Abstract
Background
Recent work suggested a role for NF-kB in the propagation of ovarian cancer cell lines, but the significance and mechanism of NF-kB in ovarian cancer is unknown. We hypothesized that the NF-kB pathway is over-activated in aggressive ovarian cancers.
Methods
We assessed the levels of three NF-kB transcription factors, the activating IkB kinases and the NF-kB target MMP-9 by immunohistochemistry in ovarian cancer specimens obtained at diagnosis from a cohort of 33 patients subsequently treated with paclitaxel, cisplatin, and cyclophosphamide. Associations were made between NF-kB pathway proteins and outcome. Validation of co-expression was performed at the gene level in two independently collected cohorts of 185 and 153 ovarian cancers, respectively.
Results
We established the presence of NF-kB proteins in newly diagnosed advanced ovarian cancers, and identified a potential association with overall survival. Transcription factors p65 and RelB were co-expressed with IKKα, one component of a key tri-molecular regulatory complex. Co-expression of the NF-kB machinery suggests activity of NF-kB signaling in these ovarian tumors. A significant association of p50 with poor overall survival was found (p=0.02). MMP9 expression showed the opposite relationship, where cases without MMP9 staining had the poorest prognosis (p=0.01), and this relationship held true at the gene expression level in an independently collected cohort of 185 ovarian cancers.
Conclusions
Deregulation of NF-κB activity may influence outcome in women treated with standard therapy for advanced ovarian cancer. Modification of the pathway could present an opportunity to improve outcome in the subset of women showing activity of the pathway.
Keywords: NF-κB, ovarian cancer, immunohistochemistry, survival, p50, MMP9, prognosis
INTRODUCTION
The NF-κB family of transcription factors is expressed in many tissue types, and has been studied extensively in lymphoid development and lymphoid malignancies. Constitutive NF-κB signaling also has been identified in tumors of epithelial origin including breast, colon, lung and ovarian carcinomas 1. Recent work suggested the importance of NF-κB in the propagation of ovarian cancer cell lines 2, but the significance and the mechanism of NF-κB signaling in ovarian cancer remains unknown.
We hypothesized that the NF-κB pathway would be over-activated in ovarian cancers with more aggressive behavior. Evidence supporting this hypothesis was derived from ovarian cancer cell lines and malignant ascites. The NF-κB pathway was implicated in ovarian cancer proliferation and cytokine secretion in vitro 2, 3, and may also contribute to chemoresistance of ovarian cancer cell lines 3–5. We therefore sought to determine the expression patterns and prognostic associations of NF-κB pathway proteins in primary ovarian cancer tissues.
The NF-κB transcription factor family consists of five subunits that join into active dimers. Homo- or hetero-dimers form the active transcription factor complex, which is retained in the cytoplasm by Inhibitors of NF-κB (I-κBs). The transcription factors are released once I-κBs are phosphorylated by I-κB kinases (IKKs) upon activation by upstream stimuli. The tumor microenvironment triggers intracellular NF-κB activation by diverse cell surface receptors, providing a link between inflammation and cancer 6. Specific inducible phosphorylation by IKKs targets the I-κBs for degradation through the proteasome. For this reason, the proteasome inhibitor bortezomib is under evaluation in clinical trials attempting to block NF-κB mediated chemoresistance and re-sensitize ovarian cancers to platinum agents 7.
Active NF-κB transcription factors alter transcription of specific target genes involved in a wide array of cellular functions including proliferation, angiogenesis and metastasis 8. MMP9 is a known target gene that contains the NF-κB consensus sequence in its promoter 9. This matrix metalloproteinase has been linked to angiogenesis and metastasis in mouse xenograft models of ovarian cancer 10, 11, prompting its consideration in the current study.
We examined the cellular expression frequency of three NF-κB subunits, the activating kinases IKKα, IKKβ, IKKε, and the NF-κB target gene MMP-9 by immunohistochemistry in an independent and blinded set of ovarian cancer specimens obtained at diagnosis. The cohort is a set of 33 patients subsequently treated with a three-drug chemotherapy clinical trial of paclitaxel, cisplatin, and cyclophosphamide at the National Cancer Institute (Sarosy, et al. in press). Outcome data was available with up to 9-year follow-up, and the association between outcomes and expression of NF-κB pathway proteins was determined. Our analysis was extended to two independent gene expression datasets in order to further validate the associations discovered in the protein expression of these factors.
PATIENTS AND METHODS
Patients
Women with advanced stage newly diagnosed epithelial ovarian cancer were treated between 1995 and 2001 using a triple-drug regimen of cisplatin, high dose paclitaxel and cyclophosphamide (12, 13 and Sarosy et al, in press). Briefly, patients received cyclophosphamide 750 mg/m2 IV on day 1, paclitaxel 250 mg/m2 24-hour infusion beginning on day 1, and cisplatin 75 mg/m2 on day 2. Cycles were repeated every 21 days. Tissue blocks of primary and/or metastatic disease from the initial staging and cytoreductive surgery were collected according to the NCI IRB-approved protocol and consent. Tissue blocks from 33 of the 62 enrolled patients (Table 1) were available and contained tumor tissue adequate for immunohistochemical staining.
Table 1.
Patient Characteristics
| Characteristic | Patients (N=33) |
|---|---|
| Age at diagnosis | |
| 40–49 | 7 |
| 50–59 | 17 |
| 60–69 | 6 |
| >70 | 3 |
| Stage | |
| III | 20 |
| IV | 13 |
| Grade | |
| 2 | 6 |
| 3 | 27 |
| Histologic subtype | |
| Serous | 22 |
| Endometrioid | 7 |
| Other | 4 |
| Response | |
| Complete | 29 |
| Partial | 2 |
| Stable | 2 |
| Median survival (mo) | |
| Overall | 65 |
| Progression-free | 15 |
Immunohistochemistry
Formalin-fixed paraffin-embedded sections were analyzed for protein expression of IKKα, IKKβ, IKKε, p50, p65, RelB, and MMP9 using immunohistochemistry. Staining was performed manually. Antibody specifications and staining conditions are detailed (Table 2). Negative controls consisted of sections that underwent similar staining procedures with a non-relevant antibody of the corresponding isotype. Positive controls consisted of solid ovarian carcinomas that demonstrated immunoreactivity for the studied antigens in a pilot study. Visualization was achieved using the EnVision ™ + peroxidase system (DAKO). Staining was considered positive and scored in either cytoplasmic or nuclear location for p50 and p65. RelB, IKKα, IKKβ, IKKε and MMP9 showed only cytoplasmic expression and were therefore scored only at this sub-cellular localization. Staining extent was scored on a scale of 0–4, as follows: 0=no staining, 1=1–5%, 2=6–25%, 3=26–75%, 4=76–100% of tumor cells. All sections contained a large number of viable tumor cells. Cases were scored by two experienced surgical pathologists (BD, AB) in a blinded fashion. Only tumor cells were scored.
Table 2.
NF-kB proteins investigated
| Protein | Gene symbol |
Function in NF-Kb pathway |
Antibody | Source | Dilution | Pre- treatment |
|---|---|---|---|---|---|---|
| P50 | NFKB1 | Transcription factor |
sc-1190 | Santa Cruz Biotechnology |
1:300 | Citrate |
| P65 | RELA | Transcription factor |
ab31481 | Abcam | 1:100 | TRSa |
| RelB | RELB | Transcription factor |
sc-226 | Santa Cruz Biotechnology |
1:200 | Citrate |
| IKKa | CHUK | Activating kinase |
sc-7218 | Santa Cruz Biotechnology |
1:50 | Citrate |
| IKKb | IKBKB | Activating kinase |
sc-8014 | Santa Cruz Biotechnology |
1:100 | Tris/EDTA |
| IKKe | IKBKE | Activating kinase |
sc-5694 | Santa Cruz Biotechnology |
1:100 | Citrate |
| MMP9 | MMP9 | Target gene | RB-1539 | Thermo Scientific |
1:100 | None |
TRS=Target Retrieval Solution (pH = 6) (Dako, Glostrup, Denmark)
Statistical methods
A Jonckheere-Terpstra trend test was used to determine statistical associations between the markers. All p-values for these associations are two-tailed and have not been adjusted for multiple comparisons. In view of the large number of comparisons in this exploratory analysis, only p< 0.005 should be interpreted as indicating statistically significant results, while those analyses for which 0.005 < p < 0.05 would reflect trends.
Reliable, complete clinical information was available on 31 of the 33 patients with available tissue blocks. We analyzed the relationships of age, survival and progression with categorical data from 9 parameters. The association between age and IHC score for each marker was determined using a Jonckheere-Terpstra test for trend if there were 3 or more categories to evaluate 14, or a Wilcoxon rank sum test if the markers were only in two categories. Associations between marker values and response (either classified as complete response (CR) vs. partial response (PR) vs. stable disease (SD) or CR vs. PR+SD) were determined using either the Jonckheere-Terpstra test for trend, a Cochran-Armitage trend test 15 or Fisher’s exact test, depending on the number of categories evaluated in each parameter.
The IHC data were typically grouped into categories to allow a minimum of 4 patients per category, and the resulting groupings were evaluated for their prognostic association with survival and progression free survival. Durations of survival and progression were computed from the on-study date until the date of death or progression, respectively, or until last known follow-up. The probability of survival or of progression-free survival was determined using the Kaplan-Meier method, and the log rank test was used to determine the statistical significance of the difference among Kaplan-Meier curves 16, 17. A Cox proportional hazards model was used to determine the statistical significance of two or more factors considered jointly on outcome, when appropriate 18. All p-values are two-tailed. The p-values are presented without adjustment for multiple testing performed, with the exception of cases in which adjusted p-values were based on pooling categories after identifying a potentially useful division in the data relative to survival.
Gene expression profiling
Data were obtained from GEO dataset GSE3149 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE3149) 19 and from personal communication 20. The following probe sets were used for analysis: MMP9 (203936_s_at), IKBKB (209341_s_at), CHUK (209666_s_at), IKBKE (204549_at), RELB (205205_at), NFKB1 (209239_at), NFKB2 (209636_at), RELA (209878_s_at). Samples were ordered by the average expression of the NF-kB transcription factors. Correlation with this value was calculated for each probe set. Strength of association (p-value) was calculated by using the website http://faculty.vassar.edu/lowry/ch4apx.html.
RESULTS
Patients and samples
The tissue samples analyzed in this study were collected prospectively from patients receiving initial therapy with cyclophosphamide, paclitaxel and cisplatin for epithelial ovarian cancer (12, 13 and Sarosy et al, in press). The regimen resulted in pathologic complete response or microscopic residual disease in 84% of patients. The median overall survival for all women enrolled on the clinical trial is 68 months and the median progression-free survival is 19.8 months. For the subset of 31 patients for whom tumor tissue and clinical data were available and adequate, the respective medians are 65.0 months and 14.7 months, which indicate that these patients are approximately representative of the larger group from which they were obtained. The patients were relatively homogenous in stage (all stage III/IV), grade (82% grade 3), and histology (67% serous; Table 1).
NF-κB transcription factors p65 and RelB are co-expressed in ovarian cancer
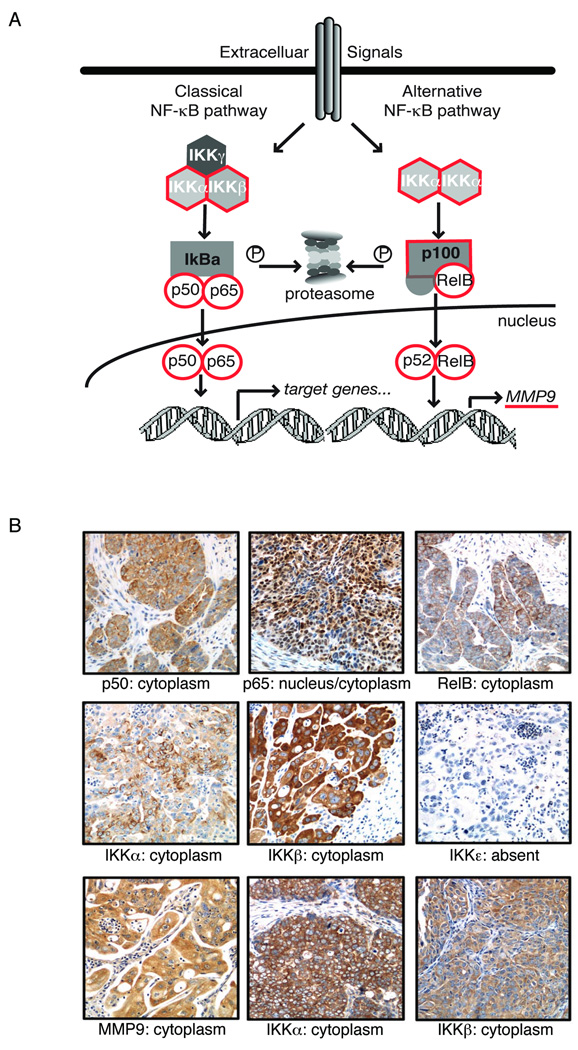
We investigated components of the NF-κB signaling pathway including three NF-κB subunits, the activating kinases IKKα, IKKβ, IKKε, and the NF-κB target gene MMP-9 (Figure 1A). Cellular frequency of expression was determined by immunohistochemistry (Table 2) in this blinded set of ovarian cancer specimens. Nine events were tabulated on the 33 evaluable cases: cytoplasmic or nuclear staining of p50 and p65 were quantified as individual events, whereas only cytoplasmic localization of RelB, IKKα, IKKβ, IKKε and MMP9 was identified and quantified (Table 3, and representative staining in Figure 1B). The two pathologists scoring the slides had good inter-observer agreement (80%), and discrepant cases (usually of one scoring level) were resolved in consensus session.
Figure 1.
NF-kB pathway proteins in ovarian cancer. A: Schematic diagram of NF-kB signaling components. B: Expression of NF-kB proteins in ovarian carcinoma using immunohistochemistry. Representative images of cytoplasmic localization of NF-kB p50; combined nuclear and cytoplasmic expression of NF-kB p65; cytoplasmic RelB expression; cytoplasmic expression of IKKa, IKKb and MMP-9, with no expression of IKKe in the same tumor.
Table 3.
Scoring of immunohistochemistry results
| Case | c-p65 | n-p65 | c-p50 | n-p50 | RelB | IKKa | IKKe | IKKb | MMP9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 3 | 3 | 0 | 2 | 2 | 0 | 2 | 3 |
| 2 | 4 | 3 | 2 | 0 | 3 | 4 | 0 | 3 | 4 |
| 3 | 2 | 3 | 3 | 0 | 3 | 0 | 0 | 4 | 0 |
| 4 | 3 | 3 | 2 | 4 | 4 | 4 | 0 | 3 | 4 |
| 5 | 0 | 1 | 4 | 0 | 2 | 3 | 0 | 4 | 3 |
| 6 | 0 | 3 | 2 | 0 | 0 | 3 | 0 | 4 | 0 |
| 7 | 3 | 1 | 1 | 0 | 2 | 2 | 0 | 4 | 2 |
| 8 | 0 | 2 | 2 | 0 | 0 | 2 | 0 | 4 | 1 |
| 9 | 3 | 4 | 4 | 0 | 4 | 4 | 0 | 4 | 4 |
| 11 | 2 | 1 | 0 | 0 | 2 | 3 | 0 | 0 | 4 |
| 12 | 4 | 3 | 3 | 0 | 4 | 4 | 0 | 3 | 4 |
| 13 | 3 | 1 | 4 | 0 | 3 | 1 | 0 | 3 | 1 |
| 14 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 4 | 1 |
| 15 | 1 | 3 | 3 | 0 | 1 | 1 | 0 | 3 | 3 |
| 16 | 1 | 3 | 3 | 0 | 2 | 4 | 0 | 4 | 0 |
| 17 | 0 | 3 | 2 | 0 | 2 | 3 | 0 | 4 | 2 |
| 18 | 1 | 4 | 4 | 0 | 3 | 4 | 0 | 4 | 3 |
| 19 | 4 | 3 | 2 | 0 | 4 | 4 | 0 | 4 | 4 |
| 20 | 3 | 3 | 4 | 0 | 3 | 3 | 0 | 3 | 4 |
| 21 | 3 | 3 | 4 | 0 | 4 | 4 | 1 | 3 | 4 |
| 27 | 0 | 1 | 3 | 0 | 0 | 2 | 0 | 4 | 3 |
| 29 | 0 | 3 | 3 | 0 | 0 | 2 | 0 | 4 | 0 |
| 30 | 2 | 1 | 1 | 1 | 4 | 4 | 0 | 1 | 3 |
| 31 | 4 | 4 | 4 | 0 | 3 | 4 | 0 | 4 | 3 |
| 32 | 3 | 3 | 3 | 0 | 4 | 3 | 0 | 3 | 2 |
| 34 | 1 | 3 | 0 | 0 | 1 | 3 | 0 | 2 | 4 |
| 35 | 0 | 2 | 1 | 0 | 1 | 2 | 0 | 3 | 1 |
| 36 | 0 | 2 | 3 | 0 | 2 | 1 | 0 | 3 | 4 |
| 40 | 4 | 3 | 2 | 0 | 4 | 4 | 0 | 4 | 3 |
| 41 | 4 | 3 | 4 | 0 | 4 | 3 | 0 | 2 | 4 |
| 42 | 0 | 1 | 1 | 0 | 3 | 3 | 0 | 4 | 3 |
| 43 | 4 | 1 | 4 | 0 | nd | 4 | 2 | nd | nd |
| 45 | 0 | 4 | 3 | 0 | 3 | 3 | 0 | 1 | 1 |
Staining was scored on a scale of 0–4: 0=no staining, 1=1–5%, 2=6–25%, 3=26–75%, 4=76–100% of tumor cells; nd = not done; c, cytoplasmic staining; n, nuclear staining; no designation indicates cytoplasmic staining only.
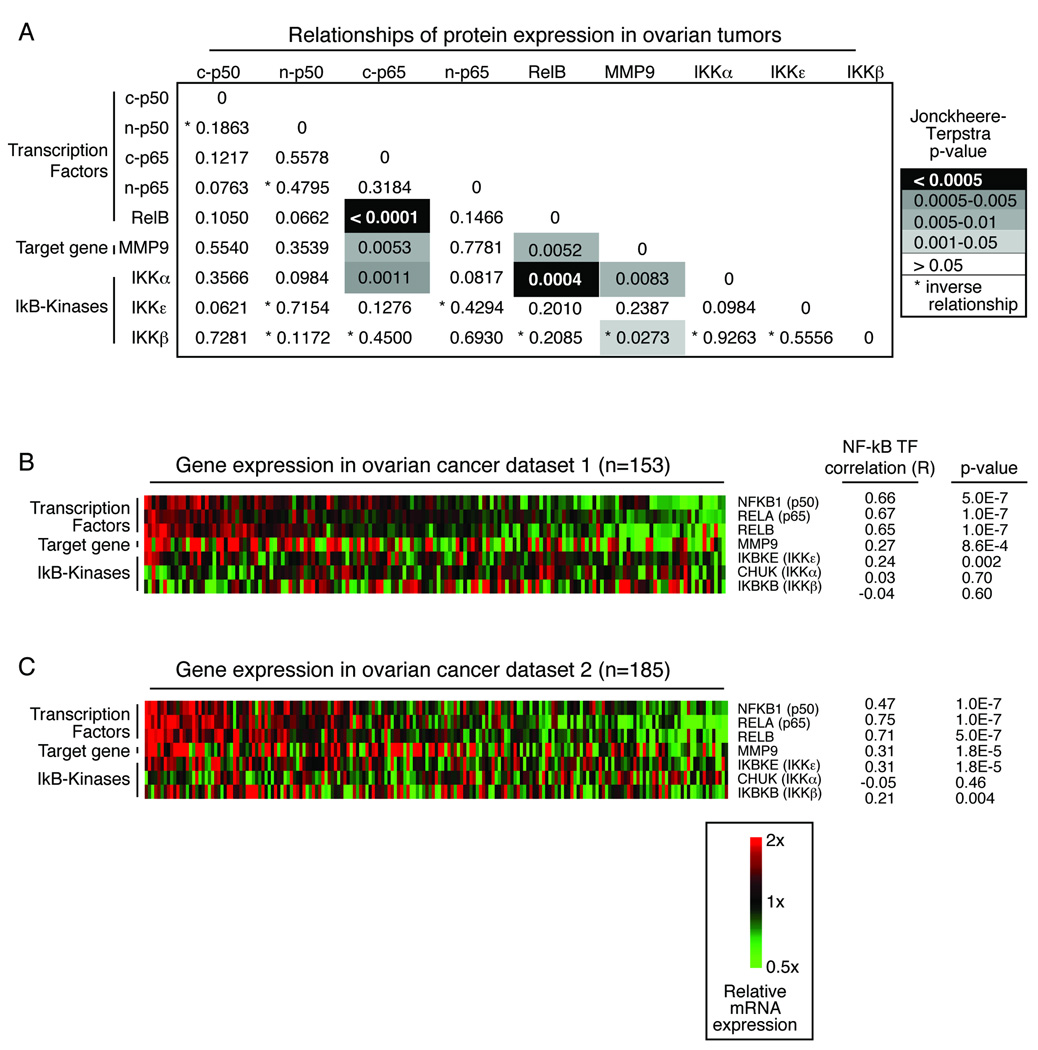
We evaluated relationships between expression levels of the individual proteins in the ovarian cancer specimens. The transcription factors p65 and RelB were closely co-expressed in the ovarian cancer specimens (Figure 2A). These two proteins were present together with IKKα, an activator of both classical and alternative NF-κB signaling 1. In view of the large number of comparisons in this exploratory analysis, only p< 0.005 should be interpreted as indicating statistically significant results, while those analyses for which 0.005 < p < 0.05 would reflect trends. As such, the trend towards co-expression of p65, RelB and IKKa with a known NF-κB target, MMP9, approached but did not reach statistical significance (p=0.0053, 0.0052 and 0.0083, respectively). These close relationships suggest that the NF-κB pathway may be active in a subset of ovarian cancers.
Figure 2.
Co-expression of NF-kB proteins in primary ovarian tumors. (A) Jonckheere-Terpstra p-values of immunohistochemistry results reported in Table 3 shows close association of NF-kB proteins. (B) Gene expression from an independent ovarian cancer dataset demonstrates a similar relationship between the transcription factors. The samples are ordered by the average expression of the transcription factors. Correlation (R) of each gene with the transcription factor average is listed, along with the p-value describing the strength of the relationship. (C) Similar analysis as (B) was calculated in a second unrelated dataset of gene expression in primary ovarian cancer samples.
Independent confirmation of activation of the NFKB pathway in ovarian cancer gene expression datasets
Associations between NF-κB components were evaluated at the gene expression level in two independent, publicly available ovarian cancer datasets (Figure 2B, C) 19, 20. An expression average was calculated for the three transcription factors NFKB1, RELA, and RELB for each dataset. Correlation (R) of each gene and level of significance (p) with the transcription factor average was calculated in order to compare the relationships seen in the two datasets (Figure 2B, 2C). In addition, correlations between individual genes were calculated within each dataset. An association between expression of the NF-κB transcription factors RELA (the gene for p65) and RELB was again evident in these two separate groups of patient samples collected at the time of initial diagnosis (dataset 1, p=6e-5; dataset 2, p=1e-7). The co-expression of RELB with MMP9 was highly significant (dataset 1, p=7e-5; dataset 2, p=5e-7), while a relationship between RELA and MMP9 was suggested (dataset 1, p=0.26; dataset 2, p=0.01). The relationship of RELA and RELB with CHUK (the gene for IKKα) expression was not re-capitulated in these datasets.
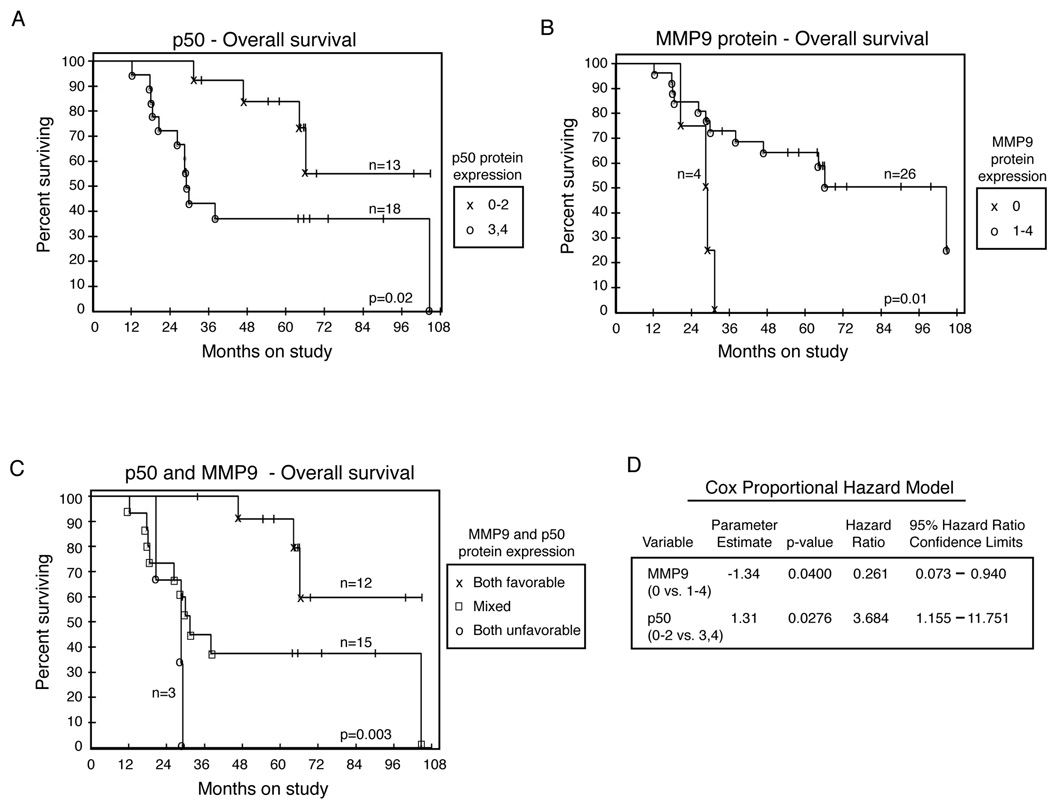
NF-κB transcription factor p50 is associated with poor survival in newly diagnosed patients
The immunhistochemistry data were grouped into categories to allow for a minimum of four patients per category. Each marker set was evaluated for relationships with overall survival and progression free survival. Elevated (3–4+, n=18) expression of c-p50 was potentially associated with shorter overall survival compared to cases with lower c-p50 (0–2+, n=13; Figure 3A, p=0.02 unadjusted; p=0.065 after adjustment). Cytoplasmic p65 was grouped into 2–3+ (n = 5) or 4+ (n = 25) as no case had fewer than 6% cells staining positive. Elevated expression of c-p65 trended toward shorter overall survival (p = 0.14) and progression-free survival (p = 0.05).
Figure 3.
NF-kB is associated with decreased overall and progression-free survival. (A–C) Kaplan-Meier plots of survival in ovarian cancer patients treated with triple therapy. (A) Median overall survival for patients with 3–4+ p50 staining was 29.1 months; median survival for patients with 0–2+ expression of p50 was not reached (p=0.02 unadjusted; p=0.065 after adjustment). (B) Median overall survival for patients with no MMP9 staining was 28.6 months; median survival for patients with 1–4+ expression of MMP9 was 67.0 months (p=0.01 unadjusted; p=0.02 after adjustment). (C) Combined analysis of p50 and MMP9 in ovarian cancer patients. Median survival for patients with unfavorable staining of both markers was 24.5 months; median survival for patients with favorable expression of both was not reached; median survival of the intermediate group was 30.4 months (p=0.003 for global comparison of all 3 arms); (D) Cox proportional hazards model for the immunoreactivity of MMP9 and p50 in ovarian cancer patients.
MMP9 protein levels showed the opposite relationship, where cases without MMP9 staining had the poorest prognosis (Figure 3B, p=0.01 unadjusted; p=0.02 after adjustment). A similar pattern, but to a lesser degree, was found in an independent dataset of 185 gene expression profiles (dataset 2) suggesting that lower MMP9 expression may be associated with shorter overall survival (data not shown).
The immunohistochemistry results indicate that the two proteins c-p50 and MMP9 are related to overall survival in patients with newly diagnosed ovarian cancer. Since each has an unadjusted p-value <0.05, the two were evaluated for their joint association with survival in a Cox proportional hazards model (Figure 3D). Each retained a p-value <0.05 when considered together and thus provide independent prognostic information for patients evaluated from the triple therapy clinical trial. Patients with both markers favorable (lower c-p50 and any MMP9 staining, n=15) had the best prognosis, while patients with both markers unfavorable (higher c-p50 and absent MMP9, n=3) had a dramatically shorter overall survival (Figure 3C, p=0.003). A similar level of significance was again evident when patients were grouped into two categories: those with both markers favorable (n=12, median survival not reached) and those with either marker unfavorable (n=18, median survival 29.1 months; p=0.0049).
DISCUSSION
NF-κB signaling contributes to the pathogenesis of cancers derived from either lymphoid or epithelial origin. We established the presence of the NF-κB pathway proteins in newly diagnosed advanced ovarian cancers, and investigated a potential link to overall survival in women treated on an experimental triple drug regimen. Interestingly, the transcription factors p65 and RelB were co-expressed with IKKα, one component of a key tri-molecular regulatory complex. This co-expression of the NF-κB machinery in the ovarian cancer tumor samples suggests NF-κB signaling occurs in a subset of tumors.
Immunohistochemistry is a semi-quantitative technique when intensity of staining is evaluated. In this analysis, however, we quantified number of cells that were positive, without introducing the subjective measure of staining intensity. The number of positive cells for a particular protein were categorized into 0–4+ and the results were evaluated as categorical variables. This method of evaluating immunohistochemical results has been used in several prior studies since it greatly reduces bias, and results in little inter-reviewer variability 21, 22. In this report, we extended our analyses to the expression of these factors in gene expression datasets in order to corroborate the immunohistochemical findings.
The co-expression of the NF-kB transcription factors was further validated in two independent datasets of gene expression in ovarian cancer specimens. Analyses of these large datasets were included in order to expand and independently verify the relationships that we found in a relatively smaller set of patient samples. Coordinate expression of the NF-kB transcription factors in both of these large datasets gives confidence to our analysis of the prospectively collected patient samples. Furthermore, the prognostic relationship of MMP9 loss was recapitulated at the gene expression level in the large set of 185 patients samples, where the survival data were available. This was an unexpected finding, since the expression of MMP9 in ovarian cancer models has been linked to poor outcome 23.
Paradoxically, the presence of any MMP9 in the ovarian cancer cells was associated with a better outcome in this group of patients compared to cases where MMP9 protein was not detected. MMP9 is a known target gene of NF-κB transcription factors, and thus was intended to serve as an indicator of NF-κB activity in ovarian cancer. Elevated MMP9 expression has been linked to enhanced angiogenesis and metastasis in several studies of ovarian cancer cell line models 10, 11, 24, 25. Increased expression of MMP9 in either the ovarian cancer cells or in the surrounding stromal cells has been associated with shorter disease-free and overall survival in advanced staged ovarian cancers 23. Thus, the opposite trend in prognosis with MMP9 in the patients on our clinical trial, therefore, was unexpected.
The trend of low MMP9 and poor prognosis, however, was corroborated by a large independent dataset of gene expression in 185 serous ovarian cancer specimens and suggests that this may be a unique subset of advanced stage ovarian cancers that depend upon other proteases for their invasive behavior. Such behavior has been demonstrated in the setting of MMP-9 knock-out, where increased angiogenesis and accelerated growth of tumors was observed 26. Given the retrospective nature of these studies, this unexpected finding is exploratory and warrants confirmation in a larger set of samples.
The cases included in the present analysis are relatively homogeneous with respect to advanced stage and grade. We thus sought to differentiate among the aggressive tumors and found that p50 was best at distinguishing prognostic groups. Frequent expression of NF-κB transcription factor p50 heralded a poor prognosis, which was worsened in the absence of MMP9. It is possible then, that loss of MMP9 signifies further de-differentiation of these already high-grade tumors and identifies a unique and differently invasive subset.
Inflammation in the microenvironment can contribute to NF-κB pathway activation in both the tumor and the stroma as well as resulting from NFKB activation. The NFKB pathway in these cancers was scored specifically in the tumor cells within the specimens. However, inflammatory signals may stimulate tumor growth and promote invasive behavior via the NF-κB pathway. The expression of TLR4 and MyD88 in ovarian cancer cells may facilitate transmission of environmental stimuli to NF-κB activation in tumors 3. In addition to stimulating growth and invasion of ovarian cancer, over-activation of NF-κB leads to resistance to standard chemotherapy agents including paclitaxel and cisplatin 3–5, two of the drugs tested in the clinical trial on which the current cohort of patients were treated. Our current work demonstrated a potentially interesting association of p50 with poor overall survival in women treated with cisplatin, paclitaxel and cyclophosphamide. This finding is consistent with NF-κB signaling as a mechanism for platinum and/or taxane resistance in ovarian cancer.
Nuclear p50 homodimers in tumor-associated macrophages can indicate suppression of anti-tumor response 27. In our study, tumor cell cytosolic p50 provided the prognostic information. It is plausible that feedback mechanisms are in place where NFKB1 (the gene for p50) is a direct target gene of p65, and becomes up-regulated for the purpose of attenuating the cascade. This feedback mechanism may be defective, however, in the ovarian cancer cells, leading to accumulation of p50 homodimers in the cytoplasm that fail to down-regulate NF-kB-targeted pro-survival factors in the ovarian cells. This phenomenon has been documented in tumor-associated macrophages 27.
A complex network of signal transduction pathways are likely to cooperate with NF-κB in promoting the aggressiveness of ovarian cancer. The precise regulation of these networks is often disrupted in cancer, allowing tumor growth, dissemination and resistance to cell death. Further investigation into the mechanism of NF-κB activity in ovarian cancer will offer new opportunities to improve outcome for women with advanced ovarian cancer.
Acknowledgements
This work was supported by the Intramural Program of the Center for Cancer Research, NCI (CMA, ECK), the Marsha Rivkin Foundation for Ovarian Cancer Research (CMA) and by grants from the Norwegian Cancer Society and the Health Region of South-Eastern Norway (BD).
Sources of support: This work was supported by the Intramural Program of the Center for Cancer Research, NCI (CMA, ECK), the Marsha Rivkin Foundation for Ovarian Cancer Researdh (CMA) and by grants from the Norwegian Cancer Society and the Health Region of South-Eastern Norway (BD).
Footnotes
Financial Disclosures: The authors have no financial disclosures.
REFERENCES
- 1.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16724054. [DOI] [PubMed] [Google Scholar]
- 2.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13(11):3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17545551. [DOI] [PubMed] [Google Scholar]
- 3.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66(7):3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16585214. [DOI] [PubMed] [Google Scholar]
- 4.Liu GH, Wang SR, Wang B, Kong BH. Inhibition of nuclear factor-kappaB by an antioxidant enhances paclitaxel sensitivity in ovarian carcinoma cell line. Int J Gynecol Cancer. 2006;16(5):1777–17782. doi: 10.1111/j.1525-1438.2006.00652.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17009971. [DOI] [PubMed] [Google Scholar]
- 5.Solomon LA, Ali S, Banerjee S, Munkarah AR, Morris RT, Sarkar FH. Sensitization of ovarian cancer cells to cisplatin by genistein: the role of NF-kappaB. J Ovarian Res. 2008;1(1):9. doi: 10.1186/1757-2215-1-9. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19025644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown M, Cohen J, Arun P, Chen Z, Van Waes C. NF-kappaB in carcinoma therapy and prevention. Expert Opin Ther Targets. 2008;12(9):1109–1122. doi: 10.1517/14728222.12.9.1109. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18694378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aghajanian C, Dizon DS, Sabbatini P, Raizer JJ, Dupont J, Spriggs DR. Phase I trial of bortezomib and carboplatin in recurrent ovarian or primary peritoneal cancer. J Clin Oncol. 2005;23(25):5943–5949. doi: 10.1200/JCO.2005.16.006. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16135465. [DOI] [PubMed] [Google Scholar]
- 8.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25(51):6817–6830. doi: 10.1038/sj.onc.1209942. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17072330. [DOI] [PubMed] [Google Scholar]
- 9.Yan C, Wang H, Aggarwal B, Boyd DD. A novel homologous recombination system to study 92 kDa type IV collagenase transcription demonstrates that the NF-kappaB motif drives the transition from a repressed to an activated state of gene expression. FASEB J. 2004;18(3):540–541. doi: 10.1096/fj.03-0960fje. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14715692. [DOI] [PubMed] [Google Scholar]
- 10.Belotti D, Calcagno C, Garofalo A, Caronia D, Riccardi E, Giavazzi R, et al. Vascular endothelial growth factor stimulates organ-specific host matrix metalloproteinase-9 expression and ovarian cancer invasion. Mol Cancer Res. 2008;6(4):525–534. doi: 10.1158/1541-7786.MCR-07-0366. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18403633. [DOI] [PubMed] [Google Scholar]
- 11.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–944. doi: 10.1038/nm1447. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16862152. [DOI] [PubMed] [Google Scholar]
- 12.Hussain MM, Seiden MV, Fuller A, Kohn EC. A two institution phase II study of paclitaxel, cyclophosphamide, cisplatin with G-CSF for patients with newly diagnosed advanced stage ovarian cancer. Proc Am Soc Clin Oncol. 2002;21 abstr 813. [Google Scholar]
- 13.Kohn EC, Sarosy GA, Davis P, Christian M, Link CE, Ognibene FP, et al. A phase I/II study of dose-intense paclitaxel with cisplatin and cyclophosphamide as initial therapy of poor-prognosis advanced-stage epithelial ovarian cancer. Gynecol Oncol. 1996;62(2):181–191. doi: 10.1006/gyno.1996.0213. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8751547. [DOI] [PubMed] [Google Scholar]
- 14.Hollander M, Wolfe D. Nonparametrical Statistical Methods. ed. 2nd. New York: John Wiley and Sons, Inc; 1999. pp. 189–269. [Google Scholar]
- 15.Agresti A. Categorical Data Analysis. New York: John Wiley and Sons, Inc; 1990. pp. 79–129. [Google Scholar]
- 16.Kaplan E, Meier P. Non-Parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chem Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 18.Cox D. Regression models and life tables. J Royal Stat Soc. 1972;34:187–202. [Google Scholar]
- 19.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439(7074):353–357. doi: 10.1038/nature04296. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16273092. [DOI] [PubMed] [Google Scholar]
- 20.Bonome T, Levine DA, Shih J, Randonovich M, Pise-Masison CA, Bogomolniy F, et al. A gene signature predicting for survival in suboptimally debulked patients with ovarian cancer. Cancer Res. 2008;68(13):5478–5486. doi: 10.1158/0008-5472.CAN-07-6595. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18593951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annunziata CM, Kleinberg L, Davidson B, Berner A, Gius D, Tchabo N, et al. BAG-4/SODD and associated antiapoptotic proteins are linked to aggressiveness of epithelial ovarian cancer. Clin Cancer Res. 2007;13(22 Pt 1):6585–6592. doi: 10.1158/1078-0432.CCR-07-0327. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18006758. [DOI] [PubMed] [Google Scholar]
- 22.Slipicevic A, Oy GF, Askildt IC, Holth A, Hellesylt E, Florenes VA, et al. Diagnostic and prognostic role of the insulin growth factor pathway members insulin-like growth factor-II and insulin-like growth factor binding protein-3 in serous effusions. Hum Pathol. 2009;40(4):527–537. doi: 10.1016/j.humpath.2008.10.003. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19121847. [DOI] [PubMed] [Google Scholar]
- 23.Sillanpaa S, Anttila M, Voutilainen K, Ropponen K, Turpeenniemi-Hujanen T, Puistola U, et al. Prognostic significance of matrix metalloproteinase-9 (MMP-9) in epithelial ovarian cancer. Gynecol Oncol. 2007;104(2):296–303. doi: 10.1016/j.ygyno.2006.09.004. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17034838. [DOI] [PubMed] [Google Scholar]
- 24.Shield K, Riley C, Quinn MA, Rice GE, Ackland ML, Ahmed N. Alpha2beta1 integrin affects metastatic potential of ovarian carcinoma spheroids by supporting disaggregation and proteolysis. J Carcinog. 2007;6:11. doi: 10.1186/1477-3163-6-11. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17567918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yagi H, Yotsumoto F, Miyamoto S. Heparin-binding epidermal growth factor-like growth factor promotes transcoelomic metastasis in ovarian cancer through epithelial-mesenchymal transition. Mol Cancer Ther. 2008;7(10):3441–3451. doi: 10.1158/1535-7163.MCT-08-0417. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18852147. [DOI] [PubMed] [Google Scholar]
- 26.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25(1):9–34. doi: 10.1007/s10555-006-7886-9. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16680569. [DOI] [PubMed] [Google Scholar]
- 27.Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, et al. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66(23):11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17145890. [DOI] [PubMed] [Google Scholar]