Abstract
Family caregivers of cancer patients receive little preparation, information, or support to carry out their caregiving role. However, their psychosocial needs must be addressed so they can maintain their own health and provide the best possible care to the patient. The purpose of this article was to analyze the types of interventions offered to family caregivers of cancer patients, and to determine the effect of these interventions on various caregiver outcomes. Meta-analysis was used to analyze data obtained from 29 randomized clinical trials, published from 1983 to March, 2009. Three types of interventions were offered to caregivers: psychoeducational, skills training, and therapeutic counseling. Most interventions were delivered jointly to patients and caregivers, but they varied considerably on dose and duration. The majority of caregivers was female (64%), Caucasian (84%), and ranged from 18 to 92 years of age (mean 55). Meta-analysis indicated that although these interventions had small to medium effects, they significantly reduced caregiver burden, improved caregivers’ ability to cope, increased their self-efficacy, and improved aspects of their quality of life. Various intervention characteristics were also examined as potential moderators. Clinicians need to deliver research-tested interventions to help caregivers and patients cope effectively and maintain their quality of life.
Keywords: Carcinoma, family caregiver, spouse caregiver, meta-analysis, emotional distress, randomized controlled trial, quality of life
Even though family caregivers are the long-term care providers to people with cancer, they receive little preparation, information, or support to carry out their vital role.1,2 Family caregivers often are expected to navigate an increasingly complex and fragmented health care system on their own and to find whatever help that may be available.3 In recent years, the caregiving responsibilities of family members have increased dramatically, primarily due to the use of toxic treatments in outpatient settings, the decline in available health care resources, and the shortage of health care providers. Family caregivers of cancer patients have participated in a limited number of intervention programs but these programs have focused almost exclusively on improving patient outcomes (e.g., symptom management, quality of life) with less attention directed to the needs of family caregivers.4 Family caregivers have psychosocial needs that must be addressed so they can maintain their own health and provide the best care possible to the patient.
The purpose of this article was to analyze the findings of randomized clinical trials to understand the type and efficacy of interventions aimed at the needs of family caregivers of cancer patients. Individual studies often have had insufficient power to draw definitive conclusions. Therefore, meta-analysis was used because it combines data from multiple studies and then determines a more accurate estimate of the effect of interventions on specific outcomes.5 We analyzed the type and content of interventions delivered to family caregivers of cancer patients, and then we examined the effect of these interventions on various family caregiver outcomes. We also identified some limitations in existing studies, and recommended directions for future research that could improve care strategies for family caregivers in practice settings.
Background
A large body of research has documented the effects that cancer can have on the emotional, social, and physical well-being of family caregivers.1,6–8 Cancer patients and their family caregivers react to cancer as one emotional system; 9,10 there is a significant reciprocal relationship between each person’s response to the illness, with family caregivers often reporting as much emotional distress, anxiety, or depression as patients.1,11–13 The advanced phase of cancer is especially difficult for family caregivers, who sometimes report more depression than patients themselves.14 However, caregivers seldom use any form of mental health services to deal with their own depression or emotional distress,1,15 and this puts them at risk for long-term health problems.
Cancer can affect the patients’ and caregivers’ family and social well-being, especially in areas related to talking about the illness, sexual well-being, changing family roles and responsibilities, and maintaining individuals’ social support systems.16,17 Problems occur when patients and caregivers hide worries from one another, and avoid talking about sensitive issues associated with cancer and its treatments. Family caregivers experience role overload when they take on patients’ household or family responsibilities, in addition to their own.18,19 Difficulty communicating and negotiating family roles can hinder patients’ and caregivers’ ability to support one another, can decrease couples’ intimacy, and have a detrimental effect on marital and family relationships.16,20,21
Cancer also can affect the physical well-being of caregivers. While caregivers’ health status is initially like the normal population, caregivers often report more problems with fatigue, sleep disturbances, and impaired cognitive function than non caregivers.1 Over time, caregivers’ burden and strain increases.22–24 Caregivers’ physical well-being is at greater risk because they have little time to rest, engage in fewer self-care behaviors (e.g., exercise), or often fail to seek medical care for themselves when sick.25,26 Over half of family caregivers have chronic health problems of their own, such as heart disease, hypertension, and arthritis,27,28 and these health problems can be exacerbated by the stress of caregiving.29,30
Despite the multiple effects of patients’ illness on family caregivers, little is known about effective interventions for caregivers to ameliorate these effects. There is need for a critical analysis of interventions conducted with family caregivers of cancer patients to determine if the interventions can improve caregivers’ quality of life, their physical, mental and social well-being, and their experiences in caregiving. Previously, five systematic reviews described interventions conducted with family caregivers of cancer patients, but did not evaluate the efficacy of interventions on multiple caregiver outcomes.4,31–34 This article presents a meta-analysis that examined interventions delivered to family caregivers of cancer patients in published randomized clinical trials, and their effects on multiple caregiver outcomes.
Research Method
Identification and selection of studies
Our literature search was aimed at identifying available research studies that assessed interventions targeting family caregivers of cancer patients. Several criteria were used to select eligible studies: 1) the intervention had to involve family caregivers, either alone, or with the cancer patient; 2) the intervention had to be psychosocially, cognitively, or behaviorally oriented; and 3) participants had to be randomly assigned to either the intervention or control arm of the study. Studies involving pediatric cancer patients were excluded because the nature of the parent-child relationship was likely to add significant heterogeneity to the studies analyzed. Pharmacological interventions also were excluded because they were not applicable to the scope of the present meta-analysis. The literature search focused solely on articles published in peer-reviewed journals to enhance the methodological rigor of the studies examined and the conclusions drawn about the efficacy of the interventions.
Studies were identified by searching multiple literature databases including CINAHL, Google Scholar, ISI Web of Knowledge, PsycINFO, and PubMed. The keywords “family caregiver,” “cancer patient,” “spouse,” “partner,” “couple,” and “intervention” were used in various combinations. When the query produced more than 200 titles, searches were further refined with the terms “random assignment” or “randomization.” Queries were limited to those involving human subjects and English language. Studies published in languages other than English were excluded due to time and resource limitations. Hand searches of reference lists of relevant literature reviews were used to complement the computer searches.4,31–33
Coding
Each research article was read and analyzed by at least two members of the research team. Data extraction was recorded on customized tables; disagreements were resolved through consensus. Since meta-analysis combines data from different instruments that measure similar variables or outcomes, a conceptual framework was used to organize extracted data in a meaningful way. The integration of Stress and Coping Theory,35 Cognitive Behavioral Theory,36 and quality of life frameworks,37,38 guided the classification of interventions and the findings of the meta-analysis into clinically applicable domains. Extracted data were initially organized into three domains: Illness Appraisal Factors, Coping Resources, and Quality of Life; within each domain, data were further categorized into specific intervention outcomes (see Table 1 for organization of the data).
TABLE 1.
Theoretical Framework for Organizing Data into Domains and Outcomes
| Domain | Illness Appraisal Factors | Coping Resources | Quality of Life |
|---|---|---|---|
| Outcomes | Caregiving burden | Coping strategies | Physical functioning |
| Caregiving benefit | Caregiver self-efficacy | Distress and anxiety | |
| Information needs | Depression | ||
| Marital-family relationships | |||
| Social functioning |
When authors used more than one instrument to measure the same outcome, extracted data were reported from the most relevant instrument, which was determined by consensus of three of the authors (LN, DM, and MK) after reviewing the wording of the items used in each instrument. A similar procedure was followed when authors reported findings on multiple subscales of instruments, rather than on global scores. For calculation of effect sizes we used outcome data from the experimental and control arm of the study. When studies had more than one experimental arm, we chose the experimental arm hypothesized by the original authors to be the most effective. Finally, since some studies assessed intervention outcomes over time, we organized the extracted data into three time frames: initial follow-up from pre-intervention (baseline) to 3 months post-intervention (T1), intermediate follow-up from greater than 3 months to 6 months post-intervention (T2), and longer-term follow-up that occurred beyond 6 months post-intervention (T3).
Statistical Analyses
Data were synthesized using meta-analytic methods.39,40 The standard mean difference, or the effect size between treatment and control groups, was calculated using Hedges’ g unbiased approach (similar to Cohen’s d statistic41). Calculation of effect sizes was based on means, standard deviations, difference in mean scores, p-values, and sample sizes of the groups. Data were statistically pooled by the standard meta-analysis approach, meaning that studies were weighted by the inverse of the sampling variance. The random effects model was used as a conservative approach to account for different sources of variation among studies. The Q statistic was used to assess heterogeneity among studies. A significant Q value indicates lack of homogeneity of findings among studies.39,40 Several intervention characteristics were identified and their effects on outcomes were examined. Categorical characteristics were treated as moderators and intervention effectiveness was compared across subgroups formed by these moderators. Continuous characteristics were examined as covariates using random-effect (method of moments) meta-regression. We also assessed publication bias using the Egger’s t-test with significance values based on one-tailed p-values.39,40 Publication bias can occur because journals are more likely to publish studies with positive results than those with negative or non significant results, authors are less likely to report null (negative or inconclusive) outcomes in multi-outcome studies, and studies with small sample sizes need to detect larger effects to be published than studies with large samples.
Comprehensive Meta-Analysis V.2© Software42 was used for the statistical analyses. Statistics reported in this meta-analysis conformed to the PRISMA Statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses), a guideline for describing meta-analyses of studies that evaluate health care interventions.5,43 Based on conventional standards, effect sizes of g = 0.20, 0.50, and 0.80 were considered small, medium, and large, respectively.41
Results
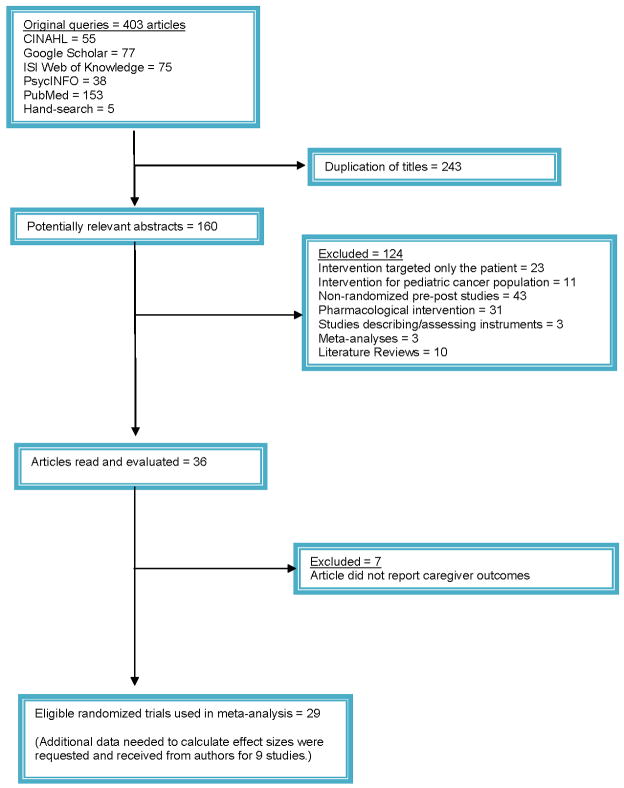
Initial queries identified a total of 403 articles from all databases and search methods. Comparison of the retrieved titles identified 243 studies that were duplicates; thus, leaving 160 abstracts for further evaluation (see Figure 1 for details). The present meta-analysis is based on data extracted from 29 studies of randomized clinical trials (RCTs) published between 1983 and March of 2009 that assessed interventions that included family caregivers of cancer patients.44–72 Among the 29 studies, additional data, not published in the reviewed manuscripts but needed to calculate effect sizes, were requested and received for nine studies. The majority of studies (27 out of 29) assessed initial outcomes during the first three months following the intervention. Approximately half (52%) assessed intermediate outcomes, and approximately one-fourth (24%) assessed longer outcomes.
FIGURE 1.
Selection Process of Randomized Trials
Characteristics and Content of Interventions for Caregivers
A content analysis of the experimental arms of the 29 randomized clinical trials included in this meta-analysis identified 35 primary intervention protocols. The number of intervention protocols, signified by the symbol k (i.e., k = 35) is greater than the number of studies (N = 29) because six studies had intervention protocols with more than one primary focus. Table 2 describes the type, the content elements, and the mode of delivery of the intervention protocols. Intervention protocols directed solely to patients are not described in Table 2. Control group protocols, most of which were some form of “Usual Care,” also were not described in Table 2.
TABLE 2.
Intervention Protocols for Caregivers of Cancer Patients
| ▪ Authors ▪ Primary Goal |
▪ Intervention Type a ▪ Contacts: # & Length ▪ Location b |
▪ Duration of Protocol ▪ Intervener |
Contents of Caregiver Intervention (as specified by investigator) | ▪ Theoretical framework ▪ Fidelity |
||
|---|---|---|---|---|---|---|
| ▪ Patient Caregiving | ▪ Marital/Family Care | ▪ Caregiver Self Care | ||||
| CG = caregivers Pt = patients |
|
RN=Nurse SW=Social worker PSY=Psychologist MD=Physician RA=Research assistant |
|
|
|
•-Indicates fidelity of intervention was discussed |
|
Badger et al.44 To reduce anxiety, depression in breast ca Pts & CGs *Interventions delivered separately to CG & Pt |
Exp Arm I:B, A: 3, 4* Three 34-min biweekly CG counseling phone calls *Pts received six calls |
6 weeks Psychiatric RN w/oncology expertise |
1, 3 Cancer information |
1, 5 Modify role responsibilities |
2, 3, 4, 6 Managing depression & anxiety, social support |
Inter-personal counseling |
|
cExp Arm II: C: 3, 4* Three 11-min biweekly CG exercise-related phone calls *Pts received six calls |
3, 4 Low-impact exercise |
None cited • |
||||
|
Baucom et al.45 To manage effects of breast ca on the marital relationship |
A, B, C: 1, 5, 6 Six 75-minute biweekly joint sessions in therapist’s office; homework |
12 weeks Advanced clinical PSY doctoral students |
1, 2, 3 Medical education; provide emotional & Pt- esteem support |
1, 2, 3, 4, 5, 6 Communication for decision-making; sharing feelings; sexual adaptation; |
2, 3, 4, 5, 9 Skills for mutual problem-solving; emotional support; find meaning |
Cognitive-behavioral approach • |
|
Blanchard et al.46 To reduce CGs’ distress |
C,A, B: 1, 4 (CWC) Four to six 1-hour CG counseling sessions in counselor’s office |
6 weeks Oncology SW |
2, 3 CGs apply problem- solving strategies to manage Pt care needs |
2 Communication w/Pt |
1, 2, 3, 4, 5, 6 Use problem-solving skills for self care; emotional support |
Problem-solving • |
|
Budin et al.47 To enhance biopsychosocial adjustment of breast ca Pts & CGs *Interventions delivered separately to CG & Pt |
cExp Arm I: A: 1, 4, 6* Four 1-hour CG viewing of standardized phase-specific videos |
About 32 weeks RNs |
1, 3 Medical information to facilitate coping & social support |
1, 4, 6 Coping, psychosocial support |
Stress & coping; crisis intervention • |
|
|
cExp Arm II: B, C: 3, 4* Four 1–2 hour CG phone counseling calls tailored to phase & recipient |
1,3 Answer Pt-care questions, facilitate coping, social support |
2 Functional communication |
1, 4, 6, 9 Manage distress by coping, support, behavior change1, 4, 6, 9 |
|||
|
Exp Arm III: A+B: 1, 4, 6* Combines Arms I & II (Four video + four phone sessions) |
1,3 All interventions I & II |
2 Functional communication |
All interventions I & II |
|||
|
Bultz et al.48 To help CGs of breast ca Pts manage Pt & self care, marital issues |
A: 2, 4, 6 Six 1.5–2 hour weekly CG group sessions in clinical site |
6 weeks PSY, MD |
1, 2, 3 Medical, psychosocial information |
1, 2, 3, 4 Discuss marital relationship, communication, sexual issues |
1, 2, 4, 5, 6, 8 Examine CGs’ fears, concerns, challenges; fears of recurrence, death & dying |
Empirical base • |
|
Campbell et al.49 To enhance self-efficacy & QOL in AA prostate ca Pts & spouse-CGs |
A, C: 3, 5* (CST) Six 1-hour joint phone sessions *Speakerphones provided for joint participation |
7 weeks on average Medical PSY |
1, 2, 3 Information re ca’s physical, emotional, & social side effects |
1, 2, 6 Effects of ca on marital relationship, communication skills; plan mutually pleasant activities |
1, 2, 5 Progressive muscle relaxation, activity-rest cycles, cognitive restructuring |
Cognitive-behavioral approaches; cultural-based health disparities • |
|
Carter50 To maximize CGs ability to improve sleep quality |
C, A: 1, 4 (CASI) One 1-hour training session plus one 1-hour booster session at CG’s choice of locations; practice at home |
4 weeks Masters’ RNs |
3, 4 Self-assess to improve sleep quality, sleep hygiene; relaxation, stimulus control |
Stress & coping; cognitive & behavioral approaches • |
||
|
Christensen51 To alleviate Pt & CG psychosocial discomfort after mastectomy |
B: 1, 5, 7; also interactive art project Four weekly couple counseling sessions in therapist’s office |
4 weeks PhD SW therapist |
1, 2, 3, 4 Sexual misconception, facilitate change, reinforce couple’s strengths |
Psycho-sexual counseling • |
||
|
Derdiarian52 To improve Pt & CG coping skills; satisfaction prior to ca treatment |
A, B: 1, 3, 5, 7 Session(s) in clinical setting w/1–2 follow-up phone calls (dose unknown) |
2 weeks RAs |
1, 2, 3 Information about disease, treatment |
1 Information re managing family concerns |
1, 3, 6, 9 Information on self-identified needs, concerns re Pt’s disease; resource information |
Psychological stress & coping • |
|
Giarelli et al. 53 To prepare CG (& Pt) for caregiving |
A, C: 1, 3, 5, 7 (SNIP) Eight 1.5-hour weekly joint home visits alternating w/eight 45-min joint phone calls |
8 weeks Advanced Practice RNs (APNs) |
1, 2, 3 Practical information, skill training to enhance Pt care |
2, 3, 4, 6 Mutual effects of Pt’s illness on marital relationship |
2, 3, 4, 6, 9 Manage personal needs, emotional effects; resources, referrals |
Labor of caregiving |
|
Given et al.54 To enhance CGs’ satisfaction & skills for caregiving |
C, A: 1, 5 plus 3, 4 Three 32-min joint sessions at clinic alternating w/two 15- min individual phone calls to CGs only |
10 weeks RNs w/oncology experience |
1, 2, 3 Assess CGs’ negative reactions to caregiving; plan & evaluate care strategies |
2 Communication as a device to help CG assist in Pt care |
1, 2, 3, 4, 5 Coping, problem-solving skills, enhance self-efficacy; cognitive reframing, self-care |
Cognitive-behavioral • |
|
Goldberg & Wool55 Social support counseling for CGs of lung ca Pts |
B: 1, 4 Twelve weekly sessions for CGs w/therapist |
12 weeks 1 MSW, 1 PSY |
3, 4 Maintain Pt’s social support system; encourage Pt autonomy; advocate for Pt in health care system |
1, 2, 5 Encourage family & social communication |
1, 4, 5, 6, 8 Manage distress, increase competence, deal w/grief, anticipatory mourning |
Social support systems • |
|
Heinrich & Schag56 To increase knowledge, psychosocial adjustment, & daily activities |
A, C: 2, 5, 6, 7 (SAM) Six 2-hour weekly couples group sessions in clinical setting |
6 weeks MD, PSY |
2, 3 Medical information; skills to manage Pt stress (coping, problem-solving), relaxation, exercise, activities |
1, 2 Increase positively-valued couple activities; Pt & CG share ideas, feelings in group discussion |
1, 2, 3, 4 Apply information, skills, & activities to enhance self care |
Cognitive-behavioral approaches |
|
Hudson et al.57 To prepare CGs to care for self & dying Pt |
A: 1, 3, 4, 6, 7 Two 1.5-hour CG home visits (Pt could be present at CG’s request), one 30-min phone call between visits |
2 weeks RNs |
1, 2, 3 Enhance CG’s ability & skills to assist in Pt’s physical & emotional care |
3, 4, 5, 6, 7, 8, 9 Diet, exercise, rest; use of social, spiritual, health care resources; optimism; emotional, bereavement support |
Stress & coping • |
|
|
Jepson et al.58 To improve CGs psychosocial status post- ca Pts’ surgery |
A, C: 1, 3, 5 (SNIP) Three 1.5-hour joint home visits alternating w/six 45-min phone calls |
4 weeks RN w/oncology expertise |
1, 2, 3 Information, skills to care for Pt’s health needs; coordinating care |
2, 3, 4, 9 Assess Pt & own personal needs; use resources; care for own health problems |
None cited • |
|
|
Keefe et al.59 Partner-guided pain management for Pts at end of life |
C, A: 1, 5, 6, 7 Three 20–90 minute (M = 56 mins) joint home visits |
1–4.6 weeks (M = 2) RN educators |
2, 3 CG coaching Pt in use of behavioral pain relief strategies |
6 Caregiving as teamwork |
1, 3, 4, 5 Apply learned skills for self-care, greater confidence in caregiving |
None cited • |
|
Kissane et al.60 To reduce distress of bereavement |
B: 1, 5 (>1 family member) Four to eight 90-min family therapy sessions in clinic or home | 9–18 months (M = 13) Family SW herapists |
3 Identify Pt’s thoughts & feelings, provide support |
1, 2, 5, 6 Family cohesion; communication; handling of conflict |
4, 8 Examine thoughts & feelings, anticipate bereavement distress |
Family-focused grief therapy • |
|
Kozachik et al.61 To reduce CG depression; enhance problem-solving skills |
A, C: 1, 5; 3, 4 Five 1-hour joint meetings alternating biweekly w/four 20-minute individual CG phone calls |
16 weeks Masters’ RNs certified in oncology |
1, 2, 3, 5 Identify symptoms & severity; select care strategies from computerized list |
2, 6 Make joint decisions; plan, evaluate care strategies for both Pt & CG problems |
2, 3, 4, 5, 9 Acquire problem-solving skills, self-efficacy for Pt care; use available resources |
Family care model • |
|
Kuijer et al.62 To restore couples’ perception of equity & exchange of support |
B: 1, 5, 7 Five 90-minute couple counseling sessions |
8 weeks PSYs |
1, 2, 3, 5, 6 Mutual support to reduce sense of inequity; enhance relationship quality & well-being |
Cognitive-behavioral; equity theory | ||
|
Kurtz et al.63 To reduce CG & Pt depression, distress by teaching skills to manage symptoms |
C, A: 1, 3, 5 Five monthly joint sessions at clinical site alternated w/five monthly CG phone calls. |
20 weeks RNs |
2, 3, 4 Information, skills to care, support Pt; communicate w/health providers; plan, evaluate care strategies |
1, 5, 6 Manage effects of role strain & CG burden on relationship |
1, 2, 3, 4, 5, 6 Apply skills to care for self, increase self-confidence, maintain social support |
Cognitive-behavioral • |
|
Manne et al.64 To reduce CGs distress & increase coping, personal growth, & marital communication |
A, C: 2, 4, 7 Six 1-hour weekly CG group sessions in clinical setting |
6 weeks MD, MSW, PSY, & nutritionist |
1,2, 3 Information re diagnosis, treatment, good nutrition, providing support |
1, 2, 3, 4 Maintaining good communication, intimacy; dealing w/sexual concerns |
1, 6, 8 Stress management & coping skills training; social support; survivorship |
Stress-coping; cognitive & social processing theories • |
|
McCorkle et al.65 To enhance CGs’ knowledge & skills in care of terminally ill lung ca Pts; anticipate distress of bereavement |
Exp Arm I:A, C: 1, 3, 5, 7 (OHC) Ten 70-min weekly home visits (on average) plus weekly follow-up phone calls |
1–24 weeks (M = 12) Masters’ RNs w/expertise in terminal ca care |
1, 2, 3, 4 Information, skills to help manage Pt’s care needs; provide emotional support, communicate w/health providers |
2, 5, 6 Facilitate marital & family communication to fulfill Pt’s role & responsibilities; teamwork |
2, 4, 6, 8, 9 (Referral to own health care provider to manage self- care needs); use of resources; receive emotional, bereavement support |
Existential plight; chronic illness trajectory |
|
cExp Arm II:A: 1, 5 (SHC) Six (on average) weekly home visits |
6 weeks Multidisciplinary team (not cancer-specific) |
2, 4 Information re Pt’s medications, when to call MD; communicate w/health providers |
2 Facilitate marital & family communication |
8, 9 Offer bereavement support; information re available resources |
||
|
McCorkle et al.66 To improve spouse-CGs’ depression, marital function w/prostatectomy Pts |
A, C: 1, 3, 5, 7 (SNIP) Eight 1.5-hour joint weekly home visits alternating w/eight 45-min weekly phone calls |
8 weeks Advanced Practice RNs |
1, 2, 3 Assist Pt to manage care needs |
1, 4, 5, 6 Examine sexual functioning issues resulting from side effects of surgery |
1, 2, 3, 4, 6, 9 Learn coping & problem-solving skills, Pt care skills, assess needs, resources |
Chronic illness & the life cycle • |
|
McMillan et al.67 To improve CGs’ QOL, burden, coping, mastery assisting Pt in hospice care |
Exp Arm I:C: 1, 4 Three 1-hour CG sessions to teach problem-solving methods |
1.2 weeks RNs & home health aides (not employed at hospice site) |
2, 3 Plan caregiving goals; receive expert information re assessing symptoms |
1, 6 Involve Pt in care planning; work as a team |
1, 2, 4, 5, 9 Creativity in problem-solving, optimism, use of respite care resources |
Family COPE model • |
|
cExp Arm II:B: 1, 4 Three 1-hour CG sessions to provide individual support |
1 Discussing relationship w/Pt |
3 Discussing CG’s fears & feelings |
||||
|
Mokuau et al.68 To increase knowledge, skills of family members of breast ca Pts |
A, C: 1, 5, 6, 7; (included many Hawaiian traditions) Six 2-hour family sessions held at participants’ preference | 13 weeks Masters SWs in teams of two |
1, 2, 3, 4 Assist Pt to manage care needs; communicate w/health care providers |
1, 2, 5 Family teamwork to meet Pt care needs & share family chores |
1, 2, 5, 7, 9 Use of conference phone & computer access for ca information |
Culturally sensitive care |
|
Northouse et al.69 To improve psychosocial & QOL outcomes in Pts w/advanced breast ca & CGs |
A, C: 1, 3, 5, 6, 7 (FOCUS) Three 90-min monthly joint home visits (initial phase); Two 30-min monthly joint phone calls (booster phase) |
Home visits 13 weeks; phone calls during next 13 weeks Masters’ RNs |
1, 2, 3, 4 Manage Pts’ care needs; provide emotional support; assist Pt to manage uncertainty, maintain optimistic attitude |
1, 2, 3, 4, 5, 6 Improve family functioning, open communication, marital relationship; adapt to role changes; work as a team |
1, 2, 3, 4, 5, 6, 9 Manage self health; effective coping w/stress; receive emotional support from Pt, manage uncertainty, use of community/social resources |
Stress & coping • |
|
Northouse et al.70 To improve appraisal, coping, symptom distress & QOL in prostate ca Pts & spouse-CGs |
A, C: 1, 3, 5, 6, 7 (FOCUS) Three 90-min monthly joint home visits alternating w/two 30-min joint phone calls |
13 weeks Masters’ RNs |
1, 2, 3 Assist Pt to manage care needs, provide support; communicate w/health providers |
2, 3, 4, 5, 6 Open communication; mutual support, healthy life-style behaviors; maintain optimism & manage uncertainty as a team |
1, 2, 3, 4, 5, 9 Maintain self health, effective coping w/stress; maintain social support system; use available resources effectively |
Stress & Coping • |
|
Scott et al.71 To enhance couple communication, coping, Pt body image, sexual adjustment in female ca Pts & their intimate partners |
Exp Arm I: C, A, B: 1, 3, 5, 7 (CanCOPE)* Five 2-hour joint home visits w/two 30-min phone calls |
7 weeks + one 6-mo follow-up session Female PSYs |
1, 2, 3 Information re caring for Pt’s needs; enhancing Pt body image |
1, 2, 3, 4 S Supportive marital communication, joint coping, sexual counseling |
1, 2, 4, 5 Coping & problem- solving skills; social support training; counseling |
Social- cognitive processing model of emotional adjustment to ca; Coping theory |
|
cExp Arm II: C, A, B: 1, 3, 4 (Pt only), 7 Four 2-hour Pt (individual) home visits w/two 30-min Pt phone calls |
CGs received no intervention in this study arm, but provided data | |||||
|
Walsh et al.72 To increase support for at-risk CGs of Pts w/advanced ca |
A, B: 1, 4 Six 50-min weekly CG home visits* (or in other location where privacy could be maintained) *Phone calls occasionally substituted for home visit |
6 weeks RN & SW (team of CG advisors) |
3, 4, 6, 8, 9 Attention to own care needs; respite; future planning, survivor benefits & finances; maintain social networks; bereavement support; social service resources |
None cited • |
||
Primary intervention focus is shown in bold type; secondary element(s) shown in regular type
All available information is reported; any missing information is unknown
Experimental arm excluded from multi-arm studies (i.e., not included in meta-analysis)
NOTE: The coding for Column 2 consists of 4 parts: A, B, C = types of interventions; 1, 2, 3 = mode(s) of presentation; 4, 5 = Caregiver alone (4) or jointly w/the Patient (5); 6, 7 = types of supplementary materials used. Thus, the string of codes for any particular intervention can be read like a sentence (e.g., looking above at the Walsh et al. study, it is described as primarily a psychoeducational intervention (A) w/secondary elements of therapeutic counseling (B), presented face-to-face (1) to the CG alone (4).
Classification of Interventions
The interventions were classified into three major types. The majority of interventions were Psychoeducational (k = 20; 57.1%), defined as protocols whose primary focus was to provide information regarding symptom management and other physical aspects of patient care as well as some attention to the emotional and psychosocial needs of patients, caregivers, and/or marital or family relationships. Skills Training (k = 9; 25.7%) was defined as protocols that focused primarily on the development of coping, communication, and problem-solving skills with some focus on behavior change. The least frequent type of intervention was Therapeutic Counseling (k = 6; 17.1%) that focused primarily on the development of a therapeutic relationship to address concerns related to cancer or caregiving. Thirty-six secondary elements, i.e., content that appeared secondary to the primary focus, also were coded, to better describe the complexity of some of the intervention protocols. The most common combinations of primary and secondary elements were those that included both psychoeducational andskills training, accounting for over two-thirds (68.6%) of all interventions.
Three measures of dose of intervention were calculated for each protocol (when available). The first measure was Total Number of Hours, M = 7.5 hours, range = 1.7 to 18 hours; the second, Total Number of Sessions/Contacts, M = 6.7, range = 2 to 16; and third, Duration of Intervention, M = 11.5 weeks, range 1.2 to 56 weeks from first to last session. Several studies had exceptionally long durations because of extended breaks between some sessions. If those outliers are excluded the average Duration of Intervention drops to 7.8 weeks. Because each of these measures was variable, the three measures represented independent assessments of the dose.
Format of Interventions
Nearly two-thirds of the interventions were offered jointly to cancer patients and their family caregivers (k = 22; 62.9%); just over one-third included only family caregivers (k = 13; 37.5%). Two of the interventions were delivered to caregivers alone while parallel protocols were delivered independently to the patients.44,47 Most interventions were delivered as face-to-face visits (k = 24; 68.6%), with two-thirds provided in the clinical setting and the remainder in the home. Telephone delivery accounted for one-fifth of the interventions (k = 7; 20.0%), while group meetings were the least frequent (k = 4; 11.3%). Face-to-face interventions often included additional contact by phone (k = 16; 66.7%). Two studies54,61 provided joint face-to-face visits but gave additional attention to the caregivers through individual phone calls. Nurses delivered the experimental intervention in 52% of the studies; social workers, 14%; and psychologists, 14%. The remaining 20% were delivered by various combinations of these professionals.
Content of Interventions
The content of the interventions for caregivers were coded into three broad areas. Patient Caregiving refers to information or skills (e.g., changing a dressing, emptying an ostomy bag) to help caregivers’ carry out their caregiving tasks, and was found in 25 (71.4%) interventions. Marital/Family Care refers to information and skills to help caregivers or couples to manage family and marital concerns, including communication, teamwork, and intimate relationships, and was the primary focus in 25 (71.4%) protocols. Caregiver Self Care refers to information, skills, and support needed by caregivers to manage their own physical and emotional health needs, gain confidence in their caregiving role, maintain their social support system, and access resources to ease caregiving burden; these issues were addressed in 27 (77.1%) of the intervention protocols. It should be noted that the degree of emphasis given to these content areas within the intervention protocols varied considerably, from high (i.e., comprising most of the content provided) to low (i.e., accounting for less than 10% of the content provided).
Characteristics of Caregivers
Across the 29 studies the number of caregivers who were enrolled and completed baseline assessments ranged from 14 to 329, with a mean sample size of 114 caregivers (median = 91) (see Table 3). Enrollment rates varied from 13% to 100% in the studies; however, not all of the studies reported the number of eligible participants who were approached. The average enrollment rate across studies was 58%. The attrition rate for caregivers ranged from 0.0% to 69%, with attrition due primarily to patients’ death. Only a few studies, mainly in palliative care, and whose intervention included a focus on managing bereavement experiences, continued to assess caregivers after the patient died. Most of the caregivers were spouses (84%); the remaining 16% were comprised of adult children, siblings, other family members, or friends. The average age of adult caregivers was approximately 55 years (range = 18 to 92 years). In three studies, family members under the age of 18 also were included.47,60,72 The majority of caregivers were female (61%) and Caucasian (84%).
TABLE 3.
Characteristics of Family Caregivers of Cancer Patientsa
| Authors/Year | Caregivers Enrolled & w/Baseline Data | Caregiver Enrollment Rate | Patient-Caregiver Relationship | Patients’ Cancer Type & Stage | Caregivers’ Demographic Characteristics | Caregiver Attritionb | ||
|---|---|---|---|---|---|---|---|---|
| Gender | Race | Age (Yr) | ||||||
| Badger et al.44 | 97 | 84% | 77% Spouse/partner 23% Other |
Breast Stage I-III |
74% Male | 87% Caucasian 11% Hispanic 2% Other |
51.7 | 12% |
| Baucom et al.45 | 14 | 13% | 100% Spouse | Breast Stage I-II |
100% Male | 86% Caucasian 14% Other |
50.0 | 43% |
| Blanchard, et al.46 | 86 | 27% | 100% Spouse | Heterogeneous Stage not specified |
52% Male | 97% Caucasian3% Other | 52.3 | 23% |
| Budin et al.47 | 184 | 36% | 54% Spouse/partner 12% Children 34% Other |
Breast Stage 0-III |
58% Male | 70% Caucasian 13% African American 7% Hispanic 10% Other |
51.6 | 32% |
| Bultz, et al.48 | 34 | 32% | 100% Spouse | Breast Stage I-II |
100% Male | NA | 51.0 | 6% |
| Campbell et al.49 | 40 | 25% | 100% Spouse | Prostate Early stage |
100% Female | 100% African American | 58.7 | 25% |
| Carter50 | 35 | 100%* | 57% Spouse 30% Adult children 13% Other |
Heterogeneous Advanced stage |
63% Female | 80% Caucasian10% African American10% Hispanic | 53.0 | 14% |
| Christensen51 | 20 | Unknown | 100% Spouse | Breast Localized stage |
100% Male | NA | 39.7 | 0% |
| Derdiarian52 | 60 | Unknown | 100% Spouse | Heterogeneous Stage I-IV |
100% Female | NA | 41.0 | Unknown |
| Giarelli et al.53 | 116 | Unknown | 100% Spouse | Prostate Early stage |
100% Female | 87% Caucasian13% Other | 54.2 | 17% |
| Given et al.54 | 237 | 39% | 65% Spouse 35% Other |
Heterogeneous 33% Early stage 67% Advanced stage |
54% Female | NA | 54.9 | 31% |
| Goldberg & Wool55 | 48 | 65% | 73% Spouse 17% Adult children 10% Other |
Lung Stage not specified |
83% Female | Most Caucasian | 49.6 | 52% |
| Heinrich & Schag56 | 28 | 78% | 100% Spouse | Heterogeneous Stage not specified |
NA | NA | n/a | 11% |
| Hudson et al.57 | 106 | 30% | 67% Spouse 16% Adult children 17% Other |
Heterogeneous Advanced stage |
65% Female | 74% Australian26% Other | 60.8 | 57% |
| Jepson et al.58 | 161 | 75% | 88% Spouse 8% Adult children 4% Other |
Heterogeneous 35% Stage III-IV |
68% Female | 85% Caucasian15% Other | 62.3 | 26% |
| Keefe et al.59 | 82 | 47% | 76% Spouse 14% Adult children 10% Other |
Heterogeneous Advanced stage |
62% Female | 79% Caucasian 20% African American 1% Other |
58.5 | 28% |
| Kissane et al.60 | 282* | 73% | 28% Partner 59% Children 3% Other |
Heterogeneous Advanced stage |
53% Female | NA | 36.6 | 24% |
| Kozachik et al.61 | 120 | 53% | 100% Spouse/partner* | Heterogeneous 48% Stage I-II 52% Stage III-IV |
51% Female | NA | 52.1 | 27% |
| Kuijer et al. 62 | 59 | 94% | 100% Spouse | Heterogeneous 56% Advanced stage |
69% Female | NA | 49.5 | 34% |
| Kurtz et al. 63 | 237 | 43% | 100% Spouse/partner | Heterogeneous 33% Early stage 67% Advanced stage |
53% Female | 92% Caucasian 5% African American 3% Other |
55.2 | 41% |
| Manne et al. 64 | 68 | 57% | 100% Spouse | Prostate 80% Stage I-II 18% Stage III-IV |
100% Female | 84% Caucasian 13% African American 3% Other |
59.6 | 12% |
| McCorkle et al. 65 | 91 | 72% | 100% Spouse | Lung Terminal stage |
Most Female | NA | 58.0 | 49% |
| McCorkle et al. 66 | 126 | 93% | 100% Spouse | Prostate Early Stage |
100% Female | 85% Caucasian 15% Other |
56.0 | 15% |
| McMillan et al. 67 | 329 | 93% | Unknown | Mixed Advanced stage |
85% Female | NA | 61.5 | 69% |
| Mokuau et al. 68 | 18 | 83% | Spouse Adult children % unknown |
Heterogeneous Stage not specified |
50% Female | 100% Hawaiian | 54.0 | 0% |
| Northouse et al. 69 | 189 | 80% | 62% Spouse 16% Adult children 22% Other |
Breast Advanced stage |
69% Male* | 77% Caucasian 19% African American 4% Other |
52.0 | 26% |
| Northouse et al. 70 | 263 | 69% | 100% Spouse | Prostate 65% Localized stage 21% Advanced stage 14% Recurrent stage |
99% Female* | 84% Caucasian 14% African American 2% Other |
59.0 | 17% |
| Scott et al. 71 | 94 | 90% | 100% Spouse/partner | Breast & Gynecological Stage I-III |
100% Male | 98% Caucasian | 53.0 | 20% |
| Walsh 72 | 271 | 68% | 64% Spouse 25% Children 11% Other |
Heterogeneous Terminal stage |
79% Female | 86% Caucasian 14% Other |
56.3 | 55% |
Information obtained from authors
When discrepancies in sample size existed within an article, the sample size used was based on the analysis section of the article.
Caregiver attrition was based on the number of caregivers who dropped out of the study between baseline and last follow-up session.
Most studies were comprised of caregivers of patients who had various types of cancer (heterogeneous) (55 %); the remaining caregivers were from homogeneous patient populations, i.e., breast cancer (21%), prostate cancer (17%) or lung cancer (7%). Of the studies that reported patients’ stage of illness, approximately one-fourth had early-stage cancer, one-third had late-stage, and the remaining studies included patients from different stages of disease.
Effect Sizes Obtained for Caregiver Outcomes
Table 4 presents an overview of study findings for the multiple domains and outcomes assessed. The table provides the pooled effect sizes for intervention outcomes, 95% Confidence Intervals, assessment of heterogeneity across studies (Q statistic), and Egger’s t-test for publication bias. Forest plots for each outcome are shown. Forest plots depict the effect sizes calculated for each study by outcome (■ symbol) as well as the overall effect size obtained for the outcome across studies (◆ symbol) at each time interval. The Forest plots also indicate whether effects obtained in each study and across studies favor the control group or the intervention group.
TABLE 4.
Pooled Effect Sizes of Outcomes for Caregivers of Cancer Patients
| Domains/Outcomes | # of Trials | # of CGs | Pooled Effect Size Hedges’ g (95% CI) | Q for Heterogeneity | Egger’s t-test for Publication Bias |
|---|---|---|---|---|---|
| ILLNESS APPRAISAL FACTORS | |||||
| Caregiving Burden: | |||||
| 0–3 months | 11 | 1172 | 0.22 (0.08 to 0.35)** | 13.15 | 1.77 |
| 3.1–6 months | 5 | 714 | 0.10 (−0.04 to 0.25) | 0.65 | 0.42 |
| >6 months | 1 | 218 | 0.08 (−0.19 to 0.34) | - | - |
| Caregiving Benefit: | |||||
| 0–3 months | 5 | 380 | 0.17 (−0.13 to 0.46) | 6.87 | 0.18 |
| 3.1–6 months | 2 | 224 | 0.31 (0.02 to 0.61)* | 1.16 | - |
| >6 months | 1 | 14 | 0.48 (−0.53 to 1.49) | - | - |
| Information Needs: | |||||
| 0–3 months | 3 | 103 | 1.36 (0.92 to 1.77)** | 1.91 | 7.62* |
| 3.1–6 months | - | - | - | - | - |
| >6 months | - | - | - | - | - |
| COPING RESOURCES | |||||
| Coping Strategies: | |||||
| 0–3 months | 10 | 790 | 0.47 (0.16 to 0.78)** | 37.64** | 1.62 |
| 3.1–6 months | 4 | 477 | 0.20 (0.02 to 0.38)* | 1.96 | 3.92* |
| >6 months | 2 | 267 | 0.35 (0.10 to 0.58)* | 1.12 | - |
| Caregiver Self-Efficacy: | |||||
| 0–3 months | 8 | 757 | 0.25 (0.03 to 0.47)* | 14.24* | 1.49 |
| 3.1–6 months | 4 | 532 | 0.20 (0.03 to 0.37)* | 0.47 | 0.38 |
| >6 months | 1 | 218 | 0.29 (0.03 to 0.56)* | - | - |
| QUALITY OF LIFE | |||||
| Physical Functioning: | |||||
| 0–3 months | 7 | 757 | 0.11 (−0.05 to 0.27) | 6.98 | 4.88* |
| 3.1–6 months | 6 | 706 | 0.22 (0.04 to 0.41)* | 6.88 | 1.81 |
| >6 months | 2 | 278 | 0.26 (0.02 to 0.49)* | 0.92 | - |
| Distress and Anxiety: | |||||
| 0–3 months | 16 | 1,119 | 0.20 (0.08 to 0.32)* | 6.30 | 0.07 |
| 3.1–6 months | 11 | 882 | 0.16 (0.03 to 0.29)* | 6.40 | 0.53 |
| >6 months | 6 | 447 | 0.29 (0.06 to 0.51)* | 6.46 | 1.46 |
| Depression: | |||||
| 0–3 months | 16 | 1,315 | 0.06 (−0.06 to 0.18) | 18.52 | 1.17 |
| 3.1–6 months | 11 | 1,133 | 0.06 (−0.05 to 0.18) | 8.23 | 0.12 |
| >6 months | 3 | 295 | −0.03 (−0.38 to 0.33) | 6.62 | −0.38 |
| Marital-Family Relationships: | |||||
| 0–3 months | 10 | 840 | 0.20 (0.02 to 0.38)* | 13.78 | 0.39 |
| 3.1–6 months | 8 | 782 | 0.13 (0.00 to 0.28)* | 7.29 | 0.63 |
| >6 months | 5 | 481 | −0.04 (−0.38 to 0.31) | 11.63* | 0.75 |
| Social Functioning: | |||||
| 0–3 months | 4 | 367 | −0.14 (−0.34 to 0.07) | 0.30 | 1.00 |
| 3.1–6 months | 6 | 416 | 0.12 (−0.06 to 0.31) | 1.67 | 1.30 |
| >6 months | 2 | 137 | 0.39 (0.03 to 0.74)* | 1.06 | - |
p<0.05
p<0.001
Illness Appraisal Domain
Appraisal of Caregiving Burden
Caregiving burden was conceptualized as: caring as a strain or demanding activity, an overinvestment, or a negative reaction to activities related to caring for the patient. Among the 11 studies that assessed caregiving burden during the first three months following the intervention, the overall effect size was small but significant, g = 0.22. Effect sizes for the 11 individual studies varied between −0.12 to 0.62. Five studies assessed caregiving burden between 3 and 6 months following the intervention, and the overall effect was small and not significant, g = 0.10. Only one study reported on longer outcomes beyond six months and the effect size was not significant. See Figure 2.
FIGURE 2.
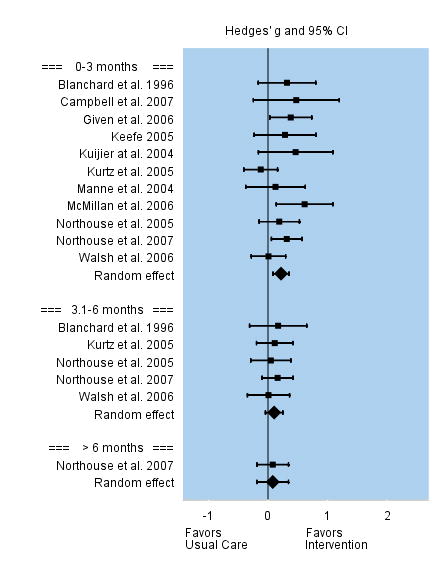
Effect Sizes for Caregiving Burden
Appraisal of Caregiving Benefit
Only a few studies addressed the appraisal of caregiving benefit as an intervention outcome. Caregiving benefit was conceptualized as: caring as an opportunity for personal growth, as a rewarding experience, as an investment, and as enhancing one’s self-esteem. Among the five studies that examined caregiving benefit during the first three months following the intervention, the overall effect size was small and not significant, g = 0.17. Effect sizes among the five individual studies varied between −0.52 to 0.61. However, based on two studies, interventions had a positive, significant effect on appraisal of caregiving benefit during 3 and 6 months following the intervention, g = 0.31. A larger but non significant effect was found beyond 6 months post intervention. See Figure 3.
FIGURE 3.

Effect Sizes for Caregiving Benefit
Information Needs
Only three studies assessed whether the intervention was effective in addressing caregivers’ appraisal of their information needs, such as information about cancer prognosis, survival, and available resources. The number of caregivers was small in these three studies, and they each reported large effect sizes. The overall effect size was large and significant, g =1.36. Effect sizes among the individual studies ranged from 0.85 to 1.87. None of the studies assessed intervention effects beyond three months. See Figure 4.
FIGURE 4.
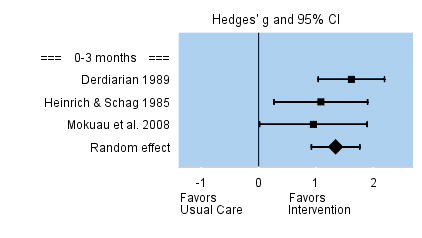
Effect Sizes for Information Needs
Coping Resources Domain
Coping Strategies
Coping strategies were conceptualized as interventions to enhance coping behavior either by promoting active coping, such as problem solving, or by reducing ineffective coping, such as avoidance and denial. Interventions were superior to the usual care in enhancing coping efforts of caregivers, and this effect appeared to be long lasting. Among the 10 studies that evaluated changes in coping efforts during the first three months post intervention, the overall effect size was moderate, but significant, g = 0.47. Effect sizes among individual studies varied between −0.47 to 1.46. Four studies evaluated changes in coping efforts between 3 and 6 months post-intervention, and the overall effect size was smaller but still significant, g = 0.20. The two studies that evaluated coping efforts beyond 6 months follow-up reported a persistent moderate effect that was significant, g = 0.35. See Figure 5.
FIGURE 5.
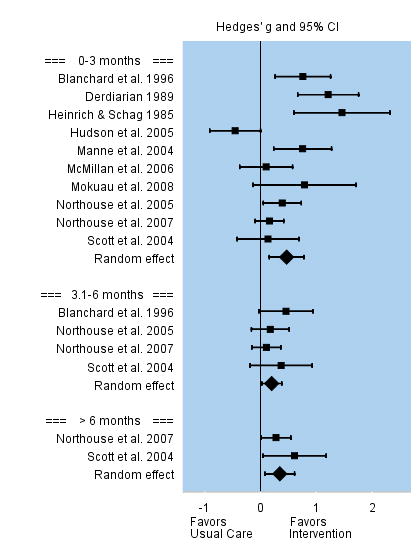
Effect Sizes for Coping
Self-Efficacy
Self-efficacy was conceptualized as the caregivers’ perceived confidence, preparation, and/or mastery to provide care and manage patients’ symptoms. Interventions were superior to the usual care. Among the eight studies that evaluated self-efficacy during the first three months post intervention, the overall effect size was small but significant, g = 0.25. Effect sizes among individual studies varied between −0.13 to 0.93. This positive significant effect persisted over time despite the fewer number of studies that assessed self-efficacy at three to six months post intervention, g = 0.20, and beyond 6 months follow-up. See Figure 6.
FIGURE 6.
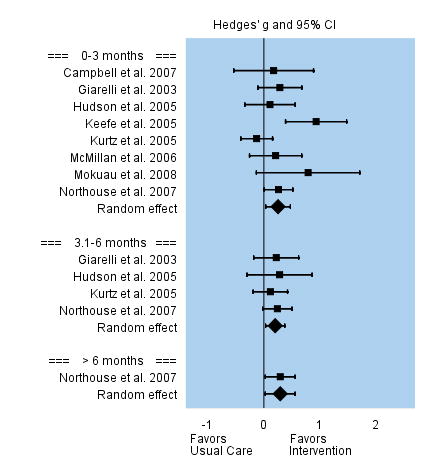
Effect Sizes for Caregiver Self-efficacy
Quality of Life Domain
Physical Functioning
Caregivers’ physical functioning was conceptualized as performance of self-care behaviors, such as an increase in exercise, participation in recreational activities, or improvement of their sleep quality. Among the seven studies that assessed caregivers’ physical functioning during the first three months following the intervention, the overall effect size was small and not significant, g = 0.11. Effect sizes among individual studies varied between −0.06 to 0.80. However, interventions were superior to usual care for improving caregivers’ physical functioning between three and six months post intervention with small but significant effect sizes, g = 0.22, and beyond 6 months follow-up, g = 0.26. See Figure 7.
FIGURE 7.
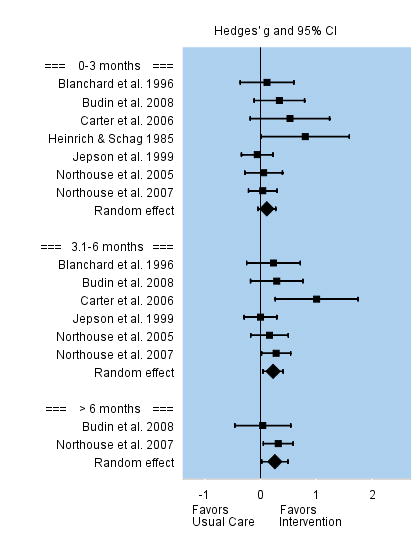
Effect Sizes for Physical Functioning
Distress and Anxiety
Distress and anxiety was conceptualized as emotional distress, worry, negative affect or mood. Interventions were superior to usual care in reducing caregivers’ distress and anxiety--and the effect appeared to last for at least 12 months. Among the 16 studies that evaluated changes in mental distress and anxiety during the first three months post intervention, the overall effect size was small but significant, g = 0.20. Effect sizes among individual studies varied between −0.18 to 0.51. Eleven studies evaluated changes in mental distress and anxiety between three and six months post intervention, and the overall effect remained small and significant, g = 0.16. The six studies that evaluated caregivers’ mental distress and anxiety beyond 6 months post intervention reported a persistent small to moderate significant effect, g = 0.29. See Figure 8.
FIGURE 8.
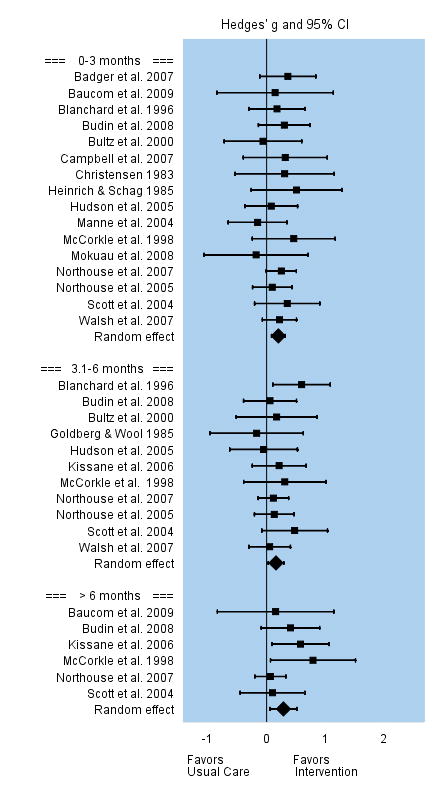
Effect Sizes for Distress and Anxiety
Depression
Interventions were not successful in reducing caregivers’ depression. Among the 16 studies that evaluated changes in caregivers’ depression during the first three months following the intervention, the overall effect size was small and not significant, g = 0.06. Effect sizes among individual studies varied between −0.25 to 0.55. Eleven studies evaluated changes in caregivers’ depression between three and six months post intervention, and the overall effect remained small and not significant, g = 0.06. Three studies that evaluated caregivers’ depression beyond 6 months follow-up reported a non significant effect, g = −0.03. See Figure 9.
FIGURE 9.
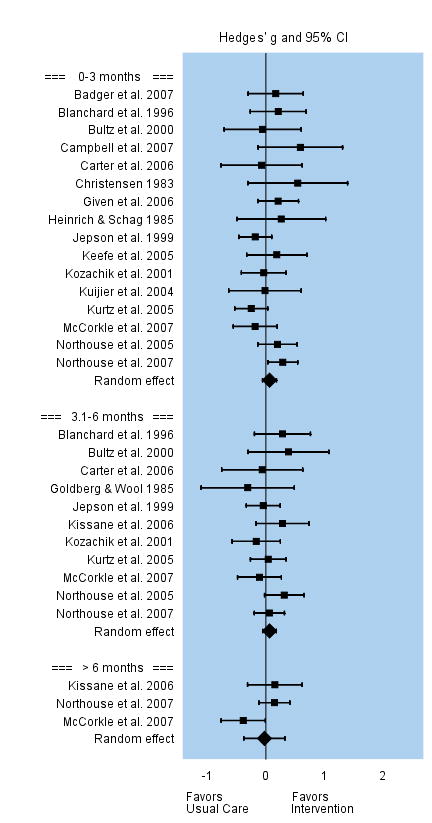
Effect Sizes for Depression
Marital-Family Relationships
Marital-family relationships were conceptualized as marital or sexual satisfaction, family support, and couple communication. Interventions were superior to usual care in improving marital-family relationships, yet this positive effect was not long lasting. Among the 10 studies that evaluated changes in marital and family relationships during the first three months following the intervention, the overall effect size was small but significant, g = 0.20. Effect sizes among individual studies varied between −0.18 to 0.47. Eight studies evaluated changes in marital-family relationships three to six months post intervention, but the overall effect was no longer significant, g = 0.13. Five studies that evaluated marital-family relationships beyond 6 months follow-up reported a non significant effect, g = −0.04. See Figure 10.
FIGURE 10.
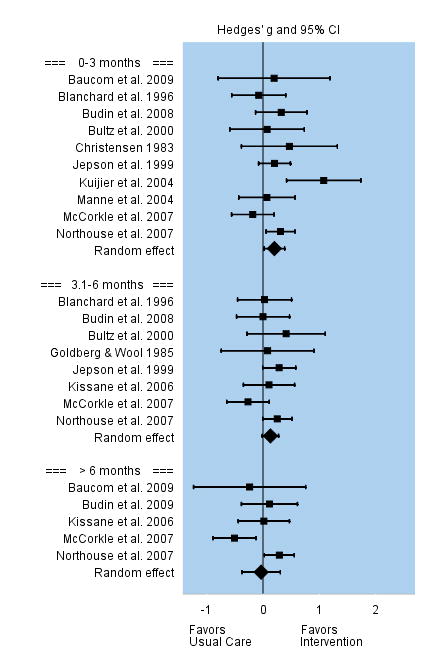
Effect Sizes for Marital-Family Relationships
Social Functioning
Caregivers’ social functioning was conceptualized as the ability to carry out domestic and family roles and increased interactions with family members, friends and peers. Interventions appear to have a delayed effect in improving caregivers’ social functioning. Among the four studies that evaluated changes in social functioning in the first three months post intervention, the overall effect size is non significant, g = −0.14. Effect sizes among individual studies varied between −0.18 to −0.04. Six studies evaluated changes in social functioning three to six months post intervention and, although the overall effect was positive, it was not significant, g = 0.12. The two studies that evaluated social functioning beyond 6 months post intervention reported an overall moderate effect, g = 0.39, that was significant. See Figure 11.
FIGURE 11.
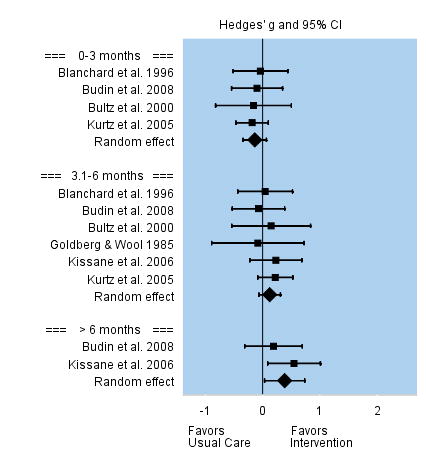
Effect Sizes for Social Functioning
Moderator Analyses for Intervention Characteristics
The moderation effects of intervention characteristics were tested on each outcome. Study characteristics examined were: 1) Intervention Participants (caregivers alone vs. caregivers with patients); 2) Mode of Delivery (face-to-face vs. phone vs. group vs. mixed); 3) Primary Content (psychoeducational vs. skills training vs. therapeutic counseling); and 4) Intervention Dose (total hours, total number of sessions, duration in weeks). Moderators of intervention outcomes were examined for the initial follow-up after the completion of the intervention when the number of studies assessed was the largest. Among the 29 studies, the initial follow-up occurred on average 5.86 weeks after completion of the intervention.
Table 5 presents intervention characteristics that significantly affected specific outcomes. For categorical intervention characteristics (e.g., type of participants), Hedges’ g for a subgroup refers to the effect of the interventions pooled across all studies with the same characteristic. A significant and positive coefficient (see asterisk) indicates that intervention was effective for that subgroup. The significance level of the Q statistic on the overall moderator line denotes whether there were significant differences in intervention effect sizes between subgroups. For continuous intervention characteristics (e.g., # of intervention hours), the sign of the coefficient indicates the direction of the relationship between study effectiveness and intervention dose level.
TABLE 5.
Moderator Analyses
| Outcomes at Initial Follow up and Moderators | # of Trials | # of CGs | Pooled Effect Size Hedges’ g (95% CI) | Q for Heterogeneity |
|---|---|---|---|---|
| Caregiving Burden | ||||
| # Intervention sessions (M=5.3) | 11 | 1192 | −0.08 (−0.13 to −0.02)* | 7.17** |
| Caregiving Benefit | ||||
| Participants | 4.77* | |||
| CGs only | 2 | 135 | 0.44 (0.10 to 0.77)* | 0.83 |
| CGs+Pts | 3 | 245 | 0.03 (−0.28 to 0.22) | 1.27 |
| Coping Strategies | ||||
| Mode of Delivery | 10.23* | |||
| Face-to-Face | 2 | 78 | 1.06 (0.42 to 1.71)** | 0.60 |
| Group | 2 | 85 | 1.01 (0.39 to 1.63)* | 1.89 |
| Mixed | 3 | 444 | 0.07 (−0.07 to 1.43) | 8.63* |
| # Intervention hours (M=7.0) | 9 | 730 | 0.08 (0.00 to 0.17)* | 3.66* |
| # Intervention sessions (M=5.2) | 9 | 730 | 0.23 (0.04 to 0.42)* | 5.42* |
| Depression | ||||
| # Intervention sessions (M=6.5) | 11 | 1448 | −0.05 (−0.08 to −0.02)* | 11.19** |
| Marital-Family Relationships | ||||
| # Intervention sessions (M=7.2) | 12 | 942 | −0.04 (−0.08 to −0.001)* | 4.09* |
p<0.05
p<0.001
Coping was found to be significantly influenced by several intervention characteristics. Studies employing face-to-face and group intervention delivery yielded better outcomes than those using mixed methods of intervention delivery. The intervention hours (M = 7.0) and the number of sessions (M = 5.2 sessions) were both positively and significantly related to the coping outcome; longer intervention hours and/or more sessions yielded better results in coping. In contrast, in the case of caregiver burden, depression, and marital-family relationship outcomes, interventions with more sessions reported significantly more negative (worse) outcomes than those with fewer sessions did. Finally, interventions that included caregivers alone reported significantly better outcomes in appraisal of caregiving benefit than interventions that included both caregivers and cancer patients.
Discussion
The present meta-analysis examined the content of 29 randomized clinical trials addressing needs of family caregivers of cancer patients, and examined the efficacy of these interventions on different caregiver outcomes. The types of interventions delivered to caregivers in the 29 RCTs were psychoeducational, skills training, and/or therapeutic counseling. Many protocols were comprehensive in scope and addressed psychoeducational and skills training activities as primary or secondary goals. The majority of these interventions included content for caregivers that addressed caring for the patient, maintaining family and marital relationships, and caring for themselves, suggesting some consensus that these are essential content areas for interventions offered to caregivers. It should be noted, however, that many of the interventions were designed to address primarily patient care. Content about caregiver self-care was a secondary focus provided incidentally or as an afterthought in some patient-focused interventions. Fewer intervention protocols were designed with a goal of focusing on content related to caregivers’ self-care.
We observed two indicators of intervention quality in the studies reviewed. First, the majority (86%) included theory-driven intervention protocols, which decreased the likelihood of isolated or chance findings. There was considerable variability, however, as some studies mentioned the theory in passing or in generic terms (e.g., cognitive-behavioral approach), while others indicated specific theories (e.g., Lazarus or Bandura) and demonstrated how the theory was utilized in the identification of hypotheses, the selection of intervention content, and choice of outcomes. Second, most studies (75%) instituted ways to examine the fidelity of the interventions, i.e., the extent to which the designated protocol was delivered by intervention staff in a consistent manner. Investigators used protocol manuals, intervention logs, tape-recorded sessions, and/or independent reviewers to assess or maintain intervention fidelity, indicating a growing understanding of the importance of adherence to standardized protocols.
The majority of interventions were delivered jointly to patients and their family caregivers, suggesting that investigators recognize that both persons are affected by the illness. Only 9 of the 29 studies focused solely on caregivers by design, and those that did, generally utilized an individual face-to-face or telephone format. Only two studies conducted caregiver groups, an approach with potential value for caregivers to interact openly with other caregivers without the presence of the patient. There was considerable variability in the intervention “dose” among protocols, both in the number of sessions (Range = 2 to 12) and duration of interventions (Range = several days to 18 months). There also was variability regarding the proportion of the intended “dose” the caregiver could miss and still be considered an evaluable case. Mode of delivery and intervention dose appear to be areas that need further evaluation or standardization within studies; otherwise it is difficult to determine if, or how much of, the dose of the intervention or mode of delivery affects study outcomes.
One of the most important findings of this meta-analysis was that interventions delivered to family caregivers of cancer patients had a significant positive effect on multiple outcomes. The multiple caregiver outcomes exemplify the multifaceted impact of caregiving and point to the diversity of intervention effects that can be achieved. Caregivers reported better outcomes in the Illness Appraisal Domain (less caregiving burden, greater caregiving benefit, fewer information needs), Coping Resources Domain (use of more effective coping strategies, and higher self-efficacy), and Quality of Life Domain (better physical functioning, less distress and anxiety, better marital-family relationships, and improved social functioning). Intervention effects were evident soon after the intervention for many outcomes, but delayed for other outcomes such as caregiver benefit, physical functioning, and social functioning in longitudinal studies. These delayed effects may be due to the additional time required for caregivers to make the necessary changes or adjustments, and to see the improvements on these outcomes as a result of their efforts. Positive and sustained intervention effects were found for coping, self-efficacy, and distress/anxiety outcomes across studies and at initial, intermediate, and long-term assessments.
The small to medium effect sizes found for interventions in this meta-analysis were similar to the effect sizes found for outcomes in other meta-analyses either with family caregivers of patients with chronic illness,73–75 or with cancer patients themselves. For example, prior meta-analyses that examined the efficacy of psychosocial interventions found an overall moderate effect on cancer patients’ quality of life, 76,77 and on cancer patients’ anxiety.76,78,79 These findings are comparable to the small to moderate intervention effects we found on most quality of life outcomes for caregivers in the present meta-analysis.
Interventions were not effective in reducing caregiver depression. Explanations provided by individual investigators included low levels of baseline caregiver depression,63 and the high rate of attrition among depressed caregivers.61 A previous meta-analysis, using all types of cancer patients, reported that interventions were not effective in reducing cancer patients’ depression,78 a finding comparable to the present study. However, another meta-analysis reported a moderate-to-strong effect in trials assessing depression in breast cancer patients.76 These conflicting reports could potentially be attributed to the effects of gender and/or type of cancer. Finally, a prior meta-analysis indicates that interventions that improve coping in cancer patients appear to be more effective than those that aim to reduce depression in cancer patients.76 This finding is directly comparable to our findings for depression and for the positive and sustained outcomes we found in the coping resources domain.
There are a number of factors that may have contributed to the small to medium effects observed in the present meta-analysis. Many of the studies we analyzed had small sample sizes (e.g., pilot studies) and high attrition rates, causing them either to be underpowered to detect intervention effects (Type 2 error) or to report inaccurate large effect sizes (publication bias).40 The only large intervention effect we found was for reducing caregivers’ need for information, and the significance of this finding is compromised by a significant Egger’s t-test, which suggests a possible publication bias. However, the meta-analysis from Sorenson et al. 75 also found large effects for improving caregivers’ ability/knowledge, which implies that this finding may not be accidental. Interestingly, even though the provision of information was provided in nearly all of the interventions analyzed in this meta-analysis, very few measured change in level of knowledge as a specific outcome. In addition, only 24% of the studies assessed intervention effects beyond six months post intervention, hindering the power to detect long term or delayed effects. Some studies were conducted with cancer patients and caregivers during a time when patients were doing well and caregiving demands were low, leaving little room for improvement in intervention outcomes.46 In some studies caregivers received fewer intervention sessions than patients (i.e., 3 vs. 6 sessions) or a less targeted intervention than patients, decreasing the likelihood of detecting intervention effects.44,66 Finally, while interventions improved caregiver outcomes in some studies, they could not cure the patient’s disease or stop the disease from progressing, which therefore remained ongoing threats for the caregiver.
Moderator analyses yielded interesting results. Studies that addressed coping as an outcome had better results with a higher intervention dose (more intervention hours and more sessions). Coping behavior was enhanced either by promoting active coping, such as problem solving, or by reducing ineffective coping, such as avoidance and denial. Thus, the finding that higher intervention dose yielded better outcomes makes intuitive sense; changing a problematic coping behavior or enhancing a good coping strategy requires engagement with the task and changes take time to occur. Interventions delivered in face-to-face or in group meetings yielded better coping outcomes than those employing a mixed method of intervention delivery. One possible explanation for this finding is that in some studies that employed a mixed method of intervention delivery (F-F and Phone), the face-to-face meetings were focused primarily on patients’ needs with the caregiver in attendance, while the phone calls were focused entirely on the caregiver alone. It is possible that this approach did not allow the patient and the caregiver to work together as a team and enhance a common coping strategy. In any case, when using a mixed mode-of-delivery, it is difficult to separate the results attributable to the phone portion of the intervention from the face-to-face portion as these two approaches are nested in one set of results.
Interventions that included only caregivers resulted in more positive appraisal of caregiving benefit. These interventions were better able to focus on caregivers’ own needs and gave them the opportunity to better reflect on the meaning and the importance of, as well as their confidence in, their caregiving role. The finding that interventions addressing caregiving burden, depression, and marital-family relationships yielded worse outcomes with higher number of sessions is more difficult to interpret. Perhaps caregivers experiencing more burden or more marital-family conflict have difficulty participating in longer interventions because they take time away from their caregiving tasks or family responsibilities and unintentionally add to their caregiver stress. It is also possible that more depressed caregivers are more likely to drop out of longer interventions lessening the effect of the intervention on caregiver outcomes. Clearly, more research is needed to fully examine the relationship between intervention length and caregiver outcomes.
Limitations of the study
First, we did not include studies published in languages other than English, unpublished studies, dissertations, or abstracts from conference proceedings. On the one hand, including only published materials ensures that higher quality, peer-reviewed studies were included in the meta-analysis; on the other hand, excluding unpublished studies is likely to introduce an upward bias into the size of the effects found, which means that calculated effect sizes are likely to be larger.40 To address this limitation, we assessed heterogeneity of findings with the Q statistic and publication bias with the Egger’s t-test statistic. Publication bias appeared only in three outcomes, and may be related to a few studies with smaller sample sizes that assessed these outcomes. However, the effect sizes we reported are comparable to effect sizes of other meta-analyses assessing cancer patients’ outcomes. Second, given the large number of moderators and the multiple outcomes we tested, we had a high chance of incidental findings of statistically significant moderators. To account for this bias, we presented and interpreted moderators that were significant at 0.05 level for an overall outcome and not those that were significant for a subgroup within a particular outcome. Third, each of the moderators was examined in separate analyses. We did not assess multiple moderators in one meta-regression model due to the small/moderate number of studies for each outcome. Finally, we limited our choice of moderators to characteristics of the interventions rather than characteristics of the caregivers (i.e., age, gender, education, etc).
Clinical Application of Findings
There are several implications from this meta-analysis for clinicians and other health professionals working with cancer patients and their family caregivers. First, clinicians need to recognize that patients and their family caregivers react to cancer as a unit, and as a result, they both have legitimate needs for help from health professionals. There is general consensus in the literature that when patients and caregivers are treated simultaneously important synergies are achieved that contribute to the well-being of each person.9,80 When caregivers’ needs are not addressed, their mental and physical health is at risk, and patients are denied the opportunity to obtain optimal care from a well-prepared family caregiver. Programs of care directed only to patients are seldom sufficient to meet patients’ needs because so much of the patient’s care depends on family caregivers. In order to provide optimal comprehensive cancer care, the care plan must focus on these patient-caregiver units.
Second, there is clear evidence from this meta-analysis that interventions provided to caregivers of cancer patients can have many positive effects on important caregiver outcomes. Even though effects were small to moderate in size, interventions show promise of achieving clinically significant outcomes. Although interventions did not improve caregivers’ overall quality of life, there is evidence that specific components of quality of life were responsive to these interventions. Interventions significantly reduced caregivers’ burden, improved their ability to cope, increased their confidence as caregivers, reduced their anxiety, and improved marital and family relationships. These interventions produce more prepared, less distressed caregivers which, in turn, is likely to result in more positive benefits for patients. Our findings are consistent with reports of interventions targeting caregivers of chronically ill patients with dementia. Caregivers of dementia patients benefited from enhanced knowledge about the disease, the caregiving role, and available resources.80 Once their information needs were met, they benefited from additional training in general problem-solving skills.80
Third, there are several theory-based, comprehensive interventions that have been developed and tested in randomized trials. To our knowledge, few, if any, of these interventions have been translated for or implemented in clinical practice settings. Both researchers and clinicians need to work together to determine ways to implement efficacious evidence-based interventions in oncology treatment sites where caregivers can benefit from them. Most of these evidence-based interventions will not move from efficacy studies (Phase III) to effectiveness studies (Phase IV) unless researchers, clinicians, and funding agencies collaborate to facilitate the implementation of these studies in practice settings.
Directions for Future Research
Based on the findings from this meta-analysis, we have identified several areas in need of further research.
Future studies need to have more racial, cultural, and socioeconomic diversity. In this meta-analysis, 16% of the participants were self-identified as members of a minority group and only two studies were tailored for a particular cultural or racial group.49,68
More studies need to examine caregivers’ self-care behaviors and the physical health outcomes that follow. Caregivers often place patients’ needs above their own needs and as a result spend less time on health promotion activities for themselves such as exercise or cancer screening. Over time this could have negative consequences on caregivers’ health.
There is a need for more research studies that identify patients and caregivers who are at higher risk for poorer outcomes, so that interventions can be targeted to them. While all caregivers should be provided with basic caregiving information as part of a comprehensive cancer care program, every effort should be made to identify those families at greater risk that are more likely to benefit the most from additional interventions.
There is a need for large, well-funded, multisite studies to obtain larger samples of patients and caregivers in a reasonable amount of time, with long-term post intervention follow-up, and with a greater ability to generalize findings. Conducting intervention studies with cancer patients and their family caregivers is challenging and requires the support of clinicians, who can inform potential participants about available studies and encourage them to participate in them. These studies also need to be integrated into clinical care to determine how effectively they can be implemented in practice settings.
Studies are also needed that assess intervention costs and their possible effect on health care resources. Of the 29 studies we examined, none of them addressed cost issues. More research is needed on how efficacious interventions can be delivered in a cost-effective manner.
There is a need for studies that assess the potential for using technology to deliver effective interventions to caregivers. In our search of the literature for this meta-analysis, we found no published studies using the Web with our target population. This may be an important mode of intervention delivery to consider for future studies.
There is a need to consider the clinical significance of interventions targeting caregivers of cancer patients in addition to their statistical significance. One major step in accomplishing this goal is to increase their methodological rigor, by being equally assured that studies are neither underpowered nor overpowered. A second step is to obtain consensus among health professionals from multiple disciplines on a set of core outcomes that are important to include and measure in all caregiver studies.80 Finally, consensus also is needed regarding the importance of the relationship between clinical and statistical significance, because even if effects are small, they may be important and associated with clinically meaningful outcomes.
In summary, findings from this meta-analysis indicate that interventions targeted to family caregivers of cancer patients can have a positive effect on many important caregiver outcomes. Researchers and clinicians need to work together to find ways to deliver research-tested interventions to patients and their caregivers so that both can cope effectively with the demands of cancer, and maintain their quality of life.
Acknowledgments
Barbara Given PhD, RN, FAAN College of Nursing, Michigan State University; Charles W. Given PhD, Department of Family Practice, Michigan State University; Bernadine Cimprich PhD, RN, FAAN, School of Nursing, University of Michigan for reviewing the manuscript and offering valuable suggestions.
References
- 1.Bishop MM, Beaumont JL, Hahn EA, et al. Late effects of cancer and hematopoietic stem-cell transplantation on spouses or partners compared with survivors and survivor-matched controls. J Clin Oncol. 2007;25:1403–1411. doi: 10.1200/JCO.2006.07.5705. [DOI] [PubMed] [Google Scholar]
- 2.Given BA, Given CW, Kozachik S. Family support in advanced cancer. CA Cancer J Clin. 2001;51:213–231. doi: 10.3322/canjclin.51.4.213. [DOI] [PubMed] [Google Scholar]
- 3.Arno PS, Levine C, Memmott MM. The economic value of informal caregiving. Health Affairs. 1999;18(2):182–188. doi: 10.1377/hlthaff.18.2.182. [DOI] [PubMed] [Google Scholar]
- 4.Cochrane B, Lewis FM. The partner’s adjustment to breast cancer: A critical analysis of intervention studies. Health Psychol. 2005;24:327–332. doi: 10.1037/0278-6133.24.3.327. [DOI] [PubMed] [Google Scholar]
- 5.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6(7):1–28. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y, Given BA. Quality of life of family caregivers of cancer survivors. Cancer suppl. 2008;112:2556–2568. doi: 10.1002/cncr.23449. [DOI] [PubMed] [Google Scholar]
- 7.Northouse LL, Mood D, Kershaw T, et al. Quality of life of women with recurrent breast cancer and their family members. J Clin Oncol. 2002;20(19):4050–4064. doi: 10.1200/JCO.2002.02.054. [DOI] [PubMed] [Google Scholar]
- 8.Wagner CD, Bigatti SM, Storniolo AM. Quality of life of husbands of women with breast cancer. Psychooncology. 2006;15:109–120. doi: 10.1002/pon.928. [DOI] [PubMed] [Google Scholar]
- 9.Hagedoorn M, Sanderman R, Bolks HN, Tuinstra J, Coyne JC. Distress in couples coping with cancer: A meta-analysis and critical review of role and gender effects. Psychol Bull. 2008;134(1):1–30. doi: 10.1037/0033-2909.134.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Segrin C, Badger T, Dorros SM, Meek P, Lopez AM. Interdependent anxiety and psychological distress in women with breast cancer and their partners. Psychooncology. 2007;16:634–643. doi: 10.1002/pon.1111. [DOI] [PubMed] [Google Scholar]
- 11.Cliff AM, MacDonagh RP. Psychosocial morbidity in prostate cancer: II. A comparison of patients and partners. BJU Int. 2000;86(7):834–839. doi: 10.1046/j.1464-410x.2000.00914.x. [DOI] [PubMed] [Google Scholar]
- 12.Given CW, Stommel M, Given BA, et al. The influence of cancer patients’ symptoms and functional status on patients’ depression and family caregivers’ reaction and depression. Health Psychol. 1993;12(4):277–285. doi: 10.1037//0278-6133.12.4.277. [DOI] [PubMed] [Google Scholar]
- 13.Kornblith AB, Herr HW, Ofman US, Scher HI, Holland JC. Quality of life of patients with prostate cancer and their spouses. The value of a data base in clinical care. Cancer. 1994;73(11):2791–2802. doi: 10.1002/1097-0142(19940601)73:11<2791::aid-cncr2820731123>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Braun M, Mikulincer M, Rydall A, Walsh A, Rodin G. Hidden morbidity in cancer: pouse caregivers. J Clin Oncol. 2007;25:4829–4834. doi: 10.1200/JCO.2006.10.0909. [DOI] [PubMed] [Google Scholar]
- 15.Vanderwerker LC, Laff RE, Kadan-Lottick NS, McColl S, Prigerson HG. Psychiatric disorders and mental health service use among caregivers of advanced cancer patients. J Clin Oncol. 2005;23(28):6899–6907. doi: 10.1200/JCO.2005.01.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badr H, Taylor C. Sexual dysfunction and spousal communication in couples coping with prostate cancer. Psychooncology. 2009;18:735–746. doi: 10.1002/pon.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manne SL, Norton TR, Ostroff JS, Winkel G, Fox K, Grana G. Protective buffering and psychological distress among couples coping with breast cancer: the moderating role of relationship satisfaction. J Fam Psychol. 2007;21:380–388. doi: 10.1037/0893-3200.21.3.380. [DOI] [PubMed] [Google Scholar]
- 18.Kuijer RG, Buunk BP, Ybema JF, Wobbes T. The relation between perceived inequity, marital satisfaction and emotions among couples facing cancer. Br J Soc Psychol. 2002;41:39–56. doi: 10.1348/014466602165045. [DOI] [PubMed] [Google Scholar]
- 19.Vess JD, Moreland JR, Schwebel AI. A follow-up study of role functioning and the psychological environment of families of cancer patients. J Psychosoc Oncol. 1985;3(2):1–13. [Google Scholar]
- 20.Manne SL, Ostroff JS, Norton TR, et al. Cancer-related relationship communication in couples coping with early stage breast cancer. Psychooncology. 2006;15:234–247. doi: 10.1002/pon.941. [DOI] [PubMed] [Google Scholar]
- 21.Porter LR, Keefe FJ, Hurwitz H, Faber M. Disclosure between patients with gastrointestinal cancer and their spouses. Psychooncology. 2005;14:1030–1042. doi: 10.1002/pon.915. [DOI] [PubMed] [Google Scholar]
- 22.Given B, Given CW. Patient and family caregiver reaction to new and recurrent breast cancer. J Amn Med Womens Assoc. 1992;47(5):201–206. [PubMed] [Google Scholar]
- 23.Given B, Wyatt G, Given C, et al. Burden and depression among caregivers of patients with cancer at the end of life. Oncol Nurs Forum. 2004;31(6):1105–1115. doi: 10.1188/04.ONF.1105-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weitzner MA, McMillan SC, Jacobsen PB. Family caregiver quality of life: differences between curative and palliative cancer treatment settings. J Pain Symptom Manage. 1999;17(6):418–428. doi: 10.1016/s0885-3924(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 25.Burton LC, Newsom JT, Schultz D, Hirsch CH, German PS. Preventive health behaviors among spousal caregivers. Prev Med. 1997;26:162–169. doi: 10.1006/pmed.1996.0129. [DOI] [PubMed] [Google Scholar]
- 26.Carter PA. Caregivers’ descriptions of sleep changes and depressive symptoms. Oncol Nurs Forum. 2002;29(9):1277–1283. doi: 10.1188/02.ONF.1277-1283. [DOI] [PubMed] [Google Scholar]
- 27.Mellon S, Northouse LL. Family survivorship and quality of life following a cancer diagnosis. Res Nurs Health. 2001;24(6):446–459. doi: 10.1002/nur.10004. [DOI] [PubMed] [Google Scholar]
- 28.Thornton AA, Perez MA, Meyerowitz BE. Patient and partner quality of life and psychosocial adjustment following radical prostatectomy. J Clin Psychol Med Settings. 2004;11(1):15–30. [Google Scholar]
- 29.Ell K, Nishimoto R, Mantell J, Hamovitch M. Longitudinal analysis of psychological adaptation among family members of patients with cancer. J Psychosom Res. 1988;32(4–5):429–438. doi: 10.1016/0022-3999(88)90026-8. [DOI] [PubMed] [Google Scholar]
- 30.Haley WE, LaMonde LA, Han B, Burton AM, Schonwetter R. Predictors of depression and life satisfaction among spouse caregivers in hospice: Application of a stress process model. J Palliat Med. 2003;6(2):215–224. doi: 10.1089/109662103764978461. [DOI] [PubMed] [Google Scholar]
- 31.Harding R, Higginson I. What is the best way to help caregivers in cancer and palliative care? A systematic literature review of interventions and their effectiveness. Palliat Med. 2003;17:63–74. doi: 10.1191/0269216303pm667oa. [DOI] [PubMed] [Google Scholar]
- 32.Hudson P. A critical review of supportive interventions for family caregivers of patients with palliative-stage cancer. J Psychoso Oncol. 2004;22(4):77–92. [Google Scholar]
- 33.Pasacreta JV, McCorkle R. Cancer care: impact of interventions on caregiver outcomes. Annu Rev Nurs Res. 2000;18:127–148. [PubMed] [Google Scholar]
- 34.Stenberg U, Ruland CM, Miaskowski C. Psychooncology. Wiley InterScience; 2009. Review of the literature on the effects of caring for a patient with cancer. [DOI] [PubMed] [Google Scholar]
- 35.Lazarus RS, Folkman S, editors. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- 36.Bandura A. Self-efficacy: The Exercise of Control. New York: Freeman; 1997. [Google Scholar]
- 37.Cella D, Tulsky D, Gray G, et al. The Functional Assessment of Cancer Therapy Scale:Development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 38.Ferrell BA, Grant M, Schmidt GM, et al. The meaning of quality of life for bone marrow transplant survivors. Part 1. The impact of bone marrow transplant of quality of life. Cancer Nurs. 1992;15(3):153–160. [PubMed] [Google Scholar]
- 39.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. West Sussex, UK: Wiley; 2009. [Google Scholar]
- 40.Lipsey MW, Wilson DB, editors. Practical Meta-Analysis. Vol. 49. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 41.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, N.J.: Lawrence Erlbaum; 1988. [Google Scholar]
- 42.Comprehensive Meta-Analysis V.2 Software [computer program]. Version V.2. Englewood, N.J.: BIOSTAT, Inc; 2009. [Google Scholar]
- 43.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):1–6. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badger T, Segrin C, Dorros SM, Meek P, Lopez AM. Depression and anxiety in women with breast cancer and their partners. Nurs Res. 2007;56(1):44–53. doi: 10.1097/00006199-200701000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Baucom DH, Porter LS, Kirby JS, et al. A couple-based intervention for female breast cancer. Psychooncology. 2009;18:276–283. doi: 10.1002/pon.1395. [DOI] [PubMed] [Google Scholar]
- 46.Blanchard C, Toseland R, McCallion P. The effects of a problem-solving intervention with spouses of cancer patients. J Psychosoc Oncol. 1996;14(2):1–21. [Google Scholar]
- 47.Budin WC, Hoskins CN, Haber J, et al. Breast cancer: Education, counseling, and adjustment among patients and partners: A randomized clinical trial. Nurs Res. 2008;57(3):199–213. doi: 10.1097/01.NNR.0000319496.67369.37. [DOI] [PubMed] [Google Scholar]
- 48.Bultz BD, Speca M, Brasher PM, Geggie PH, Page SA. A randomized controlled trial of a brief psychoeducational support group for partners of early stage breast cancer patients. Psychooncology. 2000;9:303–313. doi: 10.1002/1099-1611(200007/08)9:4<303::aid-pon462>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 49.Campbell LC, Keefe FJ, Scipio C, et al. Facilitating research participation and improving quality of life for African American prostate cancer survivors and their intimate partners. Cancer-Suppl. 2007;109(2):414–424. doi: 10.1002/cncr.22355. [DOI] [PubMed] [Google Scholar]
- 50.Carter PA. A brief behavioral sleep intervention for family caregivers of persons with cancer. Cancer Nurs. 2006;29(2):95–103. doi: 10.1097/00002820-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Christensen DN. Postmastectomy couple counseling: an outcome study of a structured treatment protocol. J Sex Marital Ther. 1983;9(4):266–275. doi: 10.1080/00926238308410913. [DOI] [PubMed] [Google Scholar]
- 52.Derdiarian AK. Effects of information on recently diagnosed cancer patients’ and spouses’ satisfaction with care. Cancer Nurs. 1989;12(5):285–292. [PubMed] [Google Scholar]
- 53.Giarelli E, McCorkle R, Monturo C. Caring for a spouse after prostate surgery: The preparedness needs of wives. J Fam Nurs. 2003;9(4):453–485. [Google Scholar]
- 54.Given BA, Given CW, Sikorskii A, Jeon S, Sherwood P, Rahbar M. The impact of providing symptom management assistance on caregiver reaction: Results of a randomized trial. J Pain Symptom Manage. 2006;32(5):433–443. doi: 10.1016/j.jpainsymman.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 55.Goldberg RJ, Wool MS. Psychotherapy for the spouses of lung cancer patients: Assessment of an intervention. Psychother Psychosom. 1985;43:141–150. doi: 10.1159/000287871. [DOI] [PubMed] [Google Scholar]
- 56.Heinrich RL, Schag CC. Stress and activity management: Group treatment for cancer patients and spouses. J Consult Clin Psychol. 1985;53(4):439–446. doi: 10.1037//0022-006x.53.4.439. [DOI] [PubMed] [Google Scholar]
- 57.Hudson PL, Aranda S, Hayman-White K. A psycho-educational intervention for family caregivers of patients receiving palliative care: A randomized controlled trial. J Pain Symptom Manage. 2005;30(4):329–341. doi: 10.1016/j.jpainsymman.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Jepson C, McCorkle R, Adler D, Nuamah I, Lusk E. Effects of home care on caregivers’ psychosocial status. Image J Nurs Sch. 1999;31(2):115–120. doi: 10.1111/j.1547-5069.1999.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 59.Keefe FJ, Ahles TA, Sutton L, et al. Partner-guided cancer pain management at the end of life: A preliminary study. J Pain Symptom Manage. 2005;29(3):263–272. doi: 10.1016/j.jpainsymman.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 60.Kissane DW, McKenzie M, Bloch S, et al. Family focused grief therapy: A randomize controlled trial in palliative care and bereavement. Am J Psychiatry. 2006;163(7):1208–1218. doi: 10.1176/ajp.2006.163.7.1208. [DOI] [PubMed] [Google Scholar]
- 61.Kozachik SL, Given CW, Given BA, et al. Improving depressive symptoms among caregivers of patients with cancer: Results of a randomized clinical trial. Oncol Nurs Forum. 2001;28(7):1149–1157. [PubMed] [Google Scholar]
- 62.Kuijer RG, Buunk BP, DeJong GM, Ybema JF, Sanderman R. Effects of a brief intervention program for patients with cancer and their partners on feelings of inequity, relationship quality and psychological distress. Psychooncology. 2004;13:321–334. doi: 10.1002/pon.749. [DOI] [PubMed] [Google Scholar]
- 63.Kurtz ME, Kurtz JC, Given CW, Given BA. A randomized, controlled trial of a patient/caregiver symptom control intervention: Effects on depressive symptomatology of caregivers of cancer patients. J Pain Symptom Manage. 2005;30(2):112–122. doi: 10.1016/j.jpainsymman.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manne S, Babb J, Pinover W, Horwitz E, Ebbert J. Psychoeducational group intervention for wives of men with prostate cancer. Psychooncology. 2004;13:37–46. doi: 10.1002/pon.724. [DOI] [PubMed] [Google Scholar]
- 65.McCorkle R, Robinson L, Nuamah I, Lev E, Benoliel JQ. The effects of home nursing care for patients during terminal illness on the bereaved’s psychological distress. Nurs Res. 1998;47(1):2–10. doi: 10.1097/00006199-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 66.McCorkle R, Siefert ML, Dowd MF, Robinson JP, Pickett M. Effects of advanced practice nursing on patient and spouse depressive symptoms, sexual function, and marital interaction after radical prostatectomy. Urol Nurs. 2007;27(1):65–77. [PubMed] [Google Scholar]
- 67.McMillan SC, Small BJ, Weitzner M, et al. Impact of coping skills intervention with family caregivers of hospice patients with cancer. Cancer. 2006;106(1):214–222. doi: 10.1002/cncr.21567. [DOI] [PubMed] [Google Scholar]
- 68.Mokuau N, Braun KL, Wong LK, Higuchi P, Gotay C. Development of a family intervention for Native Hawaiian women with cancer: A pilot study. Soc Work. 2008;53(1):9–19. doi: 10.1093/sw/53.1.9. [DOI] [PubMed] [Google Scholar]
- 69.Northouse L, Kershaw T, Mood D, Schafenacker A. Effects of a family intervention on the quality of life of women with recurrent breast cancer and their family caregivers. Psychooncology. 2005;14:478–491. doi: 10.1002/pon.871. [DOI] [PubMed] [Google Scholar]
- 70.Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer. 2007;110:2809–2818. doi: 10.1002/cncr.23114. [DOI] [PubMed] [Google Scholar]
- 71.Scott JL, Halford WK, Ward BG. United we stand? The effects of a couple-coping intervention on adjustment to early stage breast or gynecological cancer. J Consult Clin Psychol. 2004;72(6):1122–1135. doi: 10.1037/0022-006X.72.6.1122. [DOI] [PubMed] [Google Scholar]
- 72.Walsh K, Jones L, Tookman A, et al. Reducing emotional distress in people caring for patients receiving specialist palliative care. Br J Psychiatry. 2007;190:142–147. doi: 10.1192/bjp.bp.106.023960. [DOI] [PubMed] [Google Scholar]
- 73.Gitlin LN, Belle SH, Burgio LD, et al. Effect of multicomponent interventions on caregiver burden and depression. The REACH multisite initiative at 6-month follow-up. Psychol Aging. 2003;18(3):361–374. doi: 10.1037/0882-7974.18.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martire LM, Lustig AP, Schulz R, Miller GE, Helgeson VS. Is it beneficial to involve a family member? A meta-analysis of psychosocial interventions for chronic illness. Health Psychol. 2004;23(6):599–611. doi: 10.1037/0278-6133.23.6.599. [DOI] [PubMed] [Google Scholar]
- 75.Sorensen S, Pinquart M, Habil D, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42(3):356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- 76.Naaman S, Radwan K, Fergusson D, Johnson S. Status of psychological trials in breast cancer patients: A report of three meta-analyses. Psychiatry. 2009;72:50–69. doi: 10.1521/psyc.2009.72.1.50. [DOI] [PubMed] [Google Scholar]
- 77.Rehse B, Pukrop R. Effects of psychosocial interventions on quality of life in adult cancer patients: Meta-analysis of 37 published controlled outcome studies. Patient Educ Couns. 2003;50:179–186. doi: 10.1016/s0738-3991(02)00149-0. [DOI] [PubMed] [Google Scholar]
- 78.Sheard T, McGuire P. The effect of psychological interventions on anxiety and depression in cancer patients: Results of two meta-analyses. Br J Cancer. 1999;80:1770–1780. doi: 10.1038/sj.bjc.6690596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tatrow K, Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: A meta-analysis. J Behav Med. 2006;29:17–27. doi: 10.1007/s10865-005-9036-1. [DOI] [PubMed] [Google Scholar]
- 80.Schulz R, O’Brien A, Czaja S, et al. Dementia caregiver intervention research: In search of clinical significance. Gerontologist. 2002;42:589–602. doi: 10.1093/geront/42.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]