Abstract
Maintaining wild-type (WT) zebrafish stocks for research while preserving viability within the lines used presents significant challenges to zebrafish husbandry practices. Genetic homogeneity is established through inbreeding to provide continuity across experiments. This, however, leads to decreased fitness through inbreeding depression. In the laboratory setting, it is imperative that researchers consistently obtain a large number of viable embryos; thus, inbreeding depression must be suppressed. Genetic variation can be established by creating hybrid lines; however, crosses between genetically distinct lines can cause an outbreeding depression as well. There is little data describing the effects of inbreeding depression or outbreeding depression from such crosses in zebrafish. Additionally, there is a need to establish breeding standards within the zebrafish field. This study examines the susceptibility to inbreeding and outbreeding depression in crosses between four WT zebrafish lines: the inbred lines AB and Tab 14, and the F1 generation of hybrid lines TuAB and TLAB. We report that mating frequency and clutch size were significantly greater in hybrid female crosses than in inbred female crosses. Inbreeding depression in common zebrafish lines such as AB and Tab 14 used here results in fewer successful matings and smaller clutch sizes. Further, outbreeding depression caused by crossing distantly related lines, such as the inbred Tab 14 and the hybrid TLAB lines, can also influence successful zebrafish mating. These data provide evidence needed to further characterize commonly used WT zebrafish lines. We suggest that to maintain lines that mate frequently and yield large clutches, hybrid females of known backgrounds should be used.
Introduction
Two phenomena of increasing importance in zebrafish husbandry are inbreeding depression and its antithesis, outbreeding enhancement or hybrid vigor. Inbreeding depression is observed in many species as the loss of fitness in a population from increasing homozygosity.1 It can be caused by a general loss of heterozygosity in a population or by the unmasking of deleterious alleles in homozygotes, both leading to a decline in fitness.2–4 These phenomena are ubiquitous in plant and animal species,1 and although the magnitude of effects varies among populations and environments,5 it has the potential to drive small populations to extinction.6
With outbreeding enhancement, also known as hybrid vigor or heterosis, the reverse of inbreeding depression occurs.7 Heterozygosity increases the fitness of a population and remasks potential deleterious recessive alleles. Studies in several species demonstrate that outbreeding between populations has positive effects on traits that influence fitness.8–10 In many cases, when two closely related species are hybridized, the offspring are superior to at least one of the parental species.11,12
In contrast to the increased fitness observed with outbreeding enhancement, hybridization can also lead to a loss of locally adapted alleles.13 The result is that the hybrid may no longer be suited to the environment of either parent. Consequently, outbreeding depression can become apparent in the F1 generation14 or not until the F2 generation if the loss of fitness is due to disruption of epistatic interactions or loss of coadapted gene complexes.15
Inbreeding has been deemed the most important factor for genetic evaluation of a species16 and is even more apparent in captive piscine populations in which an understanding of its outcomes is necessary to appreciate the consequences of conservation actions and aquaculture practices.17 Inbreeding depression has been noted to cause body deformations and lower progeny survival in Oncorhynchus mykiss,18 reduced growth rates in Oncorhynchus kisutch,19 decreased male mating behavior and lower salinity tolerance in Poecilia reticulata,16,20–22 and reduced fertilization success and survival in Gasterosteus aculeatus.23 These consequences of inbreeding and outbreeding are receiving increasing attention by conservation biologists24 because outbreeding is often used as a technique to recover inbred wild populations25,26 or enhance desired traits in a species.27–29 However, little is known about the effects on captive zebrafish (Danio rerio) populations.
Zebrafish are a unique tool for genetic and embryonic research, and as such are maintained in colonies serving hundreds of laboratories worldwide. These colonies comprise many different zebrafish lines that exhibit ranges of fitness both between different colonies and even within a single facility. For instance, the commonly used AB line that has been maintained for multiple generations in one facility may have a very different fitness than a line derived from the same background but maintained through in-crossing in another facility. Additionally, the zebrafish field has not yet adopted standard practices for the selection of which wild-type (WT) lines to use, environmental conditions, or diets given.30–39 Thus, it is not surprising that reported clutch sizes and breeding frequencies vary wildly between publications.40 Since zebrafish are not maintained by a field-wide standard, the WT lines, even with the same name, are unique to each colony, genetically distinct from one another, and potentially varied in the degree of genetic diversity. This lack of standardization provides the opportunity to use zebrafish as a model for further study of inbreeding depression and outbreeding enhancement/depression.
We used two hybrid zebrafish lines obtained from a Tu-AB cross and a TL-AB cross and two inbred lines, AB and Tab 14, to address whether inbreeding depression exists in zebrafish lines. We hypothesized that zebrafish in captive populations display a degree of inbreeding depression and that this may be alleviated by the outbreeding to other WT lines. Moreover, to standardize and improve efficiency in zebrafish husbandry practices, it is important to define the breeding success of this species in captivity. We report that not only are inbred lines less likely to mate and produce fewer embryos, but outbreeding depression also occurs when mating two distantly related lines, such as Tab 14 inbred line crossed to the TLAB hybrid line. Therefore, it is our recommendation that each zebrafish colony maintain at least three lines of WT zebrafish and regularly generate hybrid lines to maximize the number of embryos available for experimentation.
Materials and Methods
Fish maintenance
WT lines, between 6 and 10 months of age (Table 1), were maintained at 28.5°C in 3-L AHAB tanks on a 10-h-dark–14-h-light cycle and fed three times daily with combinations of first instar Artemia nauplii, tropical flake, and Zeigler complete adult zebrafish diet. Embryos were collected from mating tanks by 2 hours postartificial dawn. Viable embryos were sorted at 6 hours postfertilization and maintained at ≤60 embryos/50 mL egg water (0.6 g/L Crystal Sea Marine Mix with 0.01 mg/L methylene blue) in 20-mm-deep Petri dishes in the dark at 28.5°C until 5 days postfertilization (dpf ). Adults were weighed and measured at the conclusion of the study. The standard length was measured from the tip of the snout to the end of the caudal peduncle, and all zebrafish were weighed after blot drying with a paper towel (Supplemental Fig. S1, available online at www.liebertonline.com).
Table 1.
Two Hybrid Zebrafish Lines Were Created by In- and Out-Crossing Several Wild-Type Lines for Comparison to In-Crossed, Inbred Zebrafish Lines
| Line | Parents | Generation | Number used | Age during study (months) | |
|---|---|---|---|---|---|
| Hybrid | TuAB | Tu × AB | F1 | 48 | 6–8 |
| TLAB | TL × AB | F1 | 32 | 8–10 | |
| Inbred | AB | First cousins | Unknown | 48 | 6–8 |
| Tab 14 | Siblings | F10 | 32 | 8–10 |
Out-crosses were performed between Tu and AB and between TL and AB to obtain hybrid zebrafish, and in-crosses were performed in AB and Tab 14 lines to obtain inbred individuals.
Experimental design
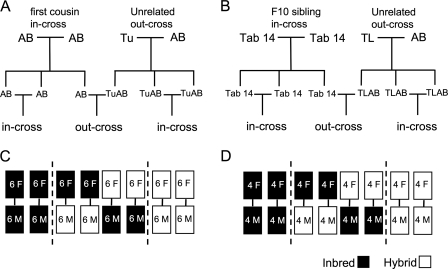
The study was performed using two inbred and two hybrid lines. Adult siblings were generated from an AB-AB first cousin in-cross (inbred AB), a Tu-AB out-cross (hybrid TuAB) (Fig. 1A), a Tab 14 F9 sibling in-cross (inbred Tab 14), and a TL-AB out-cross (hybrid TLAB) (Fig. 1B). All fish were segregated by 4 months of age according to sex. They were maintained at a density of six individuals per 3 L for crosses with AB/TuAB (Fig. 1C) or four individuals per 3 L for Tab 14/TLAB, respectively (Fig. 1D). The AB/TuAB fish were 6–8 months of age and the Tab 14/TLAB fish were 8–10 months during the study (Table 1).
FIG. 1.
Experimental design. Fish were obtained from an AB first-cousin cross and raised to adulthood as were fish from a Tu, AB out-cross, a Tab 14 F9 in-cross, and a TL, AB out-cross. Adults were segregated by sex at 5 months, and 48 male and 48 female siblings from each inbred-AB and hybrid-TuAB line (A), and 32 male and 32 female siblings from each inbred-Tab 14 and hybrid-TLAB line (B) were randomly assigned to eight same-sex, line-specific tanks at a density of six individuals for AB and TuAB (C) or four individuals for Tab 14 and TLAB (D) per tank. Pair crosses were performed with individuals from the same paired tanks, designated for in-crosses or out-crosses before the study, and fish were returned to the same tank after each setup. M, male; F, female.
Crosses
As outlined in Figure 1, individuals of each line were randomly assigned to groups for in-crossing or out-crossing and maintained in the same groups for the duration of the study. Paired crosses were performed randomly within their group 60 min after the last daily feeding, approximately 4 h before the end of the light cycle, in 1 L mating tanks with system water and a single 3–5-cm piece of artificial aquarium plant (Aquatic Habitats, Apopka, FL). Fish were crossed once per week for two consecutive weeks and then given 1 week of rest, for a total of seven crosses. Embryos were collected and counted 2 h after artificial dawn, and adults were returned to their original tanks. Crosses from AB and TuAB lines were compared separately from crosses with Tab 14 and TLAB lines.
Data analysis
Successful matings were determined by the presence or absence of fertilized embryos at 2 h postartificial dawn. The mating frequency was calculated as the number of successful matings per number of total pair-crosses within each group. Clutch sizes were determined as the number of fertilized viable embryos present at 6 hpf. Embryos were cleaned and sorted for viability on each successive day through 5 dpf, and those that did not inflate their swim bladder (SB) by 5.5 dpf were scored as nonviable.
Statistical analysis
The difference in mating frequency between the lines was analyzed using Fisher's exact test. Clutch sizes were compared using the Wilcoxon's rank-sum/Mann–Whitney U-test, and embryo viability using an unpaired t-test.
Results
We compared the mating success of two inbred and two hybrid lines that were either in- or out-crossed (Table 1). A total of 480 crosses were performed as described above, yielding 239 clutches, and a total of 30,484 embryos were obtained and sorted (Table 2). Fish ranged from 0.22 to 0.41 g (AB/TuAB females), 0.20 to 0.43 g (AB/TuAB males), 0.51 to 0.83 g (Tab 14/TLAB females), and 0.35 to 0.59 g (Tab 14/TLAB males), with no significant difference between the lines compared (Supplemental Fig. S1A, B). The standard lengths ranged from 23.0 to 27.0 mm (AB/TuAB females), 22.5 to 29.0 mm (AB/TuAB males), 29.0 to 33.0 mm (Tab 14/TLAB females), and 26.0 to 32.0 mm (Tab 14/TLAB males), with no significant difference in the length of fish of the same sex (Supplemental Fig. S1C, D).
Table 2.
Embryos Produced by In- and Out-Crosses of AB and TuAB, Tab 14, and TLAB Lines
| Line (female) | Line (male) | No. of crosses | No. of clutches | No. of embryos produced | No. of viable embryos |
|---|---|---|---|---|---|
| AB | AB | 72 | 39 | 3504 | 2804 |
| TuAB | 72 | 37 | 3746 | 3142 | |
| TuAB | TuAB | 72 | 43 | 5140 | 4355 |
| AB M | 72 | 50 | 6319 | 5246 | |
| Tab 14 | Tab 14 | 48 | 11 | 1891 | 1542 |
| TLAB | 48 | 14 | 1269 | 1096 | |
| TLAB | TLAB | 48 | 28 | 5447 | 4886 |
| Tab 14 | 48 | 17 | 3168 | 2501 | |
| Totals | 480 | 239 | 30,484 | 25,572 |
Females of each line were crossed with sibling males and males from the complementary line, AB and TuAB, Tab 14, and TLAB. A total of 480 paired crosses were performed as in-crosses or out-crosses to obtain 238 clutches with 30,484 embryos, 25,572 of which remained viable at 5 dpf.
Mating frequency
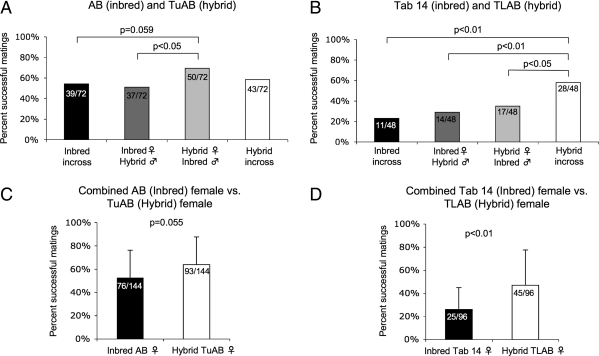
Mating frequency and clutch size dictate the number of adult fish needed to routinely obtain the large number of embryos required for developmental studies. We assessed mating frequency by comparing the number of clutches produced to the number of mating pairs within each group. Matings were considered successful if greater than five fertilized embryos at 2 h postartificial dawn were obtained, regardless of later viability. We compared the mating frequency of the inbred and hybrid lines that were in-crossed and out-crossed (Fig. 2A, B).
FIG. 2.
Mating frequency is variable between lines and is significantly improved with hybridization of TL and AB lines. Inbred and hybrid lines were in-crossed and out-crossed, and the frequency of mating success was measured as the number of clutches per number of opportunities in paired crosses. No significance was observed between incrosses of the AB and TuAB lines; however, the AB male/TuAB female out-cross was significantly higher than the TuAB male/AB female out-cross (p < 0.05), and a near significantly higher frequency (p = 0.059) was seen between the AB in-cross and the AB male/TuAB female out-cross (A). The trend toward greater mating frequency in the hybrid lines was again apparent in the Tab 14 and TLAB crosses (B). Significance was observed between the hybrid in-cross and the hybrid out-cross (p < 0.05) and inbred out-cross (p < 0.01), but not between the out-crosses and the inbred in-cross. The mating frequency of the combined in- and out-crosses was greater in the TuAB female crosses than in the AB female crosses (p = 0.055) and significantly greater (p < 0.01) in the TLAB female crosses than in the Tab 14 female crosses (C, D). p-Values were calculated using Fisher's exact test.
One indication of inbreeding depression is that mating frequency improves upon out-crossing. Alternatively, a sign of outbreeding depression is that mating frequency decreases after out-crossing. We did not find evidence of inbreeding or outbreeding depression when we compared the mating frequency of the AB and the TuAB lines: there was no difference in the mating frequency of the inbred (AB) line when it was either in-crossed (black bar; 54.2%) or when the AB female was out-crossed to the TuAB male (dark gray bar; 50.0%). Similarly, the mating frequency of the hybrid (TuAB) line was not altered by out-crossing (58.3% for the in-cross, white bar; 69.4% for TuAB female/AB male out-cross, light gray bar). In contrast, there was a nearly significant (p = 0.059) increase in mating frequency observed when comparing the results of in-crossing the inbred (AB) to the cross between a hybrid (TuAB) female and an inbred (AB) male (Fig. 2A). These data suggest that hybrid females exhibit an improved mating frequency. This is confirmed by the finding that hybrid (TuAB) females crossed to the inbred (AB) males have a significantly higher mating frequency than the inbred (AB) females crossed to the hybrid (TuAB) males (p < 0.05; Fig. 2A).
In the second data set, we observed a different trend suggestive of outbreeding depression in the Tab 14 inbred crossed to the TLAB hybrid lines. This is demonstrated by the finding that the TLAB in-cross resulted in a higher mating frequency than the out-cross (Fig. 2B; p < 0.05). This is further substantiated by the finding that out-crossing TLAB (hybrid) females to a genetically distinct male (Tab 14) did not improve mating frequency compared to the out-cross of the Tab 14 females to the TLAB males (Fig. 2A). These data suggest that the genetic distinction between the highly inbred Tab 14 line and the hybrid TLAB line may decrease their ability to mate.
Hybrid vigor describes improved fitness of a hybrid compared to an inbred line. We found evidence of hybrid vigor in both the TuAB and the TLAB lines. The TuAB females are better at mating than the inbred AB females (Fig. 2A), and the mating frequency of the TLAB line is better than any other in the experiment (Fig. 2B). The hybrid (TLAB) in-cross was significantly more successful at mating when compared to the Tab 14 in-cross (p < 0.001), the TLAB female/Tab 14 male out-cross (p = 0.024), and Tab 14 female/TLAB male out-cross (p = 0.004; Fig. 2B).
We hypothesized that females would be the strongest determinant in mating success. To address this, we analyzed the mating frequency of either the inbred or hybrid females crossed to either hybrid or inbred males in both data sets. The inbred AB females had a somewhat lower mating frequency of 52.1% compared to 63.9% for the TuAB females (Fig. 2C; p = 0.055); however, the mating frequency of hybrid TLAB females was significantly higher than that of the inbred Tab 14 females (46.9% and 26.0%, respectively, p < 0.01; Fig. 2D). There was no significant difference in the mating success of the males from any of the lines (Supplemental Fig. S2A, B, E, F, I, J, available online at www.liebertonline.com). These data indicate that two hybrid zebrafish lines, TuAB and TLAB, mate with a higher frequency than inbred lines and that the females are an important factor in determining mating frequency.
Clutch size
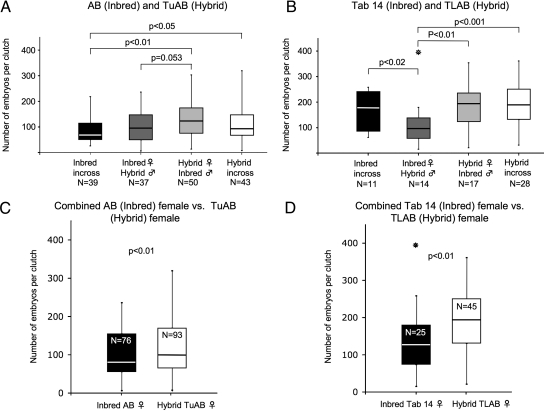
In addition to the mating frequency, another important factor in zebrafish research is the size of clutches and the ability to consistently obtain large clutches. Reduced clutch size is a direct determinant of fitness and is indicative of inbreeding or outbreeding depression. To determine whether in-crossing or out-crossing affected clutch size, we counted the number of embryos produced from each successful mating. Fertilized embryos were collected 2 h after artificial dawn, from each mating pair, and healthy embryos were counted at 6 hpf. The inbred lines (AB and Tab 14) were compared to their respective hybrid lines (TuAB and TLAB). Clutch sizes at 6 h postartificial dawn across the entire data set ranged from 6 to 394 embryos.
We found that in-crossing the inbred AB line (black bar; Fig. 3A) produced an average clutch size of 90 embryos, whereas the out-cross of the AB female to the hybrid TuAB male (dark gray bar) produced slightly larger average clutch sizes of 101 embryos. The hybrid TuAB in-cross (white bar) produced average clutches of 120 embryos and the hybrid (TuAB) female/inbred (AB) male out-cross (light gray bar) produced average clutches of 126 embryos. While these data do not suggest hybrid vigor in the TuAB line, clutches from the hybrid TuAB in-cross were significantly larger than the inbred AB in-cross (p = 0.023) and clutches from the TuAb female/AB male out-cross were significantly larger than the AB in-cross (p = 0.004). In-crosses of the inbred Tab 14 line (black bar; Fig. 3B) produced an average clutches size of 172 embryos, whereas out-crossing the Tab 14 female to the TLAB male (dark gray bar) produced significantly smaller average clutches of 118 embryos (p = 0.016). The hybrid TLAB in-cross (white bar) produced average clutches of 195 embryos, whereas the out-cross of the TLAB female to the Tab 14 male (light gray bar) produced 189 embryos. The Tab 14 male/TLAB female (dark gray bar) clutches were significantly smaller than the TLAB female/Tab 14 male clutches (light gray bar; p = 0.004) and the TLAB in-cross (white bar; p < 0.001).
FIG. 3.
Hybrid females produce significantly larger clutches than inbred females. Embryos were counted from clutches obtained from in-crosses and out-crosses of Inbred and Hybrid lines (N = number of clutches). Box represents the upper and lower quartile clutch size on either side of the median clutch size; the line extends to the maximum and minimum clutch sizes. The TuAB in-cross and the TuAB female/AB male out-cross produced significantly larger clutches than the inbred (AB) in-cross (p < 0.05 and p < 0.01, respectively) and nearly significantly larger clutches than the AB female/TuAB male out-cross (p = 0.053) (A), whereas the TLAB hybrid in-cross, the Tab 14 inbred in-cross, and the TLAB female/Tab 14 male out-cross produced significantly larger clutches than the Tab 14 female/TLAB male out-cross (p < 0.001, p < 0.02, and p < 0.01, respectively) (B). Hybrid females from both TuAB and TLAB lines produced significantly larger clutches than their inbred counterparts (p < 0.01 and p < 0.01, respectively) (C, D). N = number of clutches in sample. Two tailed p-values were calculated using Mann–Whitney U-test. Outliers were removed from p-value calculations and signified with “*” if they failed Grubb's outlier test at a significance level of 0.05.
Both data sets provided evidence of hybrid vigor. The hybrid TuAB line had larger clutches than the AB line (Fig. 3A) and the TLAB produced significantly larger clutches than the Tab 14 line (Fig. 3B). These data also support the hypothesis that the genetic background of the female is consistently an important determinant in fitness. The hybrid females of both data sets produced significantly larger clutches than the inbred females. The TuAB females produced an average clutch size of 123 compared to 95 produced by the AB females (Fig. 3C); the average size of the clutches from TLAB females was 191 compared to 132 produced by the Tab 14 females (Fig. 3D). In both data sets, the hybrid female produced significantly larger clutches than the inbred female (AB/TuAB, p = 0.009; Tab 14/TLAB, p = 0.006). These data suggest that the hybridization of lines plays a more crucial role in clutch sizes than in- or out-crossing and that hybrid females produce larger clutches than inbred females.
Embryo viability
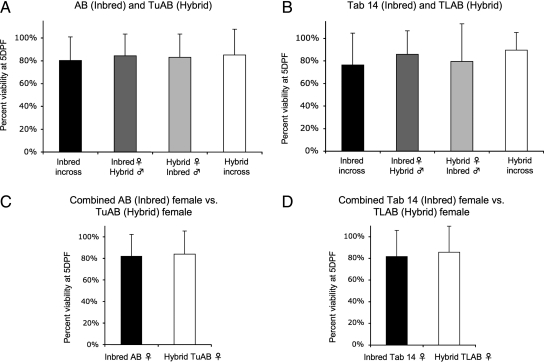
Embryos were collected from paired matings 2 h postartificial dawn and sorted for abnormalities at 6 hpf. Dead and abnormal embryos were removed each day until 5 dpf and remaining embryos were scored SB positive or negative. SB-negative embryos were counted as nonviable along with the dead and abnormal embryos removed before 5 dpf. The total numbers of healthy embryos were compared to the initial number of healthy embryos at 6 hpf to determine viability. Embryo viability between 0 and 5 dpf declined to near 80% across all lines and crosses (Table 1 and Fig. 4A, D). No difference was observed in embryo viability between in-crossing and out-crossing or between the hybrid and inbred strains. Therefore, embryo viability is not a variable measure of fitness in our zebrafish lines.
FIG. 4.
There is no difference in embryo mortality between in-crossed and out-crossed clutches or between clutches from Inbred or Hybrid Females. There was no difference in embryo viability between in-crosses and out-crosses or between clutches from hybrid and inbred females (all p > 0.10) (A–D). Viability was defined as the number of swim-bladder-positive, healthy embryos at 5 days postfertilization compared to the initial number counted at 6 h postfertilization. p-Values reported were calculated using an unpaired t-test.
The genetic background of male zebrafish does not influence mating success
Similar analysis to assess the effects of male genetic background revealed that there was no significant difference between hybrid male and inbred male mating frequency, clutch sizes, or embryo mortality (Supplemental Fig. S2A–J, available online at www.liebertonline.com), although the hybrid TLAB males tended to have a better mating frequency than their inbred (Tab 14) counterparts (43.8%–29.2%; Supplemental Fig. S2B). The genetic background of the male did not yield any differences in mating frequency or embryo viability in-crossed or out-crossed (Supplemental Fig. S2); however, a significant increase in clutch sizes was observed in in-crosses of the TLAB and Tab 14 lines when compared to out-crosses of the same lines (188–148, p = 0.017; Supplemental Fig. S2H). While this suggests that male TLAB zebrafish demonstrate hybrid vigor, the combined data from all of the lines in our study reveal that the females (Figs. 2C, D and 3C, D) are the most dominant factor affecting mating success.
Discussion
Inbreeding depression is the loss of heterozygosity in a population leading to a decline in fitness,1 and can occur easily in small populations. Conversely, hybrid vigor or outbreeding enhancement is the rescue of inbreeding depression by increasing heterozygosity in a population. These phenomena are ubiquitous among plant and animal species, and their effects have been widely reported. We hypothesized that zebrafish in captive populations display a degree of inbreeding depression and that this may be alleviated by outbreeding WT lines. The data presented here support this hypothesis.
As with many domestic fish populations, zebrafish colonies may suffer an increased incidence of inbreeding depression due to genetic bottlenecks that occur during the foundation of colonies,16 low overall population size, and selection pressure. Although many deleterious alleles may exist in large natural populations,41 they are generally rare in individuals in all but the smallest populations where they have the opportunity to become homozygous in inbred individuals.42 The conditions in which zebrafish colonies are founded and maintained are typical environments for such inbreeding depression from increased homozygosity to occur. Zebrafish have 1.5–2.0 morphologically overt, early acting, completely penetrant, recessive lethal alleles,43 and inbreeding zebrafish reduces fertilization rates and survival, lower growth rates, and cause higher instances of body deformation.44 Although these effects are commonly seen by husbandry staff, their frequency has not been reported in the literature.
Here, we document inbreeding depression in commonly used zebrafish lines that are in-crossed for several generations. Moreover, we demonstrate that hybridizing the inbred lines with other WT lines through a single generation of out-crossing provides outbreeding enhancement. These hybrid lines successfully mated with higher frequency and produced larger clutches on average than their inbred counterparts, although there was no difference in progeny survival.
Both zebrafish males and females display independent preferences in sexual selection,35 but breeding success may not be correlated with male dominance or size in individual pairings.39 Since reproductive behavior and other traits under sexual selection have been tied closely with genetic fitness in other small schooling piscine species,45 inbreeding depression could play a role in zebrafish mating frequency. We found a significantly greater mating frequency when hybrid females are used in crosses than inbred females in all the lines used. This is illustrative of hybrid vigor.
Second to mating frequency, clutch size is an important factor in measuring the health and fitness of a zebrafish colony and is critical to developmental biology research that requires large numbers of zebrafish embryos. Inbreeding depression tends to become more visible in traits associated with fecundity and survival than in morphology,46,47 predicting that decreases in clutch size associated with inbred lines would become apparent before morphological abnormalities in embryos are evident, as we observed in the hybrid lines. While clutch sizes were significantly smaller in crosses involving inbred females, those embryos that were fertilized were no more susceptible to dying or developing abnormalities by 5 dpf than the offspring of their hybrid counterparts.
These results suggest greater genetic fitness in the hybrid lines and a fitness depression in inbred lines. There are, however, some aspects of this study's design that may influence some of our data and prevent some comparisons. For instance, the low density of fish used in our study (4 or 6 fish per 3 L tank) may allow for individuals to display dominance and create a stressful environment for tank mates,46 which could lead to decreased mating behavior. Additionally, as males and females were randomly paired, it is possible that incompatible pairs were repeatedly set up or that size differences between the males and females in any given pair decreased their mating frequency. We believe these to be unlikely because no individuals displayed stress behavior during the experiment, individuals were randomly assigned to tanks initially and then again to pair tanks during setups, and the size differences were consistent within the lines compared. Differences in the size and age of fish (Table 1 and Supplemental Fig. S1) prohibited direct comparison between the TuAB/AB group and the Tab 14/TLAB group.
We have evidence of outbreeding depression in WT zebrafish: (1) the lower mating frequency observed in the TLAB female/Tab 14 male and the Tab 14 female/TLAB male out-crosses (Fig. 2B) when compared to the hybrid in-cross, and (2) the decline in clutch size in the Tab 14 female/TLAB male out-crosses (Fig. 3B) when compared to the TLAB and Tab 14 in-crosses. These may be attributed to a predisposition of incompatibility between the Tab 14 and TLAB lines, although, as they did not mate as frequently, the clutches of Tab 14/TLAB crosses were larger on average than the AB/TuAB crosses. This indicates that there is no innate incompatibility. Alternatively, the size difference between the male and female individuals is much greater in the Tab 14/TLAB crosses than in the AB/TuAB crosses (Supplemental Fig. S1). However, these were consistent across the replicates and therefore not likely to be a contributing factor. Another intriguing possibility is that there is a greater genetic disparity between TLAB and Tab 14 fish and outbreeding depression in the TLAB line. This is supported by studies demonstrating that the AB strain is more closely related to the Tu strain than it is to the TL strain,49 suggesting that the hybridized TL/AB line could exhibit outbreeding depression. This may account for the data that crosses between the TLAB and Tab 14 line are less frequently successful and trend differently than crosses between the more closely related AB and TuAB lines.
Although environmental conditions may vary by facility, these findings are likely representative. The lines used varied only in heterozygosity, being more or less inbred, and were of the most commonly used lines in the zebrafish community. There was no difference in tank density, rearing, or feeding regimes between the lines that were compared, indicating that the differences in mating frequency and clutch size are due solely to the degree of heterozygosity. While the absolute mating frequency, clutch size, and embryo survival may differ by facility, the primary findings should be consistent. Since the inbreeding depression trend was confirmed in two separate lines, it is likely that inbreeding depression would be evident in other facilities, in lines at similar generations, and that these could be rescued by creating hybrids with a closely related line. However, not all hybrids are necessarily more fit, as data from other species have demonstrated that crosses between species can be less fit, exemplified by the sterile mule produced by crossing a horse and donkey.
Taken together, these results provide the basis for ongoing efforts to improve and maintain zebrafish colonies that consistently produce a large number of viable embryos. Our data indicate that hybrid vigor exists in zebrafish. Thus, creating hybrid lines in which the genetic heritages of the lines are known significantly enhances both the number of pairs that mate and the number of embryos produced. We also provide data on clutch sizes and the frequency of successful matings between several inbred and hybrid lines, which are rarely reported but important in determining genetic health and usefulness of zebrafish lines. This paves the way for zebrafish to be used to further understand inbreeding depression, hybrid vigor, and outbreeding depression. Finally, this study provides the data to allow this evolving and expanding field to use experimental evidence to establish standards for husbandry protocols and better characterization of WT lines. To maintain a facility with WT zebrafish lines that mate as frequently as possible and produce large clutches of viable embryos, we recommend hybridizing with closely related lines such as Tu and AB. We also suggest that additional work be done to further characterize WT lines as it is apparent that lines with differing genetic backgrounds exhibit different performance and fitness traits.
Supplementary Material
Acknowledgments
The authors thank Alex Mir and Duygu Erdogan for technical assistance, Liz Loughlin for editing, and Dr. James Godbold for help with statistical analyses. This study was funded by Department of Medicine, Mount Sinai School of Medicine, March of Dimes Basil O'Conner Award, and the NIDDK RO1DK080789-OA1.
Disclosure Statement
No competing financial interests exist.
References
- 1.Charlesworth D. Charlesworth B. Inbreeding depression and its evolutionary consequences. Ann Rev Ecol Syst. 1987;18:237–268. [Google Scholar]
- 2.Morton NE. Crow JF. Muller HJ. An estimate of the mutational damage in man from data on consanguineous marriages. Proc Natl Acad Sci USA. 1956;42:855–863. doi: 10.1073/pnas.42.11.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlesworth B. Charlesworth D. The genetic basis of inbreeding depression. Genet Res. 1999;74:329–340. doi: 10.1017/s0016672399004152. [DOI] [PubMed] [Google Scholar]
- 4.Roff DA. Inbreeding depression: tests of the overdominance and partial dominance hypotheses. Evolution. 2002;56:768–775. doi: 10.1111/j.0014-3820.2002.tb01387.x. [DOI] [PubMed] [Google Scholar]
- 5.Keller LF. Waller DM. Inbreeding effects in wild populations. Trends Ecol Evol. 2002;17:230–241. [Google Scholar]
- 6.Saccheri I. Kuussaari M. Kankare M. Vilkman P. Fortelius W. Hanski I. Inbreeding and extinction in a butterfly metapopulation. Nature. 1998;392:491–494. [Google Scholar]
- 7.Waser NM. Price MV. Optimal outcrossing in Ipomopsis aggregata: seed set and offspring fitness. Evolution. 1989;43:1097–1109. doi: 10.1111/j.1558-5646.1989.tb02554.x. [DOI] [PubMed] [Google Scholar]
- 8.Sheridan AK. Crossbreeding and heterosis. Anim Breed Abstr. 1981;49:131–144. [Google Scholar]
- 9.Turton JD. Crossbreeding of dairy cattle—a selective review. Anim Breed Abstr. 1981;49:293–300. [Google Scholar]
- 10.Levin DA. Inbreeding depression and proximity-dependent crossing success in Phlox drummondii. Evolution. 1984;38:116–127. doi: 10.1111/j.1558-5646.1984.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 11.Good TP. Ellis JC. Annett CA. Pierotti R. Bounded hybrid superiority in an avian hybrid zone: effects of mate, diet, and habitat choice. Evolution. 2000;54:1774–1783. doi: 10.1111/j.0014-3820.2000.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 12.Welch ME. Rieseberg LH. Habitat divergence between a homoploid hybrid sunflower species, Helianthus paradoxus (Asteraceae), and its progenitors. Am J Bot. 2002;89:472–478. doi: 10.3732/ajb.89.3.472. [DOI] [PubMed] [Google Scholar]
- 13.Templeton AR. In: Coadaptation and outbreeding depression. In: Conservation Biology: The Science of Scarcity and Diversity. Soule ME, editor. Sunderland, MA: Sinauer Associates, Inc.; 1986. pp. 105–116. [Google Scholar]
- 14.Waser NM. Price MV. Shaw RG. Outbreeding depression varies among cohorts of Ipomopsis aggregata planted in nature. Evolution. 2000;54:485–491. doi: 10.1111/j.0014-3820.2000.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 15.Templeton AR. Hemmer H. Mace GM. Seal US. Shields WM. Woodruff DS. Local adaptation, coadaptation, and population boundaries. Zoo Biol. 1986;5:115–125. [Google Scholar]
- 16.Nakadate M. Takahito S. Nobuhiko T. Inbreeding depression and heterosis in various quanititive traits of the guppy, Poecilia reticulata. Aquaculture. 2003;220:219–226. [Google Scholar]
- 17.McClelland EK. Naish KA. What is the fitness outcome of crossing unrelated fish populations? A meta-analysis and an evaluation of future research directions. Conserv Genet. 2007;8:397–416. [Google Scholar]
- 18.Waldman B. McKinnon JS. Inbreeding and outbreeding in fishes, amphibians and reptiles. In: Thornhill NW, editor. The Natural History of Inbreeding and Outbreeding: Theoretical and Empirical Perspectives. Chicago, IL: University of Chicago Press; 1993. pp. 250–282. [Google Scholar]
- 19.Gallardo JA. Neira R. Environmental dependence of inbreeding depression in cultured Coho salmon (Oncorhynchus kisutch): aggressiveness, dominance and in-traspecific competition. Heredity. 2005;95:449–456. doi: 10.1038/sj.hdy.6800741. [DOI] [PubMed] [Google Scholar]
- 20.van Oosterhout C. Trigg RR. Carvalho GR. Magurran AE. Hauser L. Shaw PW. Inbreeding depression and genetic load of sexually selected traits: How the guppy lost its spots. J Evol Biol. 2003;16:273–281. doi: 10.1046/j.1420-9101.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- 21.Mariette M. Kelley JL. Brooks R. Evans JP. The effects of inbreeding on male courtship behaviour and coloration in guppies. Ethology. 2006;112:807–814. [Google Scholar]
- 22.Ala-Honkola O. Uddstrom A. Pauli BD. Lindstrom K. Strong inbreeding depression in male mating behaviour in a poeciliid fish. J Evol Biol. 2009;22:1396–1406. doi: 10.1111/j.1420-9101.2009.01765.x. [DOI] [PubMed] [Google Scholar]
- 23.Frommen JG. Luz C. Mazzi D. Bakker TCM. Inbreeding depression affects fertilization success and survival but not breeding coloration in threespine sticklebacks. Behaviour. 2008;145:425–441. [Google Scholar]
- 24.Vrijenhoek RC. Conservation genetics of freshwater fish. J Fish Biol. 1998;53:394–412. [Google Scholar]
- 25.Heschel MS. Paige KN. Inbreeding depression, environmental stress, and population size variation in scarlet gilia (Ipomopsis aggregata) Conserv Biol. 1995;9:126–133. [Google Scholar]
- 26.Westemeier RL. Brawn JD. Simpson SA. Esker TL. Jansen RW. Walk JW, et al. Tracking the long-term decline and recovery of an isolated population. Science. 1998;282:1695–1698. doi: 10.1126/science.282.5394.1695. [DOI] [PubMed] [Google Scholar]
- 27.Hedgecock D. Davis JP. Improving Pacific oyster broostock through crossbreeding. J Shellfish Res. 2000;19:614–615. [Google Scholar]
- 28.Hayward RS. Wang HP. Inherent growth capacity and social costs of Bluegill and hybrids of Bluegill and Green Sunfish: which fish really grows faster? N Am J Aquac. 2002;63:34–46. [Google Scholar]
- 29.Kristensen TN. Sørensen AC. Inbreeding—lessons from animal breeding, evolutionary biology and conservation genetics. Anim Sci. 2005;80:121–133. [Google Scholar]
- 30.Darrow KO. Harris WA. Characterization and development of courtship in zebrafish, Danio rerio. Zebrafish. 2004;1:40–45. doi: 10.1089/154585404774101662. [DOI] [PubMed] [Google Scholar]
- 31.Gerlach G. Pheromonal regulation of reproductive success in female zebrafish: female suppression and male enhancement. Anim Behav. 2006;72:1119–1124. [Google Scholar]
- 32.Loucks E. Carvan MJ. Strain-dependant effects of developmental ethanol exposure in zebrafish. Neurotoxicol Teratol. 2004;26:745–755. doi: 10.1016/j.ntt.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 33.McClure MM. McIntyre PB. McCune AR. Notes on the natural diet and habitat of eight danionin fishes, including the zebrafish, Danio rerio. J Fish Biol. 2006;69:553–570. [Google Scholar]
- 34.Moretz JA. Martins EP. Robison B. The effects of early and adult social environments on zebrafish (Danio rerio) behavior. Environ Biol Fish. 2006;80:91–101. [Google Scholar]
- 35.Moretz JA. Martins EP. Robison B. Behavioral syndromes and the evolution of correlated behavior in zebrafish. Behav Ecol. 2007;18:556–562. [Google Scholar]
- 36.Pyron M. Female preference and male-male interactions in zebrafish (Danio rerio) Can J Zool. 2003;81:122–125. [Google Scholar]
- 37.Spence R. Fatema MK. Reichard M. Huq KA. Wahab MA. Ahmed ZF, et al. The distribution and habitat preference of the zebrafish in Bangladesh. J Fish Biol. 2006;69:1435–1448. [Google Scholar]
- 38.Spence R. Smith C. Male territoriality mediates density and sex ratio effects on oviposition in the zerbafish (Danio rerio) Anim Behav. 2005;69:1317–1323. [Google Scholar]
- 39.Spence R. Smith C. Mating preference of female zebrafish, Danio rerio, in relation to male dominance. Behav Ecol. 2006;17:779–783. [Google Scholar]
- 40.Spence R. Gerlach G. Lawrence C. Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 41.Sunyaev S. Ramensky V. Koch I. Lathe W., 3rd Kondrashov AS. Bork P. Prediction of deleterious human alleles. Hum Mol Genet. 2001;10:591–597. doi: 10.1093/hmg/10.6.591. [DOI] [PubMed] [Google Scholar]
- 42.Crow JF. Minor viability mutants in Drosophila. Genetics. 1979;92(Suppl):s165–s172. [PubMed] [Google Scholar]
- 43.McCune AR. Fuller RC. Aquilina AA. Dawley RM. Fadool JM. Houle D, et al. A low genomic number of recessive lethals in natural populations of bluefin killifish and zebrafish. Science. 2002;296:2398–2401. doi: 10.1126/science.1071757. [DOI] [PubMed] [Google Scholar]
- 44.Mrakovcic M. Haley LE. Inbreeding depression in the zebrafish Brachydanio rerio (Hamilton Buchanan) J Fish Biol. 1979;15:323–327. [Google Scholar]
- 45.Sheridan L. Pomiankowski A. Fluctuating asymmetry, spot asymmetry and inbreeding depression in the sexual coloration of male guppy fish. Heredity. 1997;79:515–523. [Google Scholar]
- 46.Falconer DS. Introduction to Quanitative Genetics. 4th. New York, NY: Longman; 1989. [Google Scholar]
- 47.DeRose MA. Roff DA. A comparison of inbreeding depression in life-history and morphological traits in animals. Evolution. 1999;53:1288–1292. doi: 10.1111/j.1558-5646.1999.tb04541.x. [DOI] [PubMed] [Google Scholar]
- 48.Lasron ET. O'Malley DM. Melloni RH., Jr Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav Brain Res. 2006;167:94–102. doi: 10.1016/j.bbr.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 49.Guryev V. Koudijs MJ. Berezikov E. Johnson SL. Plasterk RHA. Fredericus JK, et al. Genetic variation in the zebrafish. Genome Res. 2006;16:491–497. doi: 10.1101/gr.4791006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.