Abstract
Introduction
We have previously described the labeling of interleukin-2 (IL2) with 123I and 99mTc-N3S. Both radiopharmaceuticals were successfully applied in humans to image several inflammatory lesions and autoimmune diseases characterized by tissue infiltrating lymphocytes expressing the IL2 receptor (CD25). However, both radiopharmaceuticals had some specific disadvantages, such as cost and time of synthesis.
Materials and methods
Here, we describe a new improved method for labeling interleukin-2 with 99mTc using HYNIC-NHS and tricine as coligand. Several optimizations of reagent concentrations and labeling conditions were performed in order to standardize the procedure. After labeling, IL2 was purified by tC2 reverse-phase chromatography and tested in vitro and in vivo, in mice and in a normal volunteer. Results showed that this labeling procedure is cheap, fast, reliable, and reproducible, leading to a product with high specific activity (153 µCi/µg), high stability and capable of binding in vitro to CD25 positive cells. In vivo biodistribution in mice and human volunteer did not show any significant different from 99mTc-N3S-IL2.
Conclusion
In conclusion, the optimization of 99mTc-HYNIC-IL2 has a great advantage in terms of cost and time of production and a simple kit formulation can be considered for routine application to study patients affected by autoimmune diseases, graft rejection, or other chronic inflammatory disorders.
Key words: Interleukin-2, Inflammation, Radiopharmacy, Molecular imaging
Introduction
Interleukin-2 is a small single-chain glycoprotein (15.5 kDa) of 133 amino acids that is synthesized and secreted in vivo by activated lymphocytes in several pathological conditions, such as autoimmune diseases, graft rejection, and tumors characterized by chronic infiltration of lymphomononuclear cells expressing the IL2R (interleukin-2 receptor) on their surface membrane.
The IL2R is formed by three proteins α, β and γ chain, namely the CD25, CD122, CD132. The high affinity receptor is the ethero-trimer, although also CD25 and CD122 can bind IL2 alone with intermediate or low affinity [1]. The identification of these cells in vivo has important diagnostic, prognostic, and therapeutic implications. IL2 radiolabeled with 123I and 99mTc has been proven to be useful to target-activated lymphocytes expressing CD25 molecule (interleukin-2 receptor) in chronic inflammatory diseases, such as coeliac disease [2], Crohn's disease [3], type 1 diabetes [4], Hashimoto's thyroiditis, Sjogren syndrome, cutaneous melanoma [5] and head and neck carcinoma [6]. These clinical studies have demonstrated the efficacy of using scintigraphy to detect activated lymphocytes, which correlate with the severity of tissue lymphocytic infiltration and that scintigraphy with radiolabeled IL2 can be used for monitoring the efficacy of specific therapies.
Until now, Interleukin-2 (Proleukin®, Aldesleukin®) has routinely been labeled in our institution with 123I, but the cost and time of production requiring HPLC purification of final product, has limited its use to a proof of concept. We then labeled IL2 with 99mTc by means of a two-step approach based on the use of N3S ligand as bifunctional chelating agent. This synthetic approach yields 99mTc-labeled IL2 with high specific activity [7]. However, the whole process is a multi-step procedure that requires post-labeling purification and takes approximately three hours of work. This greatly limits its routine clinical application. The aim of this work was to develop and optimize a single-step radiolabeling procedure of IL-2 (Proleukin®) based on the pre-conjugation of the protein with succinimidyl-6-hydrazinopyridine-3-carboxilate (HYNIC-NHS) as a bifunctional chelating agent and tricine as coligand, based on the fact that combination of HYNIC-peptide with tricine produces a ternary ligand system which forms a stable technetium complex. This procedure could potentially be used to obtain a kit formulation available for routine clinical use without post-labeling purification. The biological activity of the new complex was also assessed in comparison with the radiopharmaceutical previously used.
Materials and Methods
Conjugation of IL-2 with HYNIC-NHS
Recombinant human Interleukin-2 (Aldesleukin®) was provided by Chiron (Novartis, Italy) as a freeze-dried powder. The heterobifunctional linker succinimidyl-6-hydrazinopyridine-3-carboxylate (HYNIC-NHS) was purchased from Solulink (USA) and conjugated to the IL2 as previously described by Abrams et al. [8] with slight modifications. Briefly, IL2 was reconstituted with nitrogen-purged water for injection to a concentration of 2.4 mg/ml. A molar excess of HYNIC-NHS ranging from 3:1 to 12:1 in dimethylformamide (DMF, Sigma-Aldrich Chemical) was then added dropwise to 100 µl of the IL2 solution and 100 µl buffer solution. Three different buffer solutions were also explored to assess the effect of pH of conjugation on labeling efficiency, as described. After stirring gently for 2 h in darkness at room temperature, the HYNIC-IL2 complex was purified by solid-phase extraction chromatography (SPE). To eliminate the excess of unbound HYNIC-NHS, the reaction mixture was passed through a tC2 Sep-Pak® Cartridge (Waters Corporation, USA) and the product was eluted with a step-gradient of H2O/acidified ethanol (25% phosphoric acid/ethanol 2/98). The eluate was divided into 15-µg aliquots and stored in liquid nitrogen for future use. The HYNIC-IL2 conjugate was analyzed by high-performance liquid chromatography (HPLC) using a C18 reverse-phase column Inertsil ODS 2 (Phenomenex, 4.6 mm × 25 mm) by simultaneously monitoring of UV absorbance (at 280 nm) and activity, at a flow rate 1.0 ml/min using 0.1% TFA/water (solvent A) and 0.1% TFA/acetonitrile (solvent B). The gradient started at 10% solvent B for 5 min, changed to 70% solvent B over 10 min, held constant for 5 min, changed to 90% solvent B over 5 min and finally back to 10% solvent B over 2 min. The average number of hydrazino groups incorporated in each molecule was spectrophotometrically determined by converting the hydrazino group into a hydrazone moiety. Briefly, 100 μl of the conjugated IL2 was diluted in 1 mL of O-sulfonic benzaldehyde (Fluka, 0.1 M sodium acetate pH 4.7) and incubated in the dark overnight at room temperature. The conversion of hydrazino groups to the corresponding hydrazones was quantified by measurement of the optical density at 343 nm using a calibration curve [8].
Protein Characterization by Matrix-Assisted Laser Desorption Ionization Time-of-Flight
To characterize the modification occurred during the conjugation and to determine how the pH of the reaction can influence the incorporation of HYNIC-NHS molecule to IL2, a Matrix-assisted laser desorption ionization time-of-flight (MALDI-ToF) mass spectrometric determination was performed. A volume of 10 µL of HYNIC-IL2 complex (prepared at different pH values as previously described) was diluted 1:10 with 3,5-dimethoxy-4-hydroxycinnamic acid matrix solution (10 mg/ml in 50% acetonitrile/1%TFA, Sigma) to a concentration of about 10 pmol/µl. An aliquot (1–2 µl) of the final solution was applied to the sample target prior to insertion into the high vacuum of the mass spectrometer Voyager-De MALDI-ToF (Applied Biosystems). MALDI-ToF operation conditions were set as follow: mode of operation was linear, polarity was positive, the acceleration voltage was 20,000 V, delayed extraction time was 100 ns and the acquisition mass range was 14,000–17,000 Da.
Radiolabeling of HYNIC-IL2 Conjugate
The HYNIC-IL2 conjugate was radiolabeled with 99mTc by adding 1 μl of tricine (100 mg/ml in 1 M sodium acetate buffer, Sigma-Aldrich) used as a coligand, 0.1–0.2 ml of freshly eluted 99mTcO−4 solution (1–10 mCi, 37–370 MBq) and 10 µl of SnCl2 solution (2 mg/ml in nitrogen-purged 0.1 M HCl, Sigma-Aldrich) to 100 µl of HYNIC-IL2 (15 µg). The reaction mixture was incubated for 30 min at room temperature. To evaluate the non-specific binding of technetium to IL2, unconjugated IL-2 was labeled using the same conditions. All the preparations were analyzed using instant thin-layer chromatography-silica gel (ITLC-SG, Pall Corporation). Thus, chromatographic strips were performed by spotting 2.5 µl of the radiolabeled complex on the strip and developed either with 0.9% NaCl, for the determination of the labeling efficiency (LE), or with acetone, as the mobile phase, for determination of the percentage of free 99mTcO4. The percentage of insoluble and “colloidal” species were also identified by ITLC, using albumin-soaked ITLC-SG strips using a mobile phase of EtOH:NH4:H2O (2:1:5). Strips were scanned using a TLC-scanner (Bioscan System). The 99mTc-HYNIC-IL2 complex was purified by SPE using a tC2 Sep-Pak® Cartridge (Water Corporation) using a step-gradient of H2O/acidified ethanol (25% phosphoric acid/ethanol 2/98), in order to remove the excess of free 99mTc-pertechnetate and uncoupled 99mTc-Tricine. Interleukin-2 (1 mg/ml) was also labeled using the method previously described by Chianelli et al. [7] in order to compare its biological activity with that of the new HYNIC-IL2 complex.
Optimization of the Radiolabeling Procedure
Influence of Conjugation Conditions on LE of HYNIC-IL2 Complex
In order to optimize the conjugation conditions and consequently to achieve the highest labeling efficiency, different molar ratios of HYNIC-NHS:IL2 ranging from 3:1 to 12:1 and several buffer systems were tested. Due to the limited stability of IL2 at pH > 7.0 for a long time during the conjugation, the optimum pH value was assessed by performing the conjugation in presence of an excess of HYNIC-NHS ranging between 3:1 to 12:1 and using the following buffer solutions (100 µl): 100 mM phosphate with 150 mM NaCl buffer solution pH 7.2; 1.0 M NaHCO3, pH 8.5; and 1.0 M Na2CO3/NaHCO3, pH 9.2. After 30 min of reaction, the resulting HYNIC-IL2 complex was purified by solid-phase extraction chromatography and preparations were labeled with 99mTc-pertechnetate and analyzed by instant thin-layer chromatography-silica gel (ITLC-SG) chromatographic strips, as described above.
Influence of Labeling Conditions on LE of HYNIC-IL2 Complex
The effects of variation in the labeling conditions on radiolabeling efficiency and the levels of technetium colloids as well as soluble 99mTc-labeled impurities produced were explored. pH values ranging from 4 to 7 and tricine:IL2 ratios ranging from 14000 to 1000 were studied. All the preparation were analyzed using instant thin-layer chromatography-silica gel (ITLC-SG) chromatographic strips for the determination of the LE, % colloids and free technetium using saline, EtOH:NH4:H2O (2:1:5) and acetone, respectively, as solvent as described above.
Stability of 99mTc-HYNIC-IL2
The stability of 99mTc-HYNIC-IL2 was evaluated by incubating 100 µl of 99mTc-HYNIC-IL2 in 900 µl of either human serum or saline for 24 h at 37°C. The percentage of intact 99mTc-HYNIC-IL2 was analyzed by ITLC-SG using saline as the mobile phase. The stability of 99mTc-HYNIC-IL2 was also evaluated in the presence of increasing amounts of DTPA, with molar ratios ranging from 1:1 to 1000:1 DTPA:99mTc-HYNIC-IL2.
SDS-PAGE of 99mTc-HYNIC-IL2
The effect of modifications to integrity of the IL2 molecule during conjugation, SPE purification and labeling reaction were tested by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) performed under non-reducing condition. Electrophoresis of recombinant IL2 (Aldesleukin®, Chiron), HYNIC-IL2 and 99mTc-HYNIC-IL2 (before and after purification) was performed using a 10-15% gradient SDS-PAGE on a PHAST system (Amersham-Pharmacia).
In vitro Evaluation of Biological Activity of the HYNIC-IL2 Complex
The biological activity of IL2 after conjugation with HYNIC-NHS was assessed by performing a proliferation assay using the IL2-dependent cytotoxic T-cell line CTLL-2, (ATCC, USA). CTLL-2 is a subclone of cytotoxic T lymphocytes from the mouse strain C57BL/6. The proliferation of CTLL-2 cells in the presence of serial dilutions of modified-IL2 or unmodified-IL2 was evaluated by the MTT proliferation assay. Dilutions of IL2 (native, HYNIC-conjugated and N3S-conjugated) were made in RPMI 1640 medium supplemented with 100 UI/ml penicillin, 100 µg/ml streptomycin and 2 mM l-glutamine (all from Gibco Life Technologies, Roskilde, Denmark). MTT (3-[4,5-dimethylthiazolyl-2]-2,5-diphenyl tetrazolium bromide; Sigma-Aldrich) was diluted to 5 mg/ml in sterile phosphate-buffered saline solution (PBS, pH 7.4) and filtered once through a 45-µm filter. The diluted MTT was kept protected from light at 4°C and used within a month.
CTLL-2 Cell Line and Culture Conditions
The IL2 dependent cell line CTLL-2 was cultured continuously and maintained at a concentration of 1–5 × 105 cells/ml in RPMI 1640 medium supplemented with 100 UI/ml penicillin, 100 µg/ml streptomycin and 2 mM l-glutamine (all from Gibco, Denmark), 10% heat-inactivated fetal calf serum (Gibco, Denmark), 1 mM sodium pyruvate (Gibco, Denmark), 25 mM HEPES (Gibco Denmark, pH 7.4), and 5.5 × 10−5 M beta-mercaptoethanol (Sigma-Aldrich). The cells were maintained in presence of a low concentration of recombinant human IL2 (10 UI/ml, Chiron, Novartis, Italy) at 37°C in 5% CO2 atmosphere for 24–48 h. Three days before use, the cells were washed three times in IL2-free culture medium by centrifugation for 7 min at 1,200 rpm to allow internalization of any IL2 bound to its receptors and then adjusted to 1 × 106 cells/ml. The culture medium was refreshed for the last time 24 h before the bioassay and cultured without IL2.
MTT Assay
CTLL-2 cells were harvested and washed with complete medium three times then resuspended in RPMI complete medium at 5 × 105 cells/ml. CTLL-2 was pipetted into a 96-well plate (100 µl, 5 × 104 cells/well) containing 100 µl of serially diluted solutions of native IL2, HYNIC-conjugated and N3S-conjugated IL2 (from 0 to 1,000 UI/ml) and incubated at 37°C in 5% CO2 for 24 h. After 24 h the diluted MTT in PBS pH 7.4 (5 mg/ml) was added at 20 µl/well. After 4 h of incubation, 50 µl/well of solubilization solution containing 50% dimethylformamide and 10% sodium dodecyl sulfate in 0.1 M HCl (DMF/SDS, pH 4.7) was added and left to incubate overnight to dissolve the reduced MTT crystals. The absorbance was measured with a microplate reader (BioRad, Model 550) at a test wavelength of 570 nm and a reference wavelength of 620 nm (to read the MTT formazan and the unconverted MTT, respectively).
Studies in Animals
The biodistribution of 99mTc-IL2 was studied in 9 normal Balb/c mice injected i.v. with 99mTc-IL2 (approx. 3.7 MBq). Animals were killed after 30 (n = 3), 60 (n = 3), or 120 min (n = 3). Major organs (lungs, liver, kidneys, blood, and spleen) were excised, weighted and counted in a single well gamma counter, together with samples of serial dilutions of the injected 99mTc-IL2 (1:100, 1:1,000, 1:10,000, 1:100,000). Results were expressed as percentage of the injected dose per gram of tissue ± standard deviation (SD).
Biodistribution in a Normal Volunteer
Radiolabeled 99mTc-IL2 was sterilized by filtration through a 0.22 µm Millipore filter (low protein-binding filter, pre-loaded with human serum albumin) into a sterile vial containing 5 ml of 5% glucose solution and 0.3% human serum albumin. In order to compare the biodistribution of 99mTc-HYNIC-IL2 in humans with that of 99mTc-N3S-IL2, we administered intravenously 111 MBq (3 mCi, about 20 µg) of 99mTc-HYNIC-IL2 to a healthy normal volunteer after written informed consent. Anterior and posterior total body images were acquired at 30 min, 1 h, 2 h, and 3 h after injection.
Results
Conjugation of IL2 with HYNIC-NHS
Optimum conjugation was obtained by incubating IL2 with HYNIC-NHS for 2 h in darkness using a 12:1 HYNIC/IL2 molar ratio. Under these conditions, a stable HYNIC/IL2 conjugate was obtained with a mean number of 1.2 molecules of HYNIC per molecule of IL2 incorporated, as determined by the hydrazine assay. Extending the conjugation time beyond 2 h did not result in an increase in stability or labeling efficiency. The bicarbonate buffer system produced the best conjugation yield. These conjugation conditions (bicarbonate buffer, HYNIC/IL2 12:1, 2 h incubation time) were used for all further in vitro and in vivo experiments.
Protein Characterization by MALDI-ToF
MALDI-ToF analysis was used to determine the effect of pH on the number of HYNIC molecules attached to IL2 (Fig. 1). The degree of conjugation is greater at pH 8.5 than pH 7.2 or pH 9. The mass spectra of HYNIC-IL2 conjugated at pH 8.5 shows that about 50% of the IL2 molecules remain unconjugated (MWt: 15,330 Da), while the majority of the remainder carry one molecule of HYNIC-NHS (15,550 Da) and a smaller proportion two molecules (15,780 Da).
Fig. 1.
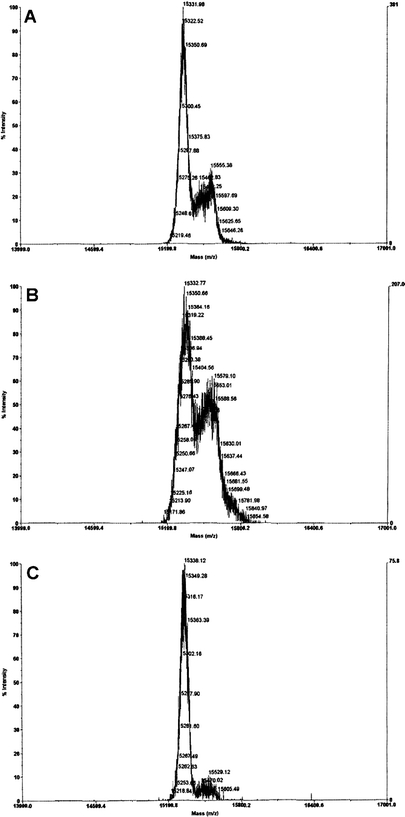
Characterization of HYNIC-IL2 by MALDI-ToF. The conjugation at pH 8.5 (b) results in a higher conjugation yield and a mean mass value of 15,600 Da for the HYNIC-IL2 complex than pH 7.4 (a) or pH 9.2 (c).
Optimization of Radiolabeling of HYNIC-IL2 Conjugate
Influence of Conjugation Conditions on LE of HYNIC-IL2 Complex
99mTc-HYNIC-IL2 labeling efficiency achieved was dependent on the molar ratio of HYNIC:IL2 and on the conjugation pH. A labeling efficiency of >90% was obtained when a molar ratio of 12:1 and a pH of 8.5 were used for the conjugation (Fig. 2) but LE ranged between 25% and 70% when lower conjugation ratios or other pH’s were used. Best results in terms of conjugation yields were obtained at an IL2 concentration of 2.4 mg/ml, while lower yields were obtained when the IL2 concentration was 1.4 mg/ml or 4.8 mg/ml (data not shown). The IL2 conjugate purified by tC2 Sep-Pak cartridge and stored in liquid nitrogen showed a good stability for at least 30 days.
Fig. 2.
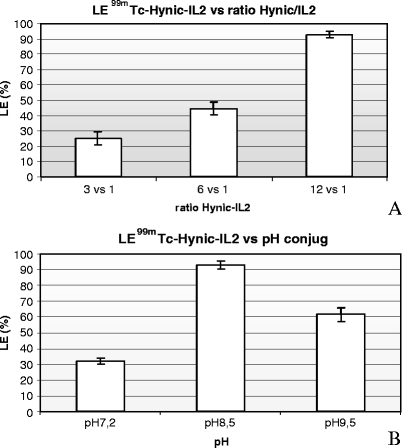
Effect of the HYNIC to IL2 ratio (upper panel) and the pH of conjugation (bottom panel) on labeling efficiency of the HYNIC-IL2 complex.
Influence of Labeling Conditions on LE of HYNIC-IL2 Complex
Best labeling results were obtained at pH 5.5 (see Fig. 3). At either pH 4 or 7 a greater percentage of technetium colloids and soluble impurities (pertechnetate and 99mTc-tricine) were produced. Reducing the tricine concentration from an excess of 14,000 to 1,000 also improved the labeling efficiency and allowed a greater specific activity to be achieved.
Fig. 3.
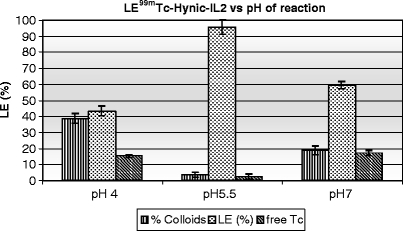
Graph showing as the labeling efficiency (LE) of 99mTc-HYNIC-IL2 is dependent on the pH of labeling. LE > 90% was obtained when the pH of reaction is 5.5, while at pH 4.0 and 7.0 a high percentage of colloids and free 99mTc was present.
The maximum labeling efficiency for the HYNIC-IL2 complex (>95%) was therefore obtained by the conjugation at pH 8.5 with a HYNIC:IL2 ratio of 12:1 and the labeling reaction in sodium acetate buffer pH 5.5 with a tricine:IL2 ratio of 1,000. This labeling efficiency was achieved before SPE purification. After tC2 Sep-Pak cartridge purification, a radiochemical purity of 99mTc-HYNIC-IL2 of around 99% was routinely achieved. These results were confirmed by HPLC analysis (Fig. 4).
Fig. 4.
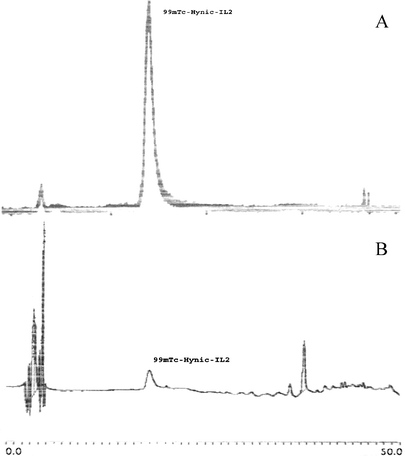
Chemical characterization of 99mTc-HYNIC-IL2 by reverse-phase HPLC. Both, the radiochromatogram (elution profile a) and the UV chromatogram (elution profile b) are presented. The peak at 18.013 min showed all the activity bound to 99mTc-HYNIC-IL2 and corresponds to the UV peak for HYNIC-IL2. In the UV profile, the peak at 37.418 min is due to a dimerization of HYNIC-IL2 that may occur because of the HPLC running buffer in which IL2 was diluted, with no corresponding radioactive peak. The peak eluted at early time in the radioactive profile is due to a little percentage of free 99mTc-tricine.
99mTc-HYNIC-IL2 Stability in Human Serum and Saline
Stability studies showed a loss of only 6% in purity when 99mTc-HYNIC-IL2 was incubated for 6 h in saline at 37°C. No detectable loss in purity was observed, during 20 h of incubation at 37°C in human serum, even when challenged with an excess of DTPA (Fig. 5).
Fig. 5.
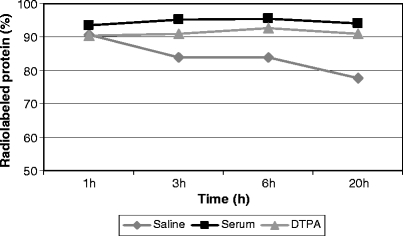
Analysis of 99mTc-HYNIC-IL2 stability in human serum, saline, and DTPA solution after purification by tC2 Sep-Pak.
SDS-PAGE of 99mTc-HYNIC-IL2
The evaluation of the integrity of IL2 was evaluated by SDS-PAGE as a result of the conjugation, radiolabeling, and purification procedures (Fig. 6). The reduction in intensity of the band in track 4 is a result of the dilution effect occurred during purification.
Fig. 6.
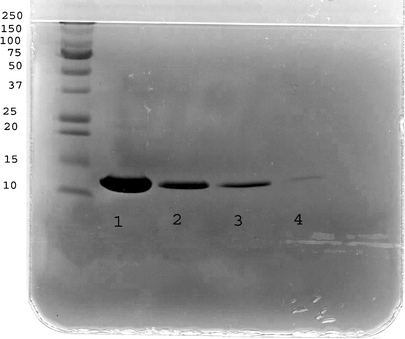
SDS-PAGE of 99mTc-IL2 performed in non-reducing conditions. Lanes 1–4 showed the unconjugated-IL2 (lane 1), the HYNIC-IL2 complex after tC2 purification (lane 2), radiolabeled IL2 before (lane 3) and after purification with tC2 cartridge (lane 4). Dimers, trimers, and 99mTc-HYNIC-IL2 aggregates are absent.
In vitro Biological Activity of HYNIC-IL2 Complex
The ability of 99mTc-HYNIC-IL2 to stimulate cell proliferation was evaluated on CTLL-2 by incubation of cells at increasing concentrations of unconjugated, HYNIC-conjugated, or N3S-conjugated IL2. No significant difference in the levels of stimulation by the different preparations was observed (Fig. 7).
Fig. 7.
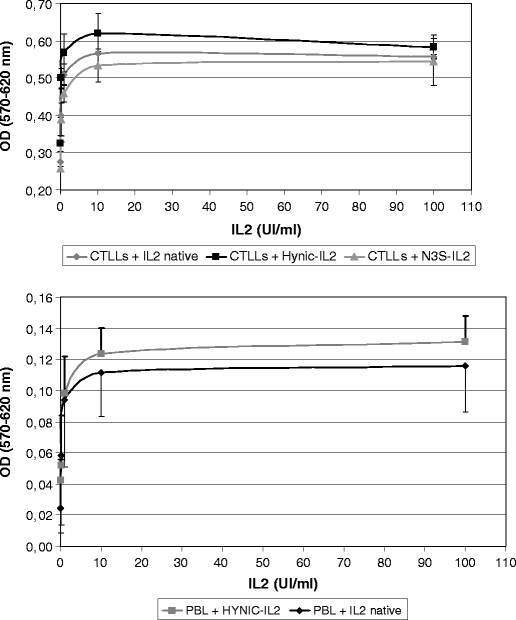
Evaluation of biological activity of HYNIC-IL2 on CTLL-2 cells by MTT assay. The maximum in the proliferation was found when adding 10 UI/ml of IL2. Profile from HYNIC-IL2 and native IL2 are comparable.
Studies in Animals
The biodistribution of 99mTc-IL2 in Balb/c mice showed that the kidneys were (as expected) the major organs of uptake (50%) of the radiotracer at both time points studied. Liver and spleen also showed a significant uptake of 99mTc-IL2. There was negligible uptake in the lungs and circulating radioactivity was rapidly cleared (data not shown).
Biodistribution in a Normal Volunteer
The biodistribution of 99mTc-HYNIC-IL2 in the normal volunteer was very similar to that seen with 99mTc-N3S-IL2 and showed fast plasma clearance with kidneys being the major organs of accumulation; the kidney uptake increased up to 1 h and declined thereafter. Liver and spleen were also detectable. No significant excretion in the bowel was observed and no uptake in the thyroid or any other organ was observed up to 3 h post-injection (Fig. 8). No adverse effects were observed.
Fig. 8.
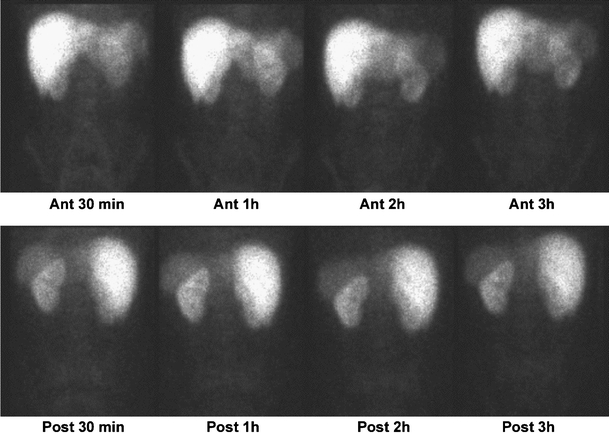
Anterior and posterior images of the abdomen acquired at 30 min, 1 h, 2 h and 3 h, after i.v. bolus injection of 185 MBq of 99mTc-HYNIC-IL2. Images are comparable to those obtained with 99mTc-N3S-IL2 and show no bowel excretion of radioactivity, with the kidney being the major catabolic organ. Faint spleen uptake can be observed due to the high number of IL2R +ve cells in this organ.
Discussion
The study of lymphocyte migration in chronic inflammation is an attractive target for molecular nuclear medicine for imaging chronic lymphocyte-mediated inflammation [9]. In particular, imaging with radiopharmaceuticals that bind to lymphocytes could be useful for the evaluation of disease activity in chronic inflammatory disorders, allowing selection of the best therapeutic option and follow-up of its efficacy [10]. To this end, we have routinely used technetium-labeled IL2 for the in vivo diagnosis of chronic inflammation, and, in particular, to evaluate the severity and extent of organ-specific autoimmune diseases and to detect peri-tumoral lymphocytic infiltration [5]. Although radiolabeled IL2 is a valuable radiopharmaceutical for diagnostic purposes, the previously described labeling procedure remains tedious, expensive, and time consuming owing to the poor solubility and stability of this cytokine.
The aim of this study was to characterize and optimize the conjugation of HYNIC-NHS to interleukin-2 in order to obtain a more readily available and stable conjugate that is useful for radiolabeling with 99mTc in a single-step labeling procedure. The effect of conjugation conditions on biological activity of the IL2 was studied. The effect of the pH of conjugation reaction and the HYNIC-NHS to IL2 molar ratio on HYNIC substitution and labeling efficiency were also investigated. Higher HYNIC-NHS-to-IL2 conjugation ratios resulted in increased conjugation yield, but could also lead to the formation of higher molecular weight aggregates and even to precipitation. A concentration of IL2 of 2.4 mg/ml resulted in efficient conjugation, but a higher concentration resulted in protein aggregation and precipitation due to the poor solubility of the protein in water after reconstitution of the powder. A 12-fold molar excess of HYNIC-NHS was required to incorporate a mean number of 1.2 molecules of ligand per IL2 molecule without aggregation or loss of biological activity. Although the bicarbonate buffer used for the conjugation is not very stable, the use of this buffer improved the conjugation yield and consequently the labeling efficiency. Using the optimized conjugation conditions, the labeling efficiency achieved for 99mTc-HYNIC-IL2 was always >90%.
A radiolabeling pH of 5.5 was necessary to achieve the highest labeling efficiency with no colloid or 99mTc impurities. Best tricine:IL2 ratio was 1,000:1 resulting in an increase in the specific activity up to 4 kBq/μg. No irreversible aggregation or fragmentation of the IL2 molecule occurred during conjugation, labeling, and purification as was shown by SDS-PAGE. In the MTT assay, the conjugate-IL2 was shown to retain its ability to interact with the IL2 receptors on the surface of the CTLL-2 cells and to promote their proliferation. The biological activity of HYNIC-IL2 was also compared with that of N3S-IL2, which was previously used as an imaging probe. A difference of less than 10% in proliferation induction was found between the two conjugates.
In conclusion, we have optimized and described the synthesis of a stable HYNIC-IL2 complex, which can be radiolabeled in high yields with 99mTc. The 99mTc-HYNIC-IL2 conjugate can be obtained with high specific activity and retained receptor binding ability. Biodistribution in mice and normal human volunteer was not different from that of the previously published 99mTc-N3S-IL2. This newly synthesized 99mTc-HYNIC-IL2 can be prepared much easier and more reproducible than 99mTc-N3S-IL2 and could, in the future, be a promising radiopharmaceutical to investigate lymphocytes migration in man affected by chronic inflammatory disease.
Acknowledgments
This work has been performed within the COST BM0607 framework. The authors wish to thank Dr G. Gentile for assistance in Mass Spectrometry analysis, Chiron Inc. (Novartis, Italy) for generous gift of recombinant rhIL2. Authors wish to thank ISORBE for support and providing scientific consultancy. This work has been partially supported by research grants of “Sapienza” University of Rome (funds from Università and Ateneo Federato).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
C. D’Alessandria and V. di Gialleonardo equally contributed to this paper.
References
- 1.Foss F. Clinical experience with denileukin diftitox (ONTAK) Semin Oncol. 2006;33(1 Suppl 3):S21–S25. doi: 10.1053/j.seminoncol.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Signore A, Chianelli M, Annovazzi A, et al. Imaging active lymphocytic infiltration in coeliac disease with iodine-123-interleukin-2 and the response to diet. Eur J Nucl Med. 2000;27(1):18–24. doi: 10.1007/PL00006657. [DOI] [PubMed] [Google Scholar]
- 3.Annovazzi A, Biancone L, Caviglia R, et al. 99mTc-interleukin-2 and 99mTc-HMPAO granulocyte scintigraphy in patients with inactive Crohn’s disease. Eur J Nucl Med. 2003;30:374–382. doi: 10.1007/s00259-002-1069-x. [DOI] [PubMed] [Google Scholar]
- 4.Signore A, Picarelli A, Chianelli M, et al. 123I-Interleukin-2 scintigraphy: a new approach to assess disease activity in autoimmunity. J Pedriatr Endocrinol Metab. 1996;9:139–144. doi: 10.1515/jpem.1996.9.s1.139. [DOI] [PubMed] [Google Scholar]
- 5.Signore A, Annovazzi A, Barone R, et al. 99mTc-interleukin-2 scintigraphy as a potential tool for evaluating tumor-infiltrating lymphocytes in melanoma lesions: a validation study. J Nucl Med. 2004;45(10):1647–1652. [PubMed] [Google Scholar]
- 6.Loose D, Signore A, Staelens L, et al. (123)I-Interleukin-2 uptake in squamous cell carcinoma of the head and neck carcinoma. Eur J Nucl Med Mol Imaging. 2008;35(2):281–286. doi: 10.1007/s00259-007-0609-9. [DOI] [PubMed] [Google Scholar]
- 7.Chianelli M, Signore A, Fritzberg AR, Mather SJ. The development of Technetium-99 m-labelled interleukin-2: a new radiopharmaceutical for the in vivo detection of mononuclear cell infiltrates in immune-mediated diseases. Nucl Med Biol. 1997;24:579–586. doi: 10.1016/S0969-8051(97)00021-8. [DOI] [PubMed] [Google Scholar]
- 8.Abrams MJ, Juweid M, tenKate CI, et al. Technetium-99 m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J Nucl Med. 1990;31(12):2022–2028. [PubMed] [Google Scholar]
- 9.Smith KA. Interleukin-2: inception, impact and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 10.Chianelli M, Parisella MG, D'Alessandria C, Corsetti F, Scopinaro F, Signore A. The developing role of peptide radiopharmaceuticals in the study of chronic inflammation: new techniques for novel therapeutic options. Q J Nucl Med. 2003;47:256–269. [PubMed] [Google Scholar]


