Abstract
Helicobacter DNA has been reported in hepatocellular carcinoma tissues in several studies from varying geographical locations, raising the possibility that helicobacter infection may contribute to the pathogenesis of hepatocellular carcinoma. Other known risk factors for hepatocellular carcinoma show significant geographical variability, but whether the same holds for helicobacter is unknown. We studied the prevalence of Helicobacter DNA in a United States cohort of HCC, where the prevalence of helicobacter infection is low in the general population. Liver tissues from 57 individuals were examined. Thirty-five individuals had paired tumor/non-tumor samples, including 21 cases of hepatocellular carcinoma, for a total of 92 samples studied. Both Helicobacter genus and Helicobacter pylori species specific PCR was performed. Helicobacter DNA was detected in 5/57 (9%) cases, all in non-neoplastic cirrhotic liver tissues from individuals with hepatitis C infection (N=4) or alcohol liver disease (N=1). Tissues from 22 hepatocellular carcinomas and 10 cholangiocarcinomas were all negative as were tissues from 8 benign primary hepatic tumors. In conclusion, Helicobacter DNA was detectable in 9% of liver tissues in this cohort, but was not found in primary benign or malignant liver tumors. These findings indicate that Helicobacter infection is unlikely to be etiologically associated with hepatocellular carcinoma in this cohort. If Helicobacter infection does contribute to the development of hepatocellular carcinoma in general, then significant regional variability must exist.
Keywords: Helicobacter, hepatocellular carcinoma, cholangiocarcinoma
Background
Helicobacter species were reported to colonize the livers of mice and cause hepatitis and hepatocellular carcinoma (HCC) in 1994 [1–3]. A subsequent report demonstrated the presence of the Helicobacter DNA in the human biliary tree [4]. Since that time, a growing body of literature has suggested a role for Helicobacter in human HCC. Specifically, individuals with HCC are more likely to have serological evidence for past Helicobacter infection [5–8] and Helicobacter DNA can be found in HCC tissues more commonly than controls [9–16]. In addition to HCC, Helicobacter DNA has also been reported in intrahepatic cholangiocarcinomas [9,12]. The potential role of Helicobacter infection in the development of HCC and intrahepatic cholangiocarcinomas has not been studied in the United States, where the prevalence of Helicobacter is low. Thus the aims of this study were to determine the frequency of Helicobacter DNA in benign liver tissues, primary HCC, and intrahepatic cholangiocarcinoma to better understand the role of Helicobacter infection, if any, in this region.
Materials and Methods
Liver tissues from the Johns Hopkins Hospital, Baltimore, Maryland were retrospectively studied. All tissue were from individuals who underwent liver transplantation (N=40) or surgery for a hepatic mass (N=17). Tissues were harvested at the time of surgery and stored at −80C prior to use.
Helicobacter DNA amplification
DNA was extracted from 20–25 mg of liver tissue using the QIAmp DNA mini kit (Qiagen, Valencia, CA, and USA). At least 100ng of liver DNA was used for each 25 ul PCR reaction, using primers listed in Table 1. To aide in comparing results from other studies, single round PCR was performed using primers reported by Rocho et. al. [15]. For helicobacter single round PCR assays using genus and species specific primers, 45 cycles of amplification were performed to enhance sensitivity. Hot start Taq was used and conditions were 95C for 10 minutes followed by 45 cycles of 95 C° for 20s, 55 C° for 30s, and 72 C° for 45s. A nested PCR assay was also performed on all liver samples (Table 1) with a first round of 50 cycles of 95 C° for 20s, 55 C° for 30s, and 72 C° for 45s. Using 1 ul of the first round product as template, a second round of PCR was performed for 45 cycles using the conditions of 95 C° for 20s, 55 C° for 30s, and 72 C° for 45s. In all samples, primers to the beta-catenin gene (CTNNB1) were used to ensure that extracted DNA was amplifiable.
Table 1.
Primers used in PCR amplification. The non-nested primers were selected from those used by Rocho et. al..[15] The original citation is also indicated below.
| Primer | Sequence 5’ to 3’ |
Target | Amplicon size (bp) |
|---|---|---|---|
| Helicobacter genus | |||
| HS-1[9] | AACGATGAAGCTTCTAGCTTGCTAG | 16S rDNA | 400 |
| HS-2 | GTGCTTATTCGTTAGATACCGTCAT | ||
| C97[4] | GCTATGACGGGTATCC | 16S rDNA | 400 |
| C98[4] | GATTTTACCCCTACACCA | ||
| Helicobacter pylori | |||
| HPY S[23] | AGGTTAAGAGGATGCGTCAGTC | 23S rDNA | 267 |
| HPY A[23] | CGCATGATATTCCCATTAGCAGT | ||
| Hemi-Nested PCR | |||
| Helicobacter genus | 163 rDNA | ||
| Outer primers[24] | TATGACGGGTATCCGGC | 375 | |
| HG-1 | ATTCCACCTACCTCTCCCA | ||
| HG-2 | |||
| Inner Primers | CTGAGACACGGTCCAGACTC | 293 | |
| HG-3 | CAAATGCAGTTCTRYRGTTAAGC | ||
| HG-2 | |||
| CTNNB1 | |||
| For | ATGGAACCAGACAGAAAAGC | Beta- catenin |
200 |
| Rev | GCTACTTGTTCTTGAGTGAAG |
Positive Controls
Three gastric biopsies (unrelated to the liver samples) with active chronic helicobacter pylori infection were micro-dissected from formalin fixed, paraffin embedded tissues, the DNA extracted, and the DNA used as controls to ensure that the helicobacter primers worked adequately The amplicons from one of the positive control were then cloned (Invitrogen, Carlsbad, CA) and PCR performed on 10 fold serial dilutions to determine the sensitivity of the PCR assays. In addition, for each PCR assay 10, 100, and 1000 copies of cloned helicobacter DNA were spiked into pooled liver DNA from five randomly selected liver samples and each PCR performed to investigate the performance of the primers in the setting of abundant liver genomic DNA.
Results
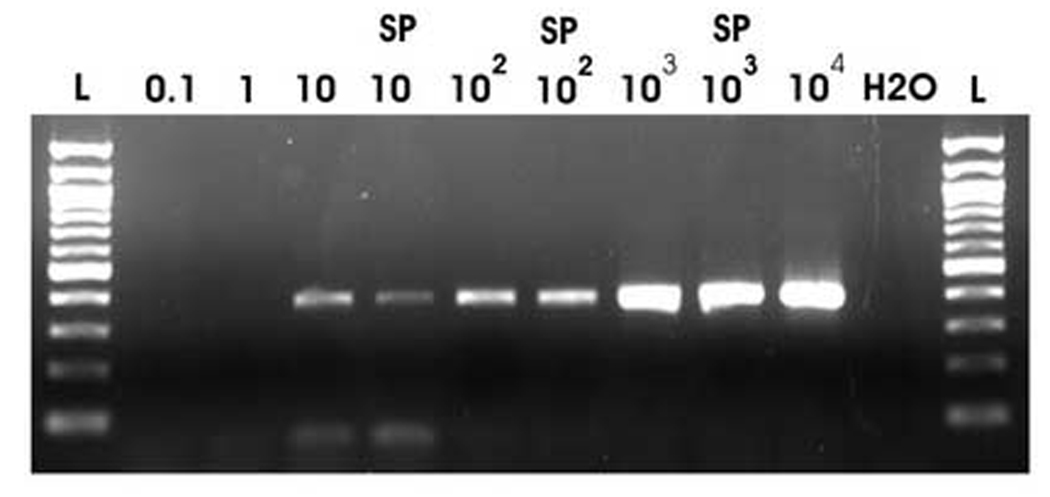
All sets of helicobacter primers amplified all positive control stomach biopsies. For the single round PCR assay, serial dilutions of the cloned amplicons demonstrated sensitivity down to 10 copies per reaction for primers C-97/C-98 and HPYS/HPYA and 100 copies per reaction for HS-1/HS-2 (Figure 1). Spiking the cloned helicobacter DNA into liver genomic DNA did not interfere with the assay’s sensitivity (Figure 1).
Figure 1.
For all three PCRs, the amplicon was cloned from one of the stomach positive controls, diluted, and PCR performed to determine sensitivity. Data for primer HS-1 and HS-2 are shown. The copy numbers of template per PCR reaction are shown above each lane. In addition, some of the assays were spiked with genomic DNA (designated by “SP”).
Liver tissues from 57 individuals were examined. 35 individuals had paired tumor/non-tumor samples, including 21 cases of HCCs, for a total of 92 samples studied. The liver tissues were from 34 men and 23 women with an average age of 52.8±14 years. The non-neoplastic livers were cirrhotic in 40 cases, mostly from chronic HCV infection (Table 2). In 11 cases, the non-neoplastic livers were not cirrhotic and contained no significant inflammation or fibrosis. The HCCs were all typical HCCs and no variants such as cholangiohepataocellular carcinomas or fibrolamellar carcinomas were examined. In addition to the HCC, 10 cholangiocarcinomas and 8 benign lesions were studied (Table 2). The cholangiocarcinomas were all intrahepatic and 9/10 had a pure glandular morphology, while one had a giant cell morphology.
Table 2.
Liver samples tested for Helicobacter species DNA.
| Type of sample | Number Samples Tested |
|---|---|
| Non-neoplastic liver, total | 51 |
| Cirrhosis, total | 40 |
| HCV | 21 |
| cryptogenic | 6 |
| HBV | 2 |
| ETOH | 5 |
| Other | 6 |
| Non-cirrhotic | 11 |
| Tumors, total | 35 |
| Hepatocellular carcinoma, total | 21 |
| HCV | 7 |
| cryptogenic | 1 |
| HBV | 2 |
| ETOH | 3 |
| Other | 4 |
| non cirrhotic background liver | 4 |
| Cholangiocarcinoma | 6 |
| Macro-regenerative nodule | 4 |
| Focal nodular hyperplasia | 2 |
| Hepatic adenoma | 1 |
| Angiomyolipoma | 1 |
|
Isolated tumors without corresponding non- neoplastic liver tissues |
6 |
| Hepatocellular carcinoma | 1 |
| Cholangiocarcinoma | 4 |
| Focal nodular hyperplasia | 1 |
| Total | 92 |
All cases showed very strong bands following amplification of the beta-catenin gene. However, no cases had detectable Helicobacter DNA using the single round PCR assays. Because of this surprising finding, the same samples were re-tested with a nested PCR assay and Helicobacter DNA was identified in 5/57 (9%) patients. Sequencing confirmed Helicobacter DNA in all cases. All of the positive cases were non-neoplastic tissues from cirrhotic livers, including 4 cases with HCV associated cirrhosis and one case of alcohol associated cirrhosis, giving an overall frequency of 13% in all cirrhotic livers and 19% in HCV associated liver cirrhosis. All of the remaining samples were negative by nested PCR.
Discussion
Our results demonstrate the presence of low levels of Helicobacter DNA in a small proportion of cirrhotic livers and suggest that Helicobacter infection did not play a major etiologically role in the development of HCC in this cohort. The absence of Helicobacter DNA in the 10 cases of intrahepatic cholangiocarcinoma further emphasizes that Helicobacter is unlikely to have an etiological role in the other major form of primary liver carcinomas in this cohort. These results significantly extend our understanding of the role of Helicobacter in HCC carcinogenesis by emphasizing that its role has important regional variations. Careful attention to positive controls and this laboratory’s experience with amplifying low-level targets from hepatic tissues [17] suggest that inadequate assay sensitivity is unlikely to explain the lack of detectable Helicobacter DNA in primary liver carcinomas. The absence of detectable Helicobacter DNA in benign neoplasms seen in this study is overall in good agreement with those studies that found no evidence for helicobacter in benign primary hepatic tumors [11,15].
As recently reviewed, Helicobacter may not necessarily be directly carcinogenic in the liver and may be a co-risk factor or even an innocent bystander [18]. To date, the possible association between HCC and helicobacter has been supported by a number of diverse observations which can be summarized as follows. First, based on serological studies as well as direct detection in liver tissues, Helicobacter is more common in those with cirrhosis than non-cirrhosis and, of those with cirrhosis, positive serology is found more frequently in those who have HCC [9–16,19]. Secondly, helicobacter DNA in HCC tissues has been reported from multiple geographic locations. Third, studies have reported positive helicobacter culture from liver tissues [20] and positive immunostaining for helicobacter on liver tissues [11,13,19]. Fourth, the lack of DNA from other gut organisms in HCC tissues, suggesting that helicobacter DNA detection does not represent non-specific DNA contamination from the portal circulation [13,15]. Finally, the absence or lower frequency of helicobacter in primary versus metastatic liver carcinomas suggests a more specific association with HCC [12,14,16]. Nevertheless, this link between HCC and Helicobacter is tempered by the observations of others that Helicobacter DNA was not detected in a group of 55 liver tissues [21]. Even if lack of assay sensitivity accounts for some of their findings [22], their results still strongly imply that any Helicobacter DNA that may have been present in the tested tissues was at a very low concentration. Our findings also indicate that Helicobacter DNA is absent in primary liver carcinomas and present in only 13% of cirrhotic liver tissues overall. While we did not quantitate the levels of Helicobacter DNA, the observation that sample positivity was found only with a nested PCR assay further implies very low Helicobacter DNA copy numbers.
Environmental, genetic, or clinical management methods may explain the discrepancy between our findings and those from other geographical locations. It seems unlikely that this discrepancy is entirely explained by Helicobacter pylori prevalences in the general population alone, as studies from France [9,15], Sweden [12], and the Netherlands [16] have also identified a higher frequency of Helicobacter DNA in HCC tissues, despite have an overall low Helicobacter prevalence, similar to the United States. Further study of this question may reveal important information on the biology of helicobacter infection as well as HCC.
In conclusion, Helicobacter DNA was detectable in 13% of cirrhotic liver tissues, but was not found in any case of primary liver cancer. Helicobacter is unlikely to have played an etiological role in the development of liver cancer in this cohort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Perumal Vivekanandan, Email: cmcvivek@yahoo.com.
Michael Torbenson, Email: mtorben@jhmi.edu.
References
- 1.Fox JG, Dewhirst FE, Tully JG, et al. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward JM, Fox JG, Anver MR, et al. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 3.Ward JM, Anver MR, Haines DC, et al. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol. 1994;145:959–968. [PMC free article] [PubMed] [Google Scholar]
- 4.Fox JG, Dewhirst FE, Shen Z, et al. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 5.Siringo S, Vaira D, Menegatti M, et al. High prevalence of Helicobacter pylori in liver cirrhosis: relationship with clinical and endoscopic features and the risk of peptic ulcer. Dig Dis Sci. 1997;42:2024–2030. doi: 10.1023/a:1018849930107. [DOI] [PubMed] [Google Scholar]
- 6.Ponzetto A, Pellicano R, Redaelli A, et al. Helicobacter pylori infection in patients with Hepatitis C Virus positive chronic liver diseases. New Microbiol. 2003;26:321–328. [PubMed] [Google Scholar]
- 7.Ponzetto A, Pellicano R, Leone N, et al. Helicobacter infection and cirrhosis in hepatitis C virus carriage: is it an innocent bystander or a troublemaker? Med Hypotheses. 2000;54:275–277. doi: 10.1054/mehy.1999.0987. [DOI] [PubMed] [Google Scholar]
- 8.Leone N, Pellicano R, Brunello F, et al. Helicobacter pylori seroprevalence in patients with cirrhosis of the liver and hepatocellular carcinoma. Cancer Detect Prev. 2003;27:494–497. doi: 10.1016/j.cdp.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Avenaud P, Marais A, Monteiro L, et al. Detection of Helicobacter species in the liver of patients with and without primary liver carcinoma. Cancer. 2000;89:1431–1439. [PubMed] [Google Scholar]
- 10.Dore MP, Realdi G, Mura D, et al. Helicobacter infection in patients with HCV-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Dig Dis Sci. 2002;47:1638–1643. doi: 10.1023/a:1015848009444. [DOI] [PubMed] [Google Scholar]
- 11.Fan XG, Peng XN, Huang Y, et al. Helicobacter species ribosomal DNA recovered from the liver tissue of chinese patients with primary hepatocellular carcinoma. Clin Infect Dis. 2002;35:1555–1557. doi: 10.1086/344770. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson HO, Mulchandani R, Tranberg KG, et al. Helicobacter species identified in liver from patients with cholangiocarcinoma and hepatocellular carcinoma. Gastroenterology. 2001;120:323–324. doi: 10.1053/gast.2001.21382. [DOI] [PubMed] [Google Scholar]
- 13.Ito K, Nakamura M, Toda G, et al. Potential role of Helicobacter pylori in hepatocarcinogenesis. Int J Mol Med. 2004;13:221–227. [PubMed] [Google Scholar]
- 14.Pellicano R, Mazzaferro V, Grigioni WF, et al. Helicobacter species sequences in liver samples from patients with and without hepatocellular carcinoma. World J Gastroenterol. 2004;10:598–601. doi: 10.3748/wjg.v10.i4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha M, Avenaud P, Menard A, et al. Association of Helicobacter species with hepatitis C cirrhosis with or without hepatocellular carcinoma. Gut. 2005;54:396–401. doi: 10.1136/gut.2004.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhoef C, Pot RG, de Man RA, et al. Detection of identical Helicobacter DNA in the stomach and in the non-cirrhotic liver of patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2003;15:1171–1174. doi: 10.1097/00042737-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Kannangai R, Molmenti E, Arrazola L, et al. Occult hepatitis B viral DNA in liver carcinomas from a region with a low prevalence of chronic hepatitis B infection. J Viral Hepat. 2004;11:297–301. doi: 10.1111/j.1365-2893.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu XZ, Chen D. Helicobacter pylori and hepatocellular carcinoma: Correlated or uncorrelated? J Gastroenterol Hepatol. 2006;21:345–347. doi: 10.1111/j.1440-1746.2006.04245.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Fan XG, Wang ZM, et al. Identification of helicobacter species in human liver samples from patients with primary hepatocellular carcinoma. J Clin Pathol. 2004;57:1273–1277. doi: 10.1136/jcp.2004.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Magalhaes Queiroz DM, Santos A. Isolation of a Helicobacter strain from the human liver. Gastroenterology. 2001;121:1023–1024. doi: 10.1053/gast.2001.28574. [DOI] [PubMed] [Google Scholar]
- 21.Coppola N, De Stefano G, Marrocco C, et al. Absence of Helicobacter spp in the liver of patients with primary or metastatic liver cancer. Hepatology. 2002;36:1300–1301. doi: 10.1053/jhep.2002.36367. [DOI] [PubMed] [Google Scholar]
- 22.Wadstrom T, Abu Al-Soud W, Ljungh A, et al. Absence of Helicobacter species in the liver of patients with primary or metastatic liver cancer. Hepatology. 2003;38:532–533. doi: 10.1053/jhep.2003.50262. author reply 33. [DOI] [PubMed] [Google Scholar]
- 23.Menard A, Santos A, Megraud F, et al. PCR-restriction fragment length polymorphism can also detect point mutation A2142C in the 23S rRNA gene, associated with Helicobacter pylori resistance to clarithromycin. Antimicrob Agents Chemother. 2002;46:1156–1157. doi: 10.1128/AAC.46.4.1156-1157.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulajic M, Maisonneuve P, Schneider-Brachert W, et al. Helicobacter pylori and the risk of benign and malignant biliary tract disease. Cancer. 2002;95:1946–1953. doi: 10.1002/cncr.10893. [DOI] [PubMed] [Google Scholar]