Abstract
Wear debris causes biological response which can result in periprosthetic osteolysis after total joint replacement surgery. Nuclear factor-kappa B (NFκB), a representative transcription factor involved in inflammation, is believed to play an important role in this event by regulating the production of proinflammatory mediators and osteoclastogenesis. In this study, we sought to determine whether activation of NFκB in response to stimulation by particles could be visualized by in vivo imaging. We loaded polyethylene (PE) particles onto the calvaria of NFκB/luciferase transgenic mouse, and detected luminescence generated by activation of NFκB. On day 7 after loading, the level of luminescence was maximal. Levels of luminescence were significantly correlated with the levels of luciferase activity, proinflammatory mediator mRNAs, and bone resorption parameters. This system, which enabled us to evaluate particle-induced inflammation and osteolysis without sacrificing mice, constitutes a useful tool for evaluating the efficacy of prophylaxis or treatments for particle-induced osteolysis.
1. Introduction
Wear debris-induced osteolysis is one of the principal causes of implant failure and consequent revision surgery in total joint arthroplasty [1, 2]. The osteolytic process is believed to start with the activation of macrophages and foreign-body giant cells and with phagocytosis of particulate wear debris. This appears to induce the release of proinflammatory cytokines and mediators that provoke the differentiation of phagocyte precursors into osteoclasts, which, in turn, results in periprosthetic osteolysis and implant loosening [3, 4]. Several proinflammatory mediators known to stimulate osteoclastic bone resorption, including interleukin (IL)-1, tumor necrosis factor (TNF)-α, IL-6, and prostaglandin (PG) E2 have been found in periprosthetic tissues [5–8]. These mediators are thought to promote the differentiation of precursor cells into mature osteoclasts by stimulating stromal cell expression of receptor activator of nuclear factor kappa-B ligand (RANKL), which is also abundant in the periprosthetic tissues around a loosened joint prosthesis [9].
Nuclear factor-kappa B (NFκB) is a critical signaling molecule for an array of biological processes including inflammation, cellular differentiation, apoptosis, and tumorigenesis [10–12]. NFκB also plays an important role in a majority of the pathophysiologic steps in particle-induced osteolysis. Namely, NFκB is believed to be one of the transcription factors involved in regulating gene expression of proinflammatory cytokines including TNF-α and IL-1 in the monocyte/macrophage cell line [13]. Prior studies demonstrated particle-induced activation of NFκB signaling, which preceded production of TNF-α or IL-6 [14, 15]. NFκB is also important in regulating osteoclast differentiation [15–17]. It has also been reported that blockade of NFκB signaling results in the inhibition of particle-induced osteoclastogenesis in vitro and osteolysis in vivo [15, 18, 19]. Therefore, it seems reasonable to employ NFκB activation, whose significance is well established in this field, as an indicator of particle-induced inflammation and osteolysis.
In recent years, several highly-sensitive imaging technologies have been developed to detect and quantitate in vivo fluorescence and luminescence without sacrificing animals, and these technologies are coming to be widely used. NFκB/luciferase transgenic (NFκB/luc tg) mice, which carry a transgene-containing six NFκB responsive elements and modified firefly luciferase complementary deoxyribonucleic acid (cDNA), exhibit luminescence at inflammatory sites in inflammatory disease models including arthritis and sepsis. Ho et al. evaluated the host-biomaterial interaction of genipin-cross-linked gelatin implant using this mouse [20]. We hypothesized that this might be applicable to the in vivo evaluation of inflammation and osteolysis induced by particles of bearing surface materials without sacrificing mice. The aim of this study was to test this hypothesis.
2. Materials and Methods
2.1. Animals
NFκB/luc tg mice were purchased from Caliper Lifesciences (CA, USA) and maintained under specific pathogen-free conditions. In all experiments, 7-8 week old mice were used. All experiments were performed according to the guidelines of the Hokkaido University animal welfare committee.
2.2. Particles
Polyethylene (PE) particles (Ceridust 3615, mean diameter 7 μm) were generously provided by Clariant Japan (Tokyo, Japan). The particles were processed under sterile conditions. They were washed for 24 h twice in 75% ethanol at room temperature using a rocking device and afterward dried in a vacuum desiccator according to a previously reported method [21]. The absence of endotoxin was confirmed by a Limulus assay (Limulus Amebocyte Lysate QCL-1000, Cambrex, NJ, USA). The particles were subdivided into separate use and stored at 4°C.
2.3. In Vivo Mouse Calvaria Resorption Model and Experimental Design
The murine calvarial model is a representative model of particle-induced osteolysis in which wear particles of various materials, such as polymethyl methacrylate, titanium, or PE, are implanted onto mouse calvaria to induce bone resorption [22]. We treated mice with pentobarbital anesthesia (50 mg/kg of body weight, intraperitoneal injection), and then a 1 cm × 1 cm area of calvarial bone was exposed by making a midline sagittal incision over the calvaria. The periosteum was peeled of the external cortex of the calvaria and PE particles were spread over the area. The incision was then closed with simple 5–0 nylon sutures. Sham-operated animals were treated as described above except that no particles were implanted.
Firstly, five mice were subjected to histological analysis on day 7 after implantation of 5 mg PE particles. Secondly, 10 mice were divided into two groups (sham or 5 mg PE, n = 5, resp.), and luminescence was detected on days 0, 3, 7, 10, and 14 after PE implantation. Thirdly, 15 mice were divided into five groups (sham, 0.5 mg PE, 2 mg PE, 5 mg PE, and 10 mg PE, n = 3, resp.), and luminescence was detected on day 7 after PE implantation. Fourthly, 19 mice were implanted with 5 mg PE particles, from which three to five mice were subjected to the evaluation of luminescence and subsequent sacrifice on days 0, 3, 7, 10, or 14, respectively. The retrieved calvaria was cut into two pieces along the sagittal suture, which were then subjected to a luciferase assay or to real time reverse transcription-polymerase chain reaction (RT-PCR), respectively. And fifthly, 12 mice were divided into three groups (n = 4 per group, sham, 0.5 mg PE, and 5 mg PE), and following the evaluation of luminescence on day 7 after PE implantation, the calvariae were retrieved and were subjected to bone histomorphometry.
2.4. Histology
The calvariae were fixed in formalin, decalcified in ethylenediaminetetraacetic acid (EDTA), and embedded in paraffin. The calvaria were then sectioned into 5 μm mid-frontal sections that were made such that the midline suture was at the center of the cross-section. For histological analysis, the sections were stained with hematoxylin/eosin (HE) and for tartrate-resistant acid phosphatase (TRAP) with use of the Diagnostics Acid Phosphatase Kit (Sigma Chemical, St. Louis, MO, USA).
2.5. In Vivo Imaging
Following intravenous injection of 200 μL of d-luciferin (D-Luciferin Potassium Salt, Wako Chemicals, Kanagawa, Japan) solution (10 mg/ml phosphate buffered saline (PBS)), we detected luminescence over the calvaria using an IVIS imaging system (IVIS spectrum, Xenogen, CA, USA). After setting the range of interest (ROI) to cover the luminescent area, the level of luminescence was quantified and expressed as total influx.
2.6. Luciferase Assay
Luciferase activity of the calvaria was measured using a commercially available luciferase assay kit (PicaGene, TOYO B-Net, Tokyo, Japan) according to the manufacturer's protocol. In brief, the samples were soaked in lysis buffer (complete, mini, EDTA-free (Roche, Mannheim, Germany), 1 tablet in 10 ml of 50 mM Tris HCl pH 6.8) whose volume was fivefold the weight of the samples, homogenized using a pestle homogenizer, and centrifuged at 15000 rpm for 10 min. Finally, 20 μl of the supernatant was mixed with 100 μl of substrate, and luciferase activity was measured using a luminometer (Sirius luminometer, Berthold, Germany).
2.7. RNA Isolation and Real-Time RT PCR
Total ribonucleic acid (RNA) was extracted using the RNeasy minikit (Qiagen Inc., CA, USA) according to the manufacturer's instructions. Total RNA (1 μg per sample) was reverse transcribed into single-stranded cDNA using the PrimeScript RT reagent Kit (TakaraBio, Shiga, Japan). Primers were obtained from the Perfect Real Time support system (Takara Bio, Shiga, Japan) on the basis of the published messenger RNA (mRNA) sequences, and their sequences are listed in Table 1. RT-PCR was then performed using a Thermal Cycler Dice TP800 (TakaraBio, Shiga, Japan) and SYBR Premix Ex Taq (TakaraBio, Shiga, Japan) with 5 ng of cDNA template in a final volume of 25 μl. The cDNA was amplified by 40 amplification cycles, and fluorescence changes were monitored with SYBR Green after each cycle. The results were evaluated using the Thermal Cycler Dice Real Time System software program. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were used to normalize the results.
Table 1.
List of primers used in real-time RT-PCR.
| Primer ID | Primers(5′–3′) | Amplicon size (bp) | Accession no. |
|---|---|---|---|
| NFκB (p100/p52)-F | GCT GAT GGC ACA GGA CGA GA | 103 | NM019408.2 |
| NFκB (p100/p52)-R | AGC GTG ATA AAT GAC GTG GGC TA | ||
| TNF-α-F | AAG CCT GTA GCC CAC GTC GTA | 122 | NM013693 |
| TNF-α-R | GGC ACC ACT AGT TGG TTG TCT TTG | ||
| IL-1β-F | TCC AGG ATG AGG ACA TGA GCA C | 105 | NM008361 |
| IL-1β-R | GAA CGT CAC ACA CCA GCA GGT TA | ||
| RANKL-F | CAT GTG CCA CTG AGA ACC TTG AA | 107 | NM011613 |
| RANKL-R | CAG GTC CCA GCG CAA TGT AAC | ||
| COX-2-F | GCC AGG CTG AAC TTC GAA ACA | 91 | NM011198 |
| COX-2-R | GCT CAC GAG GCC ACT GAT ACC TA | ||
| GAPDH-F | TGT GTC CGT CGT GGA TCT GA | 150 | NM001001303 |
| GAPDH-R | TTG CTG TTG AAG TCG CAG GAG |
NFκB: nuclear factor-kappa B, TNF: tumor-necrosis factor, IL: interleukin, RANKL: receptor activator of nuclear factor kappa-B ligand, COX: cyclooxygenase, GAPDH: glyceroaldehyde-3-phosphate dehydrogenase, bp: base pair.
2.8. Bone Histomorphometry
The retrieved calvariae were fixed with 70% ethanol, stained with Villanueva bone stain for 7 days, dehydrated in graded concentrations of ethanol, and embedded in methyl methacrylate (Wako Chemicals, Kanagawa, Japan) without decalcification. Plastic blocks of 200 μm thick midfrontal sections of the calvaria were cut with a precision bone saw such that the midline suture was in the center of the cross-section. Sections were mounted on plastic slides and ground to a thickness of 15 μm using a precision lapping machine (Maruto, Tokyo, Japan) and hand ground according to the method of Frost [23]. The sections were analyzed using a semiautomatic image analyzing system (System Supply, Nagano, Japan) and a fluorescent microscope (Optiphot; Nikon, Tokyo, Japan) set at a magnification of 200x. The parameters measured for bone resorption were the number of osteoclasts per bone surface (N.Oc/BS, /mm), the osteoclast surface per bone surface (Oc.S/BS, %), and the eroded surface per bone surface (ES/BS, %).
2.9. Statistics
The data were evaluated for statistical significance by one-way analysis of variance (ANOVA) using Fisher's test as a post hoc test. The analysis of correlation was performed using the Pearson's product moment correlation coefficient. A P value of <.05 was considered to be statistically significant.
3. Results
3.1. Loading of PE Particles onto the Calvaria Induces Inflammatory Reaction and Osteoclastogenesis
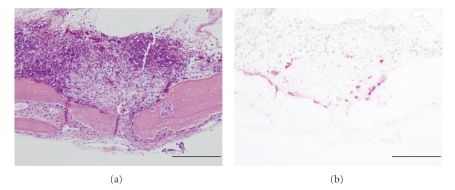
Histological evaluation of murine calvaria on day 7 after loading of 5 mg of PE particles revealed the formation of fibrous granulomatous tissues centered around the sagittal suture area, which was accompanied by massive bone resorption and the formation of osteoclasts bordering the cortex (Figures 1(a) and 1(b)).
Figure 1.
Histological analysis of murine calvaria retrieved on day 7 after PE particle implantation. Midfrontal sections of parietal bone were made as described in Section 2. Magnification x100 and scale bar represents 200 μm in all figures. A representative of five murine calvarial samples is shown. (a) Hematoxylin/eosin (HE). (b) Tartrate-resistant acid phosphatase (TRAP) staining.
3.2. Loading of PE Particles onto the Calvaria Increases Luminescence
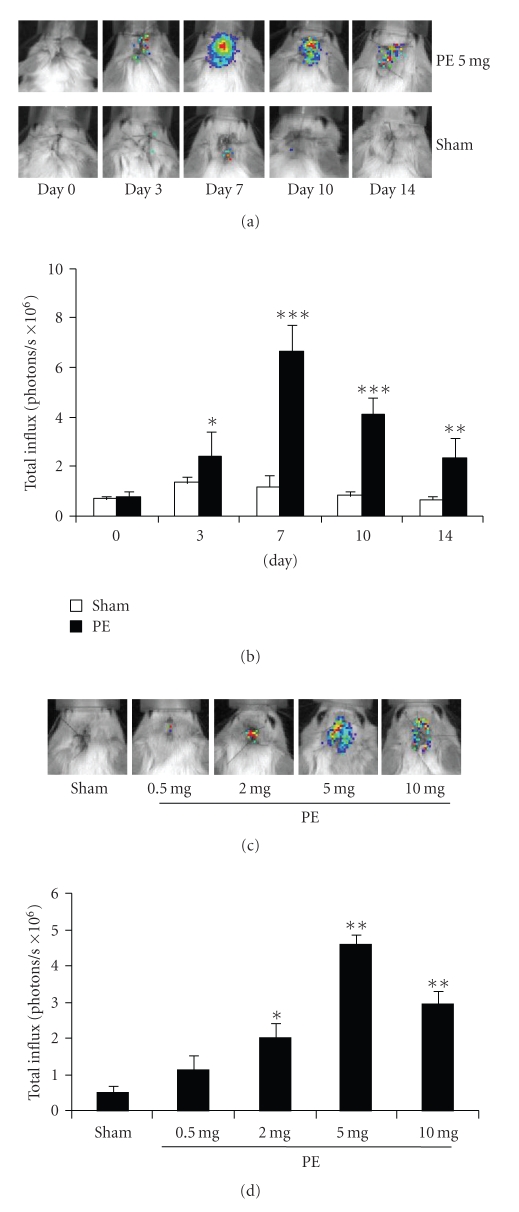
In vivo imaging at the indicated number of days after loading of 5 mg PE particles showed a prominent increase in luminescence, reaching a maximum on day 7 (Figure 2(a)). In the sham group, only a slight increase in luminescence was observed. Quantitative analysis showed a significant increase in total influx in calvariae that received PE particles than those that received sham surgery (Figure 2(b), *P < .05, **P < .001, ***P < .0001 versus sham at each time point). Imaging analysis performed on day 7 after loading of PE particles showed a dose-dependent increase in luminescence, reaching a maximum at 5 mg (Figure 2(c)). Quantitative analysis showed a significant increase in luminescence in response to loading of more than 2 mg of particles, reaching a maximum at 5 mg (Figure 2(d), *P = .0005, **P < .0001 versus sham).
Figure 2.
(a) In vivo imaging analysis was performed on the indicated days after loading of 5 mg PE. (b) Quantitative analysis of luminescence in (a) (n = 3 per bar, Mean ± SD, *P < .05, **P < .001, ***P < .0001, 5 mg PE versus sham at each time point). (c) In vivo imaging analysis performed on day 7 after loading of the indicated amounts of PE. (d) Quantitative analysis of luminescence in (c) (n = 3 per bar, Mean ± SD, *P = .0005, **P < .0001 versus sham).
3.3. Exposure to PE Particles Increases Calvaria Luciferase Activity, which Is Positively Correlated with Total Influx
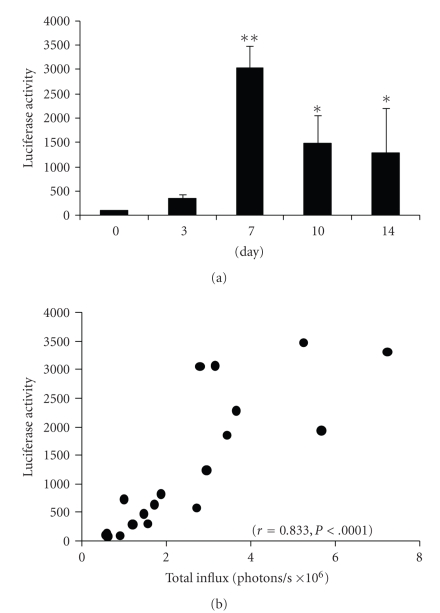
Luciferase activity was significantly enhanced on days 7, 10, and 14 (Figure 3(a), *P < .05, **P < .0001 versus values on day 0). We examined the correlation between luminescence and luciferase activity, and found that the levels of total influx were positively correlated with the luciferase activity of the calvariae (Figure 3(b), n = 19, r = 0.833, P < .0001), indicating that luminescence actually reflected levels of luciferase activity.
Figure 3.
(a) Luciferase activity of calvarial tissues retrieved on the indicated days and then subjected to a luciferase assay (n = 3–5 per bar, Mean ± SD, *P < .05, **P < .0001 versus value on day 0). (b) Correlation between total influx and luciferase activity of the calvariae (n = 19, r = 0.833, P < .0001).
3.4. Exposure to PE Particles Induces Upregulation of mRNA for NFκB (p100/p52) and Bone-Resorbing Mediators, All of which Are Positively Correlated with Total Influx
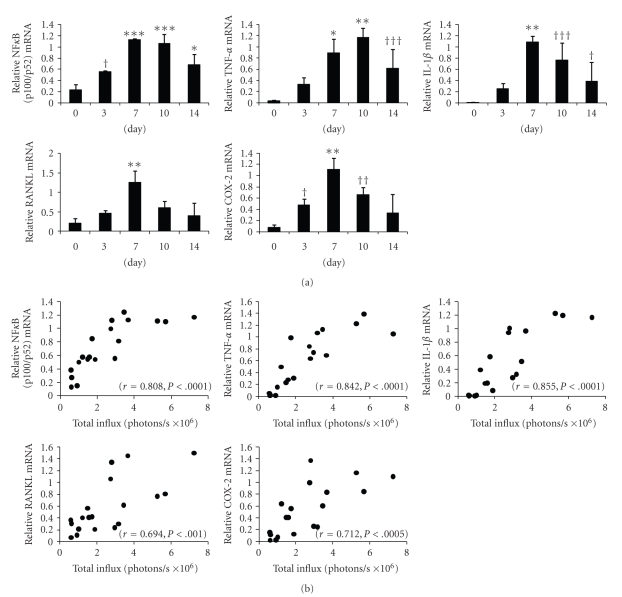
NFκB/Rel transcription factors regulate their own synthesis. The promoter regions of nfkb contain binding sites for NFκB that respond positively to activation of NFκB/Rel [24]. To test if NFκB is actually activated after particle loading, we evaluated NFκB (p100/p52) mRNA expression. We also investigated mRNA levels of downstream bone-resorbing mediators TNF-α, IL-1β, RANKL, and cyclooxygenase (COX)-2, an inducible form of COX and is induced at inflammation sites and produces proinflammatory PGs. These mediators were nominated because of their pivotal role especially in murine osteolysis models, since selective inhibition of these factors were reported to result in the suppression of osteolysis using murine osteolysis models [25–28]. Real-time RT-PCR showed that NFκB (p100/p52) mRNA level was significantly upregulated on days 3 to 14. The mRNA levels of TNF-α, IL-1β, RANKL, and COX-2 were also significantly upregulated (Figure 4(a), †P < .05, ††P < .01, †††P < .005 *P < .001, **P < .0005, ***P < .0001, versus value on day 0).
Figure 4.
(a) The mRNA levels of NFκB (p100/p52), TNF-α, IL-1β, RANKL, and COX-2 in calvarial tissues retrieved on the indicated days and subjected to real-time RT-PCR (n = 3–5 per bar, Mean ± SD, †P < .05, ††P < .01, †††P < .005 *P < .001, **P < .0005, ***P < .0001 versus value on day 0). (b) Correlation between total influx and mRNA levels of NFκB (p100/p52), TNF-α, IL-1β, RANKL, and COX-2. The correlation coefficient and P values were r = 0.808, P < .0001 for NFκB (p100/p52); r = 0.842, P < .0001 for TNF-α; r = 0.855, P < .0001 for IL-1β; r = 0.694, P < .001 for RANKL; r = 0.712, P < .0005 for COX-2.
We examined the correlation between luminescence and mRNA levels, and found that the mRNA levels of all these factors were positively correlated with total influx. The correlation coefficient and P values were r = 0.808, P < .0001 for NFκB (p100/p52); r = 0.842, P < .0001 for TNF-α; r = 0.855, P < .0001 for IL-1β; r = 0.694, P < .001 for RANKL; and r = 0.712, P < .0005 for COX-2 (Figure 4(b)).
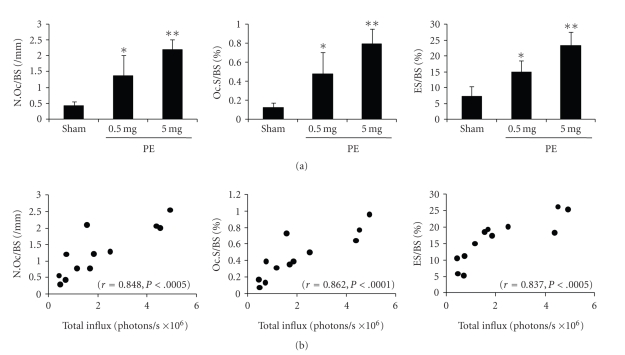
3.5. Exposure to PE Particles Increases Bone Resorption Parameters, All of which Are Positively Correlated with Total Influx
Most studies using murine calvarial model have evaluated bone resorption on day 7 after particle implantation, which is consistent with the report that osteolysis is reported to reach maximum on this day in this model [29]. Accordingly, to investigate the correlation between luminescence and osteolysis, we evaluated bone resorption on day 7. All the parameters for bone resorption were significantly enhanced by loading of more than 0.5 mg of PE particles (Figure 5(a), *P < .05, **P < .005, versus sham). We examined the correlation between luminescence and levels of bone resorption parameters, and found that the levels of these parameters were positively correlated with total influx. The correlation coefficients and P values were r = 0.848, P < .0005 for N.Oc/BS; r = 0.862, P < .0001 for Oc.S/BS; and r = 0.837, P < .0005 for ES/BS (Figure 5(b)).
Figure 5.
(a) Levels of bone resorption parameters (N.Oc/BS, Oc.S/BS, and ES/BS) as assessed by bone histomorphometry in calvarial tissues retrieved on day 7 after PE loading (n = 4 per bar, Mean ± SD, *P < .05, **P < .005 versus sham). (b) Correlation between total influx and levels of bone resorption parameters. The correlation coefficient and P values were r = 0.848, P < .0005 for N.Oc/BS; r = 0.862, P < .0001 for Oc.S/BS; r = 0.837, P < .0005 for ES/BS.
4. Discussion
Understanding the complex cellular and tissue mechanisms and interactions which occur in periprosthetic osteolysis requires multiple experimental approaches, each of which has its own set of advantages and limitations [30]. Among murine osteolysis models, calvarial model was developed to investigate the biology of wear debris-induced osteolysis independent of the critical mechanical and biomechanical components of aseptic loosening [31]. This model is limited by the fact that it does not exactly mimic the clinical situation responsible for osteolysis in humans, as the particles are implanted at a single-time point and the calvaria is a flat, membranous bone that is not exposed to synovial fluid. Nevertheless, this murine model is frequently used because it permits the use of a large array of molecular reagents and genetically defined strains and of highly quantitative outcome measures of osteoclast formation and bone resorption. Using this approach, a variety of laboratories has shown the prophylactic effects of inhibitors for TNF, RANKL, and COX-2, and of chemical agents, including bisphosphonates. Generally, the results of these experiments have been generated mainly by histological or molecular analysis. Accordingly, the use of many animals has been unavoidable for the evaluation of temporal changes after particle loading. Also, it has been difficult to observe any real-time changes that may occur in a single mouse.
In vivo imaging is an extremely useful approach that allows researchers to monitor biological reactions and evaluate the efficacy of drugs without sacrificing experimental animals. This technique, which allows the real-time visualization of pathological or physiological conditions including inflammation, tissue regeneration, angiogenesis, neoplasm, and others, has been widely used for the evaluation of experimental disease models; however, there have been few reports looking at particle-induced osteolysis using this technique. Very recently, Ren et al. published a report on the trafficking of intravenously administered luciferase-expressing macrophages after injection of cement particles in the femoral cavity of nude mice [32]. This report, which showed significant accumulation of macrophages at the femoral site where particles were loaded, may be the first one that observed particle-induced inflammation using in vivo imaging. NFκB/luc tg mouse, which was first reported in 2002 and which has since been widely used for the evaluation of arthritis, sepsis, ultraviolet-induced dermatitis, and other diseases [33], is very useful for the assessment of inflammatory disease models. In this study, we were able to use this mouse to detect luminescence in the murine calvarial osteolysis model using PE particles. The luminescence reached maximum on day 7 and then gradually decreased, which was correlated with the kinetic change of mRNA levels of NFκB (p100/p52) and downstream proinflammatory mediators. These suggested that the luminescence reflected the activation of NFκB and subsequent inflammation. We also examined the correlation between bone resorption parameters and luminescence on day 7 and found that all these parameters were positively correlated with total influx. This suggested that the luminescence also reflected bone resorption.
Microcomputed tomography (μCT) is a useful and promising method to precisely evaluate the amount of osteolysis induced by particle loading. Recently, Tsutsumi et al. evaluated the effect of bisphosphonate and osteoprotegerin on particle-induced osteolysis by this method [34]. In evaluating the amount of bone resorption, μCT might be superior to in vivo imaging. On the other hand, the advantage of in vivo imaging is that it can provide molecular or biochemical information that are related to osteolysis, such as promoter activity of proinflammatory cytokines, enzymatic activity of proteases, and cellular trafficking of ex vivo administered cells depending on the need. Moreover, it might be more time saving than μCT in that it acquires images almost in a moment. Therefore, these two methods should be compatible when evaluating experimental osteolysis model.
We found in this study that the luminescence was weaker in mice that received 10 mg of PE than in those that received 5 mg of PE; however, the inflammatory reaction in mice loaded with 10 mg of PE was similar to that in mice loaded with 5 mg with respect to mRNA levels of inflammatory cytokines (data not shown), which leads us to conclude that the thickness of the PE layer resulting from loading of greater amounts of PE particles might have attenuated the luminescence. The attenuation of light photons is the biggest obstacle in optical imaging. Approximately 90% of bioluminescence signal flux is lost per each centimeter of tissue thickness, and thus, the photon intensities detected by CCD cameras may not accurately reflect endogenous reporter gene expression in the inner organs [35]. Thus, it is important to be aware of this limitation, and to take into consideration the nature or the quantity of particles loaded.
5. Conclusion
We have performed in vivo imaging analysis of the murine calvarial osteolysis model using NFκB/luc tg mice to visualize the inflammatory reaction and osteolysis caused by foreign particles. This system could be a useful tool for screening therapeutic approaches to treat particle-induced inflammation and osteolysis.
Acknowledgments
The present paper was performed under the support of Health and Labor Sciences Research Grants in Research on Chemical Substance Assessment from the Ministry of Health, Labor and Welfare of Japan (Principle investigator: F. Watari Ph.D.,H18-Chemistry-General-006). This paper was also supported by a Grant-in-Aid from the Ministry of Science and Education of Japan (19591746).
References
- 1.Friedman RJ, Black J, Galante JO, Jacobs JJ, Skinner HB. Current concepts in orthopaedic biomaterials and implant fixation. Instructional Course Lectures. 1994;43:233–255. [PubMed] [Google Scholar]
- 2.Amstutz HC, Campbell P, Kossovsky N, Clarke IC. Mechanism and clinical significance of wear debris-induced osteolysis. Clinical Orthopaedics and Related Research. 1992;276:7–18. [PubMed] [Google Scholar]
- 3.Sabokbar A, Fujikawa Y, Neale S, Murray DW, Athanasou NA. Human arthroplasty derived macrophages differentiate into osteoclastic bone resorbing cells. Annals of the Rheumatic Diseases. 1997;56(7):414–420. doi: 10.1136/ard.56.7.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neale SD, Sabokbar A, Howie DW, Murray DW, Athanasou NA. Macrophage colony-stimulating factor and interleukin-6 release by periprosthetic cells stimulates osteoclast formation and bone resorption. Journal of Orthopaedic Research. 1999;17(5):686–694. doi: 10.1002/jor.1100170510. [DOI] [PubMed] [Google Scholar]
- 5.Wooley PH, Petersen S, Song Z, Nasser S. Cellular immune responses to orthopaedic implant materials following cemented total joint replacement. Journal of Orthopaedic Research. 1997;15(6):874–880. doi: 10.1002/jor.1100150613. [DOI] [PubMed] [Google Scholar]
- 6.Chiba J, Rubash HE, Kim KJ, Iwaki Y. The characterization of cytokines in the interface tissue obtained from failed cementless total hip arthroplasty with and without femoral osteolysis. Clinical Orthopaedics and Related Research. 1994;300:304–312. [PubMed] [Google Scholar]
- 7.Jones LC, Frondoza C, Hungerford DS. Immunohistochemical evaluation of interface membranes from failed cemented and uncemented acetabular components. Journal of Biomedical Materials Research. 1999;48(6):889–898. doi: 10.1002/(sici)1097-4636(1999)48:6<889::aid-jbm19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Goodman SB, Chin RC, Chiou SS, Schurman DJ, Woolson ST, Masada MP. A clinical-pathologic-biochemical study of the membrane surrounding loosened and nonloosened total hip arthroplasties. Clinical Orthopaedics and Related Research. 1989;244:182–187. [PubMed] [Google Scholar]
- 9.Horiki M, Nakase T, Myoui A, et al. Localization of RANKL in osteolytic tissue around a loosened joint prosthesis. Journal of Bone and Mineral Metabolism. 2004;22(4):346–351. doi: 10.1007/s00774-003-0493-8. [DOI] [PubMed] [Google Scholar]
- 10.Baeuerle PA, Baltimore D. Nf-κB: ten years after. Cell. 1996;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen F, Castranova V, Shi X. New insights into the role of nuclear factor-κB in cell growth regulation. American Journal of Pathology. 2001;159(2):387–397. doi: 10.1016/s0002-9440(10)61708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80(4):529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 13.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annual Review of Immunology. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima Y, Sun D-H, Trindade MCD, et al. Signaling pathways for tumor necrosis factor-α and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. Journal of Bone and Joint Surgery. American. 1999;81(5):603–615. doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz EM, Lu AP, Goater JJ, et al. Tumor necrosis factor-α/nuclear transcription factor-κB signaling in periprosthetic osteolysis. Journal of Orthopaedic Research. 2000;18(3):472–480. doi: 10.1002/jor.1100180321. [DOI] [PubMed] [Google Scholar]
- 16.Franzoso G, Carlson L, Xing L, et al. Requirement for NF-κB in osteoclast and B-cell development. Genes and Development. 1997;11(24):3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nature Medicine. 1997;3(11):1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 18.Clohisy JC, Hirayama T, Frazier E, Han S-K, Abu-Amer Y. NF-kB signaling blockade abolishes implant particle-induced osteoclastogenesis. Journal of Orthopaedic Research. 2004;22(1):13–20. doi: 10.1016/S0736-0266(03)00156-6. [DOI] [PubMed] [Google Scholar]
- 19.Ren W, Li XH, Chen BD, Wooley PH. Erythromycin inhibits wear debris-induced osteoclastogenesis by modulation of murine macrophage NF-κB activity. Journal of Orthopaedic Research. 2004;22(1):21–29. doi: 10.1016/S0736-0266(03)00130-X. [DOI] [PubMed] [Google Scholar]
- 20.Ho T-Y, Chen Y-S, Hsiang C-Y. Noninvasive nuclear factor-κB bioluminescence imaging for the assessment of host-biomaterial interaction in transgenic mice. Biomaterials. 2007;28(30):4370–4377. doi: 10.1016/j.biomaterials.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Wedemeyer C, Neuerburg C, Pfeiffer A, et al. Polyethylene particle-induced bone resorption in substance P-deficient mice. Calcified Tissue International. 2007;80(4):268–274. doi: 10.1007/s00223-007-9005-5. [DOI] [PubMed] [Google Scholar]
- 22.Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-α mediates orthopedic implant osteolysis. American Journal of Pathology. 1999;154(1):203–210. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frost HM. Preparation of thin undercalcified bone sectios by rapid manual method. Stain Technology. 1958;33(6):272–276. doi: 10.3109/10520295809111862. [DOI] [PubMed] [Google Scholar]
- 24.Liptay S, Schmid RM, Nabel EG, Nabel GJ. Transcriptional regulation of NF-κB2: evidence for κB-mediated positive and negative autoregulation. Molecular and Cellular Biology. 1994;14(12):7695–7703. doi: 10.1128/mcb.14.12.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Morham SG, Langenbach R, et al. Evidence for a direct role of cyclo-oxygenase 2 in implant wear debris-induced osteolysis. Journal of Bone and Mineral Research. 2001;16(4):660–670. doi: 10.1359/jbmr.2001.16.4.660. [DOI] [PubMed] [Google Scholar]
- 26.Childs LM, Goater JJ, O’Keefe RJ, Schwarz EM. Efficacy of etanercept for wear debris-induced osteolysis. Journal of Bone and Mineral Research. 2001;16(2):338–347. doi: 10.1359/jbmr.2001.16.2.338. [DOI] [PubMed] [Google Scholar]
- 27.Childs LM, Paschalis EP, Xing L, et al. In vivo RANK signaling blockade using the receptor activator of NF-κB:Fc effectively prevents and ameliorates wear debris-induced osteolysis via osteoclast depletion without inhibiting osteogenesis. Journal of Bone and Mineral Research. 2002;17(2):192–199. doi: 10.1359/jbmr.2002.17.2.192. [DOI] [PubMed] [Google Scholar]
- 28.Yang S-Y, Wu B, Mayton L, et al. Protective effects of IL-1Ra or vIL-10 gene transfer on a murine model of wear debris-induced osteolysis. Gene Therapy. 2004;11(5):483–491. doi: 10.1038/sj.gt.3302192. [DOI] [PubMed] [Google Scholar]
- 29.Kaar SG, Ragab AA, Kaye SJ, et al. Rapid repair of titanium particle-induced osteolysis is dramatically reduced in aged mice. Journal of Orthopaedic Research. 2001;19(2):171–178. doi: 10.1016/S0736-0266(00)00033-4. [DOI] [PubMed] [Google Scholar]
- 30.Bostrom M, O'Keefe R. What experimental approaches (eg, in vivo, in vitro, tissue retrieval) are effective in investigating the biologic effects of particles? The Journal of the American Academy of Orthopaedic Surgeons. 2008;16(supplement 1):S63–S67. doi: 10.5435/00124635-200800001-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wooley PH, Schwarz EM. Aseptic loosening. Gene Therapy. 2004;11(4):402–407. doi: 10.1038/sj.gt.3302202. [DOI] [PubMed] [Google Scholar]
- 32.Ren P-G, Lee S-W, Biswal S, Goodman SB. Systemic trafficking of macrophages induced by bone cement particles in nude mice. Biomaterials. 2008;29(36):4760–4765. doi: 10.1016/j.biomaterials.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlsen H, Alexander G, Austenaa LMI, Ebihara K, Blomhoff R. Molecular imaging of the transcription factor NF-κB, a primary regulator of stress response. Mutation Research. 2004;551(1-2):199–211. doi: 10.1016/j.mrfmmm.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Tsutsumi R, Hock C, Bechtold CD, et al. Differential effects of biologic versus bisphosphonate inhibition of wear debris-induced osteolysis assessed by longitudinal micro-CT. Journal of Orthopaedic Research. 2008;26(10):1340–1346. doi: 10.1002/jor.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang JH, Chung J-K. Molecular-genetic imaging based on reporter gene expression. Journal of Nuclear Medicine. 2008;49(supplement 2):164S–179S. doi: 10.2967/jnumed.107.045955. [DOI] [PubMed] [Google Scholar]