Abstract
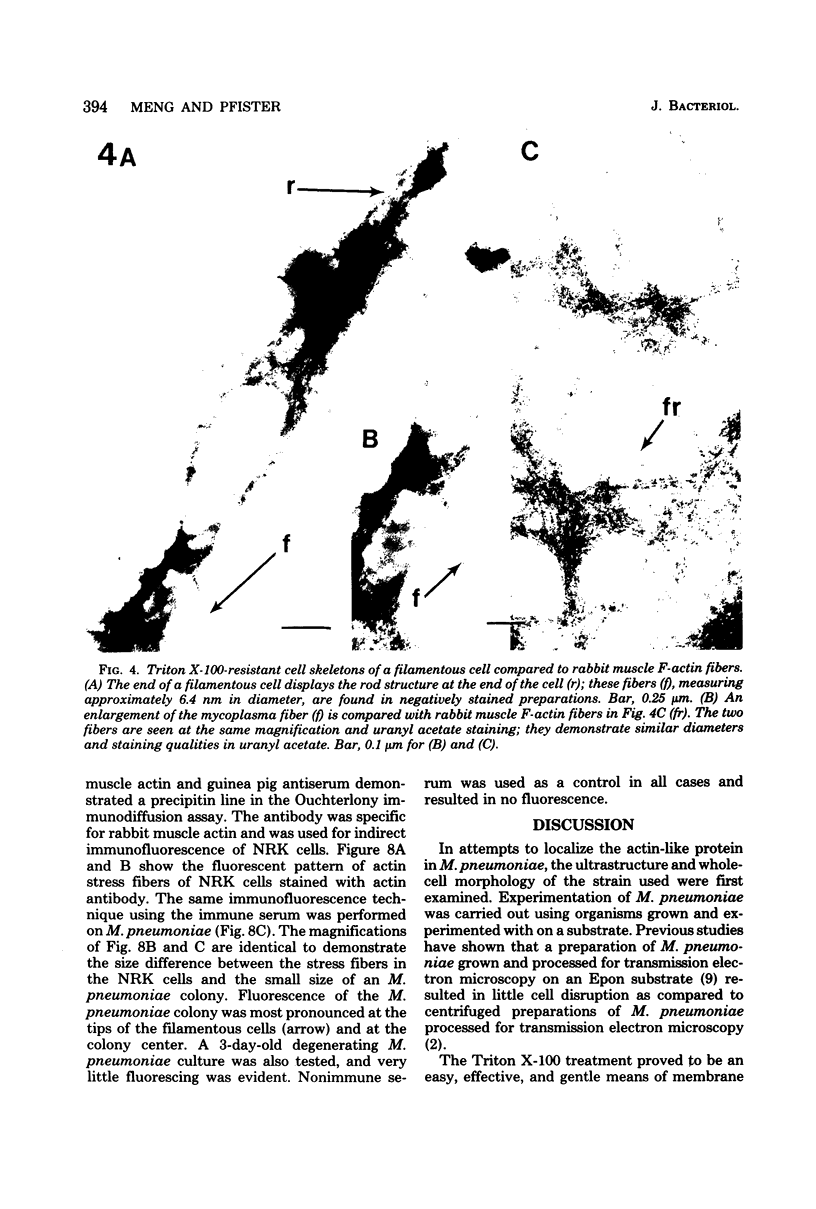
Mycoplasma pneumoniae was grown on Formvar- and carbon-coated electron microscope grids and treated with the nonionic detergent Triton X-100 to gently remove the membrane and cytoplasm. The detergent mixture was composed of 0.5% Triton X-100 in SSR-2 broth base. After this treatment, the grids were rinsed in a mixture of 0.1 M KCl, 5 mM MgCl2, and 6 mM potassium phosphate buffer (pH 7.05) and negatively stained with uranyl acetate. The Triton X-100-resistant remains of M. pneumoniae after gentle removal of the membrane and cytoplasm consisted of fibrous structures oriented similarly to the undisrupted cells. The thin fibers displayed a negative staining quality and diameter analogous to that of rabbit muscle F-actin. The fibrous moieties ended in rodlike condensations which appeared striated in negatively stained and shadowed preparations. These striations were regular, and the majority of rod structures had lengths of 220 to 300 nm and widths of 50 to 80 nm. Specific antibody to rabbit muscle actin, produced in guinea pigs, was used in indirect immunofluorescence of the M. pneumoniae colonies. Fluorescence was detected, with concentrations at the colony center and at the tips of filamentous cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biberfeld G., Biberfeld P. Ultrastructural features of Mycoplasma pneumoniae. J Bacteriol. 1970 Jun;102(3):855–861. doi: 10.1128/jb.102.3.855-861.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman E. S., Kenny G. E. Morphology and ultrastructure of Mycoplasma pneumoniae spherules. J Bacteriol. 1971 Jun;106(3):1005–1015. doi: 10.1128/jb.106.3.1005-1015.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. K., Pfister R. M., Somerson N. L. Electron microscopy of Mycoplasma pneumoniae microcolonies grown on solid surfaces. Appl Environ Microbiol. 1977 Nov;34(5):591–594. doi: 10.1128/aem.34.5.591-594.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Maupin-Szamier P., Pollard T. D. Actin filament destruction by osmium tetroxide. J Cell Biol. 1978 Jun;77(3):837–852. doi: 10.1083/jcb.77.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neimark H. C. Extraction of an actin-like protein from the prokaryote Mycoplasma pneumoniae. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4041–4045. doi: 10.1073/pnas.74.9.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Radestock U., Bredt W. Motility of Mycoplasma pneumoniae. J Bacteriol. 1977 Mar;129(3):1495–1501. doi: 10.1128/jb.129.3.1495-1501.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell A. W., Peterson J. E., Rodwell E. S. Striated fibers of the rho form of Mycoplasma: in vitro reassembly, composition, and structure. J Bacteriol. 1975 Jun;122(3):1216–1229. doi: 10.1128/jb.122.3.1216-1229.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerson N. L., James W. D., Walls B. E., Chanock R. M. Growth of Mycoplasma pneumoniae on a glass surface. Ann N Y Acad Sci. 1967 Jul 28;143(1):384–389. doi: 10.1111/j.1749-6632.1967.tb27680.x. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Manchee R. J. Adherence of mycoplasmas to glass and plastic. J Bacteriol. 1967 Nov;94(5):1781–1782. doi: 10.1128/jb.94.5.1781-1782.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky N., Leaman D. H., Jr, Kallman B. J. A simple method for recovering and concentrating fractionated sample components from cylindrical gels. Anal Biochem. 1975 Aug;67(2):507–514. doi: 10.1016/0003-2697(75)90325-5. [DOI] [PubMed] [Google Scholar]
- Wilson M. H., Collier A. M. Ultrastructural study of Mycoplasma pneumoniae in organ culture. J Bacteriol. 1976 Jan;125(1):332–339. doi: 10.1128/jb.125.1.332-339.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]











