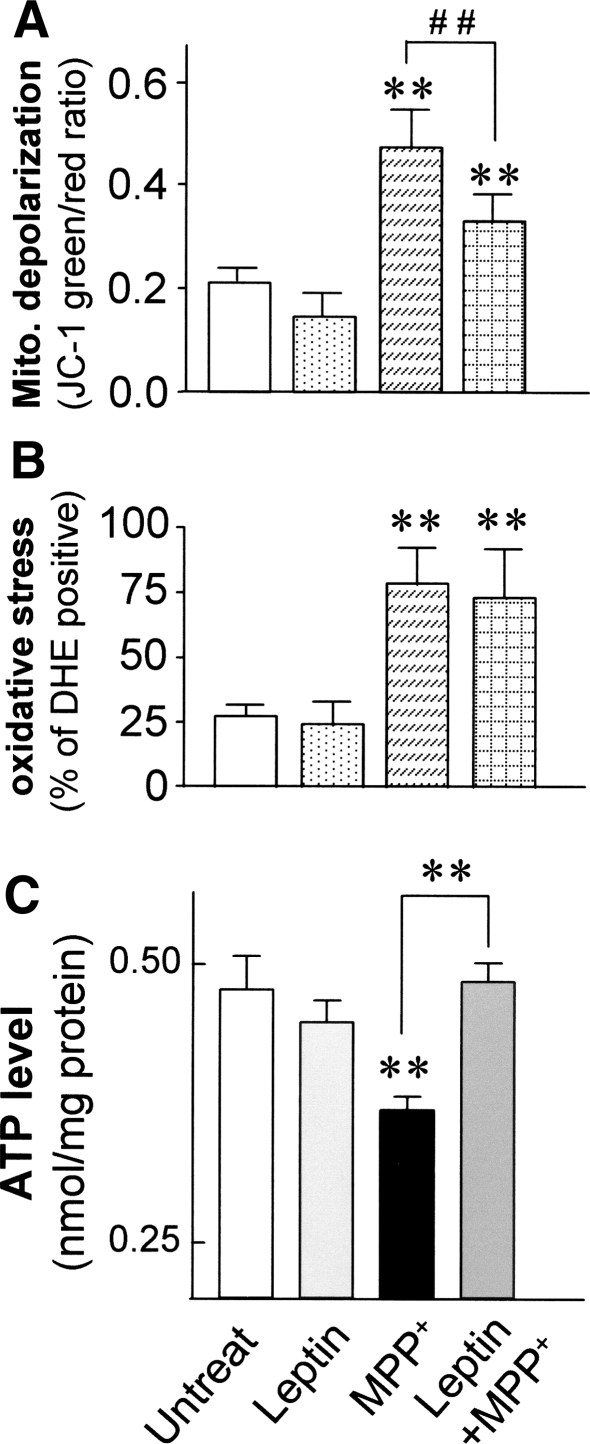
Relative MMP was determined by JC-1 staining following exposure to MPP+, with and without leptin. Incubation with leptin alone did not change MMP levels compared with untreated controls (Fig. 2a). At 24 h, MPP+ (0.5 mM) caused significant mitochondrial membrane depolarization (from 0.21 ± 0.03 to 0.47 ± 0.08; P < 0.01). Leptin significantly attenuated this MPP+-induced mitochondrial membrane depolarization (by 32%; P < 0.01; Fig. 2a).
Fig. 2.
Leptin prevented MPP+-induced depolarization of MMP, preserved ATP levels, but no effect on oxidative stress in SH-SY5Y cells. SH-SY5Y cells were exposed to 0.5 mM MPP+ for 24 h with and without leptin. a Cells after treatment were stained with JC-1, and the relative MMP was expressed as the ratio of green to red fluorescence measured by flow cytometry. b Cells after treatment were stained with DHE, and the level of oxidative stress was expressed as the level of red fluorescence measured by flow cytometry. c Total intracellular ATP levels were measured by utilizing luciferin-luciferase bioluminescene assay, 24 h after treatments. Results are expressed as mean ± SEM of three separate experiments (N = 3). Statistical significance at the level of * P < 0.05; ** P < 0.01, compared to untreated controls. Statistical significance at the level of ** P < 0.01, between two treatment groups indicated
Leptin treatment alone did not increase the ROS level compared with untreated cells. MPP+ (0.5 mM) significantly increased ROS levels (by 3-fold) compared with untreated controls at 24 h (P < 0.01), but leptin did not attenuate this increase in ROS levels induced by MPP+ (Fig. 2b).
Leptin Preserved Cellular ATP Levels in MPP+-Induced Toxicity
Incubation with leptin alone for 24 h did not change the basal ATP level compared with untreated controls. MPP+ (0.5 mM) significantly decreased the intracellular ATP level (by 23%) compared with untreated controls (from 0.477 ± 0.03 to 0.369 ± 0.01 nmol/mg; P < 0.05). Leptin treatment significantly preserved ATP levels under MPP+ toxicity, similar to controls without MPP+ (P < 0.01; Fig. 2c).
Both Leptin and MPP+ Increased UCP2 and UCP4 Expression, but not UCP5 in Control Cells
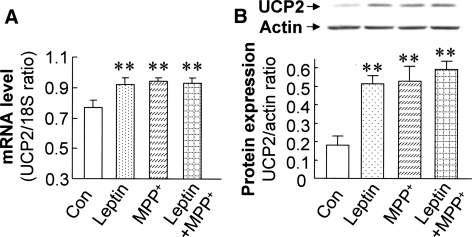
The role of UCP2 in mediating the protective effects of leptin was explored by measuring UCP2 mRNA and protein expression after treatment with leptin and MPP+. In control cells, incubation with leptin for 24 h increased UCP2 mRNA (by 32%; P < 0.01) and protein levels (by 184%; P < 0.01). MPP+ (0.5 mM) at 24 h also significantly increased UCP2 mRNA (by 37%; P < 0.01) and protein expression (by 193%; P < 0.01) compared with untreated controls. Combined treatment with both leptin and MPP+ did not further increase UCP2 expression, compared with cells treated with either leptin or MPP+ alone (Fig. 3a, b).
Fig. 3.
Leptin and MPP+ induced UCP2 mRNA and protein expression in SH-SY5Y cells. a The steady-state UCP2 mRNA expression was measured and normalized by rRNA-18S level by quantitative RT-PCR. Leptin treatment and MPP+ exposure for 24 h increased UCP2 mRNA expression compared to the untreated controls. b Under the same treatment, leptin and MPP+ increased UCP2 protein expression as shown by western blot. Combination of leptin and MPP+ did not further increased UCP2 mRNA and protein expression. Results are mean ± SEM of four separate experiments (N = 4). Statistical significance at the level of ** P < 0.01, as compared to untreated controls
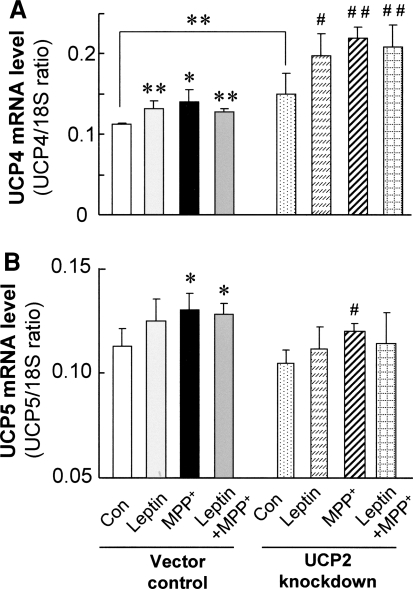
We have previously reported neuroprotective effects of overexpressing UCP4 and potential compensatory effects between UCP2 and UCP4 expression in MPP+ toxicity (Chu et al. 2009). Hence, we explored whether leptin could also affect UCP4 expression in response to changes in UCP2 expression. Here, we showed that leptin alone induced the steady-state UCP4 mRNA level (by 26%; P < 0.01) compared with untreated controls. MPP+ significantly increased UCP4 mRNA level (by 19%; P < 0.01; Fig. 5a) compared with untreated controls at 24 h, consistent with our previous report (Ho et al. 2005). Combined treatment with leptin and MPP+ did not alter UCP4 expression further compared with either leptin or MPP+ alone (Fig. 5a).
Fig. 5.
Steady-state mRNA expression of UCP4 (a) and UCP5 (b) in both UCP2 knockdown and vector control cells. Leptin treatment and MPP+ exposure increased UCP4 mRNA expression. Stable knockdown of UCP2 also increased UCP4 mRNA expression, but not UCP5. UCP5 mRNA expression was only induced by MPP+ exposure. Results are mean ± SEM of three separate experiments (N = 3). Statistical significance at * P < 0.05; ** P < 0.01, as compared to untreated vector controls. # P < 0.05; ## P < 0.01, as compared to untreated UCP2 knockdown cells
For UCP5, leptin alone had no significant effect on UCP5 mRNA levels in control cells, whereas exposure of these cells to MPP+ toxicity caused a significant increase (by 13%; P < 0.05; Fig. 5b). Combine treatment with leptin and MPP+ increased UCP5 mRNA levels to a similar extent to that caused by MPP+ alone (Fig. 5b).
Stable Knockdown of UCP2 Expression Increased MMP
Our above results in control cells showed that either leptin or MPP+ alone, and combined treatment increased UCP2 mRNA and protein expression (Fig. 3). We also showed that leptin significantly reduced MPP+ toxicity (Fig. 1), indicating that UCP2 may be involved in the protective properties of leptin. Hence, we developed a cell model in which UCP2 expression was stably knocked down by transfection of a plasmid overexpressing miRNA against human UCP2. In this cell model, UCP2 knockdown was demonstrated by a 48% reduction in UCP2 protein expression (Fig. 4a). The MMP of these UCP2-knockdown cells relative to the empty-vector controls was measured by flow cytometry after TMRM staining. Under normal conditions, UCP2-knockdown cells showed a significantly higher MMP compared with vector controls as shown by a 5.8-fold stronger TMRM staining in UCP2-knockdown cells (P < 0.01) (Fig. 4b). These results demonstrated classical uncoupling properties of UCP2 (Fleury et al. 1997).
Fig. 4.
Stable knockdown of UCP2 in SH-SY5Y cells decreased UCP2 protein expression (a), and increased MMP under normal untreated condition (b), as compared to vector controls. The relative MMP between UCP2 knockdown and vector control cells was expressed as levels of red fluorescence measured by flow cytometry after staining with 200 nM TMRM for 15 min. Results are mean ± SEM of three separate experiments (N = 3). Statistical significance at the level of ** P < 0.01, as compared to untreated vector controls
Effects of Knockdown of UCP2 Expression, Leptin, and MPP+ on UCP4 and UCP5 Expression
To explore whether UCP2-knockdown affected other neuronal UCPs (namely UCP4 and 5), their mRNA levels were determined by quantitative RT-PCR after UCP2-knockdown. Treatment with either leptin or MPP+ significantly increased UCP4 mRNA levels in UCP2-knockdown cells similar to those observed in the vector control cells as described above. Interestingly, the magnitude of differences in UCP4 mRNA levels between the various treatments and their respective controls appeared to be greater in UCP2-knockdown cells compared with changes seen in vector control cells. In untreated conditions, UCP4 mRNA levels were 35% higher in UCP2-knockdown cells, compared with vector controls (P < 0.01) (Fig. 5a). In UCP2-knockdown cells, leptin increased UCP4 mRNA expression by 31% (P < 0.05), and MPP+ toxicity by 47% (P < 0.01), compared with untreated controls. Only MPP+ toxicity alone caused a small but significant increase in UCP5 mRNA (Fig. 5b).
Knockdown of UCP2 Expression Reduced ATP Levels and Negated the Protective Effects of Leptin Against ATP Deficiency
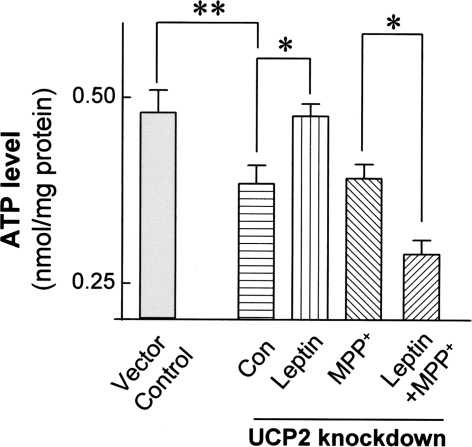
Knockdown of UCP2 expression significantly decreased total ATP levels by 26%, compared with vector controls (P < 0.01; Fig. 6). Furthermore, unlike vector control cells (Fig. 2c), leptin failed to preserve cellular ATP levels in UCP2-knockdown cells exposed to MPP+ toxicity. Surprisingly, ATP levels in leptin-treated UCP2-knockdown cells were even lower than untreated vector control cells exposed to MPP+ (P < 0.05; Fig. 6).
Fig. 6.
Knockdown of UCP2 expression decreased intracellular ATP levels compared to the vector controls. UCP2 knockdown also abolished leptin protection against MPP+-induced ATP deficiency and further decreased the ATP level as compared to the group exposure to MPP+ only. Combination of leptin and MPP+ in UCP2 knockdown cells further decreased ATP level as compared to the group exposure to MPP+ only. Results are mean ± SEM of four separate experiments (N = 4). Statistical significance at * P < 0.05; ** P < 0.01, as compared to untreated controls
Knockdown of UCP2 Expression Abolished Leptin Protection Against Cell Death and Mitochondrial Depolarization Induced by MPP+
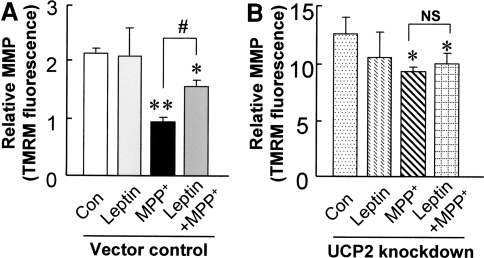
Our results above showed that leptin can protect against mitochondrial depolarization induced by MPP+ in normal SH-SY5Y cells. To further explore the role of UCP2 in the protective properties of leptin, both UCP2-knockdown and vector control SH-SY5Y cells were challenged with MPP+ (0.5 mM), with or without leptin (100 nM) for 24 h. Cells after treatment were stained with TMRM to measure the relative MMP. Similar to our results obtained above using JC-1 staining, MPP+ (0.5 mM) caused significant mitochondrial membrane depolarization (from 2.15 ± 0.06 to 0.94 ± 0.09; P < 0.01). Leptin significantly attenuated this MPP+-induced mitochondrial membrane depolarization (by 41%; P < 0.05; Fig. 7a). However, this protective effect of leptin against mitochondrial depolarization was not observed in UCP2-knockdown cells (Fig. 7b), indicating that UCP2 is critical in mediating protection of leptin against mitochondrial depolarization induced by MPP+ toxicity.
Fig. 7.
Leptin attenuated MPP+-induced depolarization of MMP in normal SH-SY5Y cells (a), but not in UCP2 knockdown cells (b). SH-SY5Y cells were exposed to 0.5 mM MPP+ for 24 h, with and without leptin. Cells after treatment were stained with 200 nM TMRM for 15 min., and the relative MMP was expressed as levels of red fluorescence measured by flow cytometry. Results are expressed as mean ± SEM of three separate experiments (N = 3). Statistical significance at the level of * P < 0.05; ** P < 0.01, compared to the respective untreated controls. Statistical significance at the level of # P < 0.01, between two treatment groups indicated
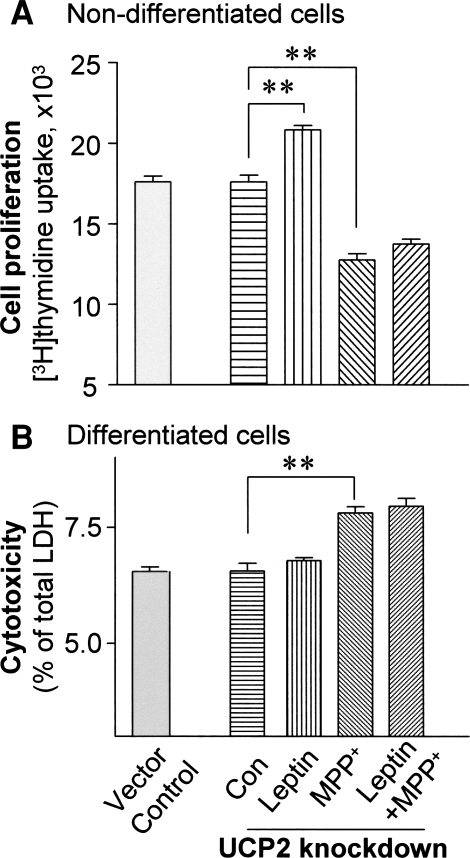
Moreover, stable knockdown of UCP2 expression did not affect the rate of cell proliferation in nondifferentiated cells (Fig. 8a), and did not cause cytotoxicity in differentiated cells (Fig. 8b) compared with empty-vector controls under normal conditions. Similar to vector control cells (Fig. 1a), leptin treatment alone significantly increased proliferation of UCP2-knockdown cells (by 20%; P < 0.01) compared with untreated controls (Fig. 8a). Following combined treatment with leptin and MPP+, unlike the results in vector control cells, the protective effects of leptin against cell death were not observed in either nondifferentiated or differentiated UCP2-knockdown cells (Fig. 8a, b).
Fig. 8.
a Effects of UCP2 knockdown on cell proliferation in nondifferentiated SY-SY5Y cells. Knockdown of UCP2 expression did not affect the rate of cell proliferation under normal culture condition, but abolished leptin protection against MPP+ toxicity. b Effects of UCP2 knockdown on leptin protection against MPP+ toxicity in differentiated SY-SY5Y cells. Results are mean ± SEM of four separate experiments (N = 4). Statistical significance at ** P < 0.01, as compared to untreated controls
Leptin Protection Against MPP+ Toxicity was not Associated with Mitochondrial Proliferation
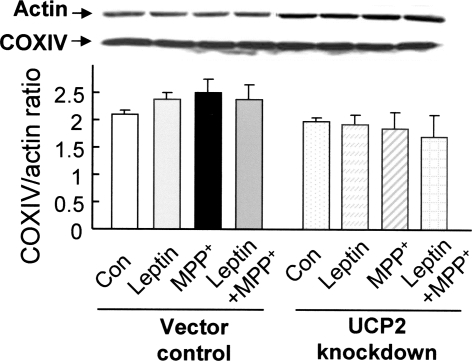
Because leptin preserved ATP levels in vector control but not in UCP2-knockdown cells exposed to MPP+, we determined whether the protective effects associated with leptin were due to mitochondrial proliferation. We compared the expression of a mitochondrial marker, COX-IV, with that of a nuclear encoded housekeeping gene product, actin, by western blotting. The relative amount of mitochondria was expressed as the ratio of COX-IV to actin. The relative amounts of mitochondria between vector control and UCP2-knockdown cells were similar under normal culture conditions and in leptin-treated cells exposed to MPP+ (Fig. 9), indicating that UCP2-knockdown did not affect mitochondrial proliferation rates, and that the preservation of ATP levels by leptin was not due to mitochondrial proliferation.
Fig. 9.
UCP2 knockdown did not affect mitochondrial proliferation as estimated by the protein expression of COX-IV/actin ratio. Results are mean ± SEM of three separate experiments (N = 3)
Discussion
Mitochondrial dysfunction, as a consequence of mitochondrial Complex I inhibition, causes oxidative stress and ATP deficiency in neurons (Beal 2003; Dawson and Dawson 2003; Dauer and Przedborski. 2003). Despite distinctive expression profiles among the neuronal UCPs (Andrews et al. 2005; Fleury et al. 1997; Mao et al. 1999; Sanchis et al. 1998), their physiological functions are unclear. We and others have shown that increased expression of these UCPs reduced the level of cell death under different insults, including MPP+, MPTP, and 6-hydroxydopamine toxicity (Chu et al. 2009; Andrews et al. 2006; Chan et al. 2006; Conti et al. 2005). Because human SH-SY5Y neuroblastoma cells express the leptin receptor, they represent an appropriate homogeneous culture model to study the effects of leptin on neuronal cells (Lu et al. 2006; Benomar et al. 2005).
To demonstrate the neuroprotective effects of leptin on mitochondrial dysfunction, MPP+ was used to induce ATP deficiency and oxidative stress. MPP+ specifically blocks mitochondrial Complex I activity, which impairs oxidative phosphorylation and subsequently suppresses ATP production and generates ROS. We previously showed that MPP+ toxicity significantly induced expression of UCP2, 4 and 5 in neuronal culture (Ho et al. 2005), which we interpreted as defensive responses to counteract the effects of mitochondrial dysfunction induced by this toxin. In this study, we found that treatment with leptin protected neuronal cells against MPP+-induced toxicity. This was accompanied by stabilization of MMP at levels similar to untreated cells, and by significant preservation of total intracellular ATP concentrations. We not only showed the ability of leptin to modulate MMP and ATP production in our neuronal culture, our findings also demonstrated an inductive effect of leptin in increasing expression of UCP2 and UCP4, but not UCP5. Nevertheless, leptin did not have any direct effects in suppressing MPP+-induced ROS production in these cells. These observations suggest the existence of mitochondrial pathways, potentially activated by leptin, to promote cell survival via its effects on neuronal UCPs.
UCP2 expression was induced by MPP+, consistent with our previous report (Ho et al. 2005). UCP2 expression was also significantly induced after incubation with leptin alone, to a level similar to the cells exposed to MPP+. Nevertheless, incubation of leptin alone did not cause increased cytotoxicity. Cell death induced by MPP+ was remarkably reduced by co-treatment with leptin. Interestingly, combined leptin and MPP+ treatment did not further increase the expression of UCP2, when compared with levels in cells exposed to either leptin or MPP+. We also observed protective effects of leptin, in that levels of cell survival, MMP, and intracellular ATP were preserved. These effects were abolished after UCP2 knockdown. Thus, the protective effects of leptin were dependent on the presence of UCP2. Nevertheless, whether UCP2 directly interacts with proteins in the mitochondrial respiratory chain in response to leptin signaling is still unclear.
The increase in UCP2 expression after MPP+ toxicity was likely due to increased ROS generation mediated by mitochondrial Complex I inhibition by this toxin, as previously proposed (Echtay et al. 2002; Brand et al. 2004; Krauss et al. 2003). Moreover, induction of UCP2 expression was also observed after leptin exposure under normal culture conditions (Fig. 3). In our study, the increase in UCP2 expression was not due to an increase in ROS, because leptin treatment did not significantly increase the ROS level. Leptin can modulate the phosphatidylinositol-3-kinase-phosphodiesterase 3B-cAMP pathway (Sahu 2004). Multiple cAMP-response element binding protein (CREB) response elements have been reported in human UCP2 promoter region (Tu et al. 1999). It is possible that leptin-induced UCP2 expression occurs via the cAMP signaling pathway. Furthermore, because we did not observe significant additive effects on the level of UCP2 expression after combined treatment with leptin and MPP+, it is reasonable to propose that the protective effects of leptin were not dependent on a still further increase in UCP2 expression.
There is considerable evidence to indicate that UCP2 is neuroprotective in various experimental models. UCP2 has been shown to play an important role in neurons to diminish free radicals (Andrews et al. 2006; Conti et al. 2005), to decrease calcium influx into mitochondria (Mattiasson et al. 2003), and to elevate cellular ATP levels (Diano et al. 2003). Exactly how UCP2 and leptin interact to achieve the protective effect reported here is still poorly understood. Functional linkages have been observed between leptin and UCP2 in peripheral tissues. For example, leptin diminished UCP2 abundance in pancreas but not brain during neonatal development (Gnanalingham et al. 2005), whereas significant upregulation of UCP2 by leptin was observed in preadipocytes (Luo et al. 2008). UCP2 was shown to promote fatty acid oxidation and limit glycolysis-derived pyruvate catabolism (Pecqueur et al., 2008; Tajima et al. 2005) and to regulate the choice of mitochondrial substrates for bioenergetics (Criscuolo et al. 2006), but it is uncertain whether these actions would occur in a terminally differentiated neuronal cell which has very limited ability to utilize biofuels other than glucose. Knockdown of UCP2, if it functions simply as an uncoupling protein, should limit the ability of protons to bypass Complex V (ATP synthase). Thus, protons re-entering the mitochondrial matrix would be expected to be more likely to stimulate ATP production. In fact, knockdown of UCP2 decreased ATP synthesis, consistent with a previous report (Diano et al. 2003), which showed that overexpression of UCP2 led to increased ATP synthesis. In our study, the combination of leptin and MPP+ treatment in UCP2 knockdown cells led to a greater reduction in ATP levels than seen in these cells exposed to MPP+ only. The reasons for this observation are unclear. Nevertheless, we postulate that this further decrease in intracellular ATP level contributes to the abolition of leptin protection in UCP2 knockdown cells.
The observed leptin protection via increased UCP2 expression is not the only protective action of leptin. Effects outside mitochondria have been reported by other groups (Esler et al. 1998; Scarpace et al. 2000; Ceddia et al. 2000). Leptin is known to activate AMPK, which is known to increases β-oxidation and thus modulate cellular ATP/ADP ratio (Blázquez et al. 1999). Nevertheless, glucose is by far the major provider of neuronal energy needs (Edmond et al. 1987), whereas other energy substrates, such as fatty acids, represent only up to 20% of brain oxidative energy production (Ebert et al. 2003). It is unlikely that the attenuation of MPP+-induced ATP deficiency by leptin in our neuronal culture was solely due to stimulation of β-oxidation by leptin-activated AMPK activity (Blázquez et al. 1999). Instead, we observed a significant preservation of MMP by leptin in our control cells, which contributed to the attenuation of ATP deficiency induced by MPP+. This protective effect against mitochondrial depolarization by leptin was completed abolished after UCP2-knockdown. UCP2 has been shown to play a critical role in maintaining mitochondrial functions in neurons (Arsenijevic et al. 2000; Andrews et al. 2006). Although we observed a higher MMP after UCP2-knockdown, these cells have significantly lower ATP levels, even under normal culture conditions. The reasons for this observation are unclear. However, it is clear that suppressing UCP2 expression failed to maintain normal mitochondrial functioning. Similar observations were observed in immortalized embryonic fibroblasts from UCP2 knockout mice which exhibited a significant decrease in ATP/ADP ratio as compared with cells from wild-type littermates (Pecqueur et al. 2008). Our current findings support UCP2 as a critical candidate in regulating ATP levels by leptin.
Another factor which might involve in the protective leptin pathway is the increase of UCP4 expression in this MPP+ toxicity model. UCP4 is a neuronal specific UCP homologue which shares about 33% amino acid identity with UCP2 (Mao et al. 1999). Functional studies of UCP4 have been described (Chu et al. 2009; Chan et al. 2006; Liu et al. 2006; Zhang et al. 2006; Yu et al. 2000). In this study, knockdown of UCP2 in neuronal culture significantly increased UCP4 expression; however, this inductive effect was not observed in another neuronal UCP homologue, UCP5 (Kim-Han et al. 2001; Ho et al. 2006). This supports the concept of differentiation in functions among these UCP homologues as we suggested previously (Ho et al. 2005).
We recently reported that UCP4 overexpression protected neuronal cells against MPP+ toxicity by preserving ATP levels and suppressing oxidative stress (Chu et al. 2009). Classical mitochondrial uncoupling was suggested to depolarize MMP and decrease ATP levels (Andrews et al. 2005; Brookes, 2005), but UCP4 overexpression significantly increased ATP production by promoting respiration rate (Chu et al. 2009). In this study, MPP+ depolarized the inner mitochondrial membrane and reduced ATP levels in vector control cells, whereas in UCP2-knockdown cells, MPP+ had no effect on ATP levels, possibly because of a compensatory increase of UCP4 level after knocking down UCP2, thus maintaining ATP levels similar to its respective untreated control. We hypothesize that this finding is a reflection of the differing interactions between UCP2 and UCP4, in preserving mitochondrial homeostasis, and might partly contribute to the protective effects observed in this current model. Such synergistic interaction between UCP2 and UCP4 has been previously reported (Yasuno et al. 2007).
In conclusion, leptin is protective against MPP+-induced mitochondrial depolarization and ATP deficiency in neuronal cells. Our findings showed a crucial link between UCP2 expression and the neuroprotective effects of leptin against MPP+ toxicity. The absence of these protective effects by leptin after knockdown of UCP2 is the first evidence to demonstrate a critical role of mitochondrial UCP2 in mediating such effects of leptin in neuronal cells to preserve energy supply. It is possible that the restoration of ATP levels was attributed to a potential facilitation of oxidative phosphorylation as shown by preservation of MMP by the presence of UCP2, and a compensatory increase of UCP4, which likely help to preserve ATP levels in MPP+ toxicity. It might be possible to develop therapeutic strategies using leptin or its structural analogs to increase mitochondrial efficiency to prevent or delay neuronal degeneration as a consequence of mitochondrial dysfunction.
Acknowledgments
We gratefully acknowledge the invaluable support from the Henry G Leong Professorship in Neurology (SLH), the Research Grants Council, Hong Kong (HKU 7661/07M; SLH), the Donation Fund for Neurology Research (SLH), and the Small Project Funding (HKU 200707176087; PWLH). PWL Ho is supported by a Research Assistant Professorship; WY Zhang is supported by a Postdoctoral Fellowship; HF Liu, X Ge, and JWM Ho are supported by PhD Studentships from the University of Hong Kong. KHH Kwok PhD studentship was fully supported by the Donation Fund for Neurology Research (SLH).
References
- Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci. 2005;6:829–840. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Rivera A, Elsworth JD, Roth RH, Agnati L, Gago B, Abizaid A, Schwartz M, Fuxe K, Horvath TL. Uncoupling protein-2 promotes nigrostriatal dopamine neuronal function. Eur J Neurosci. 2006;24:32–36. doi: 10.1111/j.1460-9568.2006.04906.x. [DOI] [PubMed] [Google Scholar]
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann N Y Acad Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- Benomar Y, Roy AF, Aubourg A, Djiane J, Taouis M. Cross down-regulation of leptin and insulin receptor expression and signalling in a human neuronal cell line. Biochem J. 2005;388:929–939. doi: 10.1042/BJ20041621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez C, Woods A, Ceballos ML, Carling D, Guzmán M. The AMP-activated protein kinase is involved in the regulation of ketone body production by astrocytes. J Neurochem. 1999;73:1674–1682. doi: 10.1046/j.1471-4159.1999.731674.x. [DOI] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Brookes PS. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Casteilla L, Rigoulet M, Pénicaud L. Mitochondrial ROS metabolism: modulation by uncoupling proteins. IUBMB Life. 2001;52:181–188. doi: 10.1080/15216540152845984. [DOI] [PubMed] [Google Scholar]
- Ceddia RB, William WN, Jr, Lima FB, Flandin P, Curi R, Giacobino JP. Leptin stimulates uncoupling protein-2 mRNA expression and Krebs cycle activity and inhibits lipid synthesis in isolated rat white adipocytes. Eur J Biochem. 2000;267:5952–5958. doi: 10.1046/j.1432-1327.2000.01664.x. [DOI] [PubMed] [Google Scholar]
- Chan SL, Liu D, Kyriazis GA, Bagsiyao P, Ouyang X, Mattson MP. Mitochondrial uncoupling protein-4 regulates calcium homeostasis and sensitivity to store-depletion-induced apoptosis in neural cells. J Biol Chem. 2006;281:37391–37403. doi: 10.1074/jbc.M605552200. [DOI] [PubMed] [Google Scholar]
- Chu ACY, Ho PWL, Kwok KHH, Ho JWM, Chan KH, Liu HF, Kung MHW, Ramsden DB, Ho SL. Mitochondrial UCP4 attenuates MPP+- and dopamine-induced oxidative stress, mitochondrial depolarization, and ATP deficiency in neurons and is interlinked with UCP2 expression. Free Radic Biol Med. 2009;46:810–820. doi: 10.1016/j.freeradbiomed.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Conti B, Sugama S, Lucero J, Winsky-Sommerer R, Wirz SA, Maher P, Andrews Z, Barr AM, Morale MC, Paneda C, Pemberton J, Gaidarova S, Behrens MM, Beal F, Sanna PP, Horvath T, Bartfai T. Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J Neurochem. 2005;93:493–501. doi: 10.1111/j.1471-4159.2005.03052.x. [DOI] [PubMed] [Google Scholar]
- Couce ME, Burguera B, Parisi JE, Jensen MD, Lloyd RV. Localization of leptin receptor in the human brain. Neuroendocrinology. 1997;66:145–150. doi: 10.1159/000127232. [DOI] [PubMed] [Google Scholar]
- Criscuolo F, Mozo J, Hurtaud C, Nübel T, Bouillaud F. UCP2, UCP3, avUCP, what do they do when proton transport is not stimulated? Possible relevance to pyruvate and glutamine metabolism. Biochim Biophys Acta. 2006;1757(9–10):1284–1291. doi: 10.1016/j.bbabio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Diano S, Matthews RT, Patrylo P, Yang L, Beal MF, Barnstable CJ, Horvath TL. Uncoupling protein 2 prevents neuronal death including that occurring during seizures: a mechanism for preconditioning. Endocrinology. 2003;144:5014–5021. doi: 10.1210/en.2003-0667. [DOI] [PubMed] [Google Scholar]
- Distelmaier F, Koopman WJ, Testa ER, Jong AS, Swarts HG, Mayatepek E, Smeitink JA, Willems PH. Life cell quantification of mitochondrial membrane potential at the single organelle level. Cytometry A. 2008;73(2):129–138. doi: 10.1002/cyto.a.20503. [DOI] [PubMed] [Google Scholar]
- Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2003;23:5928–5935. doi: 10.1523/JNEUROSCI.23-13-05928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415(6867):96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Edmond J, Robbins RA, Bergstrom JD, Cole RA, Vellis J. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res. 1987;18:551–561. doi: 10.1002/jnr.490180407. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. doi: 10.1002/(SICI)1096-9861(19980615)395:4<535::AID-CNE9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Esler M, Vaz M, Collier G, Nestel P, Jennings G, Kaye D, Seals D, Lambert G. Leptin in human plasma is derived in part from the brain, and cleared by the kidneys. Lancet. 1998;351:879. doi: 10.1016/S0140-6736(05)70289-0. [DOI] [PubMed] [Google Scholar]
- Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, Warden CH. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gnanalingham MG, Mostyn A, Wang J, Webb R, Keisler DH, Raver N, Alves-Guerra MC, Pecqueur C, Miroux B, Stephenson T, Symonds ME. Tissue-specific effects of leptin administration on the abundance of mitochondrial proteins during neonatal development. J Endocrinol. 2005;187:81–88. doi: 10.1677/joe.1.06251. [DOI] [PubMed] [Google Scholar]
- Guo Z, Jiang H, Xu X, Duan W, Mattson MP. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J Biol Chem. 2008;283:1754–1763. doi: 10.1074/jbc.M703753200. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Ho PWL, Chan DYL, Kwok KHH, Chu ACY, Ho JWM, Kung MHW, Ramsden DB, Ho SL. Methyl-4-phenylpyridinium ion modulates expression of mitochondrial uncoupling proteins 2, 4, and 5 in catecholaminergic (SK-N-SH) cells. J Neurosci Res. 2005;81:261–268. doi: 10.1002/jnr.20569. [DOI] [PubMed] [Google Scholar]
- Ho PWL, Chu ACY, Kwok KHH, Kung MHW, Ramsden DB, Ho SL. Knockdown of uncoupling protein-5 in neuronal SH-SY5Y cells: Effects on MPP+-induced mitochondrial membrane depolarization, ATP deficiency, and oxidative cytotoxicity. J Neurosci Res. 2006;84:1358–1366. doi: 10.1002/jnr.21034. [DOI] [PubMed] [Google Scholar]
- Kim-Han JS, Reichert SA, Quick KL, Dugan LL. BMCP1: a mitochondrial uncoupling protein in neurons which regulates mitochondrial function and oxidant production. J Neurochem. 2001;79:658–668. doi: 10.1046/j.1471-4159.2001.00604.x. [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. A significant portion of mitochondrial proton leak in intact thymocytes depends on expression of UCP2. Proc Natl Acad Sci USA. 2002;99:118–122. doi: 10.1073/pnas.012410699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest. 2003;112(12):1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6(3):248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- Liu D, Chan SL, Souza-Pinto NC, Slevin JR, Wersto RP, Zhan M, Mustafa K, Cabo R, Mattson MP. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med. 2006;8:389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- Lu J, Park CS, Lee SK, Shin DW, Kang JH. Leptin inhibits 1-methyl-4-phenylpyridinium-induced cell death in SH-SY5Y cells. Neurosci Lett. 2006;407:240–243. doi: 10.1016/j.neulet.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Luo GF, Yu TY, Wen XH, Li Y, Yang GS. Alteration of mitochondrial oxidative capacity during porcine preadipocyte differentiation and in response to leptin. Mol Cell Biochem. 2008;307:83–91. doi: 10.1007/s11010-007-9587-2. [DOI] [PubMed] [Google Scholar]
- Mao W, Yu XX, Zhong A, Li W, Brush J, Sherwood SW, Adams SH, Pan G. UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett. 1999;443:326–330. doi: 10.1016/S0014-5793(98)01713-X. [DOI] [PubMed] [Google Scholar]
- Maratos-Flier E. The long reach of leptin. Nat Med. 2008;14:604–606. doi: 10.1038/nm0608-604. [DOI] [PubMed] [Google Scholar]
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, Ricquier D, Miroux B, Thompson CB. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J. 2008;22:9–18. doi: 10.1096/fj.07-8945com. [DOI] [PubMed] [Google Scholar]
- Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP, AtUCP. J Biochem. 2000;345:161–179. doi: 10.1042/0264-6021:3450161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145:4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- Sahu A. Minireview: a hypothalamic role in energy balance with special emphasis on leptin. Endocrinology. 2004;145:2613–2620. doi: 10.1210/en.2004-0032. [DOI] [PubMed] [Google Scholar]
- Sanchis D, Fleury C, Chomiki N, Goubern M, Huang Q, Neverova M, Gregoire F, Easlick J, Raimbault S, Levi-Meyrueis C, Miroux B, Collins S, Seldin M, Richard D, Warden C, Bouillaud F, Ricquier D. BMCP1, a novel mitochondrial carrier with high expression in the central nervous system of humans and rodents, and respiration uncoupling activity in recombinant yeast. J Biol Chem. 1998;273:34611–34625. doi: 10.1074/jbc.273.51.34611. [DOI] [PubMed] [Google Scholar]
- Scaduto RC, Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76(1 Pt 1):469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Moore RL, Kumar MV. Modulation of uncoupling protein 2 and uncoupling protein 3: regulation by denervation, leptin and retinoic acid treatment. J Endocrinol. 2000;164:331–337. doi: 10.1677/joe.0.1640331. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/S0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Tajima D, Masaki T, Hidaka S, Kakuma T, Sakata T, Yoshimatsu H. Acute central infusion of leptin modulates fatty acid mobilization by affecting lipolysis and mRNA expression for uncoupling proteins. Exp Biol Med (Maywood) 2005;230:200–206. doi: 10.1177/153537020523000306. [DOI] [PubMed] [Google Scholar]
- Tu N, Chen H, Winnikes U, Reinert I, Marmann G, Pirke KM, Lentes KU. Molecular cloning and functional characterization of the promoter region of the human uncoupling protein-2 gene. Biochem Biophys Res Commun. 1999;265:326–334. doi: 10.1006/bbrc.1999.1663. [DOI] [PubMed] [Google Scholar]
- Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, Yin XM, Chen J. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem. 2007;282:34479–34491. doi: 10.1074/jbc.M705426200. [DOI] [PubMed] [Google Scholar]
- Yasuno K, Ando S, Misumi S, Makino S, Kulski JK, Muratake T, Kaneko N, Amagane H, Someya T, Inoko H, Suga H, Kanemoto K, Tamiya G. Synergistic association of mitochondrial uncoupling protein (UCP) genes with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(2):250–253. doi: 10.1002/ajmg.b.30443. [DOI] [PubMed] [Google Scholar]
- Yu XX, Mao W, Zhong A, Schow P, Brush J, Sherwood SW, Adams SH, Pan G. Characterization of novel UCP5/BMCP1 isoforms and differential regulation of UCP4 and UCP5 expression through dietary or temperature manipulation. FASEB J. 2000;14:1611–1618. doi: 10.1096/fj.14.11.1611. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang B, Ni YH, Liu F, Fei L, Pan XQ, Guo M, Chen RH, Guo XR. Overexpression of uncoupling protein 4 promotes proliferation and inhibits apoptosis and differentiation of preadipocytes. Life Sci. 2006;79:1428–1435. doi: 10.1016/j.lfs.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]