Abstract
Objective
Informed decision-making requires that parents and research subjects understand the risks and benefits of a study, yet research suggests that comprehension of these elements is often poor. This study was designed to examine the effect of factors including manipulation of risk/benefit tradeoffs, numeracy, and socio-demographics on parents’ understanding of risks and benefits.
Methods
4,685 parents completed an Internet survey in which they were randomized to receive information about the risks and benefits of a hypothetical pain treatment study presented in one of four different scenarios. Parents’ gist (essential) and verbatim (exact) understanding and their perceptions of the risks and benefits, were compared across scenarios. The effects of parental socio-demographics and numeracy were also examined.
Results
Participants randomized to consider a research study offering the possibility of improved outcomes had higher gist and verbatim understanding of the information than participants considering studies that only offered reductions in the risk of side effects. Furthermore, these parents perceived the risks of the study to be significantly lower compared with the scenarios that offered the same risks but less benefit. Caucasian race, college education, and higher numeracy were all associated significantly with improved gist and verbatim understanding.
Conclusions
Research studies which offer only improved outcomes to participants may be evaluated more thoroughly than those that only offer reduced risks, and individual characteristics significantly moderate parents’ ability to comprehend risk/benefit information. These results are important toward developing strategies to improve the ways in which risks and benefits are communicated to parents and research subjects.
Keywords: Risk/benefit trade-offs, pediatric research, Decision-making, numeracy
INTRODUCTION
Comprehension of the risks and benefits of research is central to the informed consent process yet, several studies have shown that the interpretation and understanding of risks and benefits is often incomplete.1-3 Not surprisingly, many research participants are ill-prepared for the complexity of the information provided. Indeed, Peters et al. note that a “hierarchy of skills” is required for informed decision-making including the ability to understand the information, make calculations, weigh factors, and make inferences and trade-off decisions.4 Of note too, is the fact that parents may weigh decisions for their children differently than they would for themselves.5-7
Critical to the process of understanding research information is the manner by which it is presented. Recent studies have shown that risks and benefits are better understood when presented in graphical format rather than text and tables,8, 9 as incremental change rather than relative or absolute values10 and, as percentages rather than frequencies (e.g., 15 out of 100).11 Despite this, there is much to learn regarding the optimal methods of presenting risks and benefits to research participants and, in particular, parents of child subjects.
Of note, little is known about how the characteristics of a specific treatment or intervention (in psychological terms; the “option set”) affect research subjects’ willingness to participate. For example, many clinical studies involve comparing a standard treatment to a newer alternative. Yet, there are good theoretical reasons to believe that subjects may not view all such studies alike. In some studies, the new treatment alternative is believed to offer increased benefits to the subject (i.e., a “gain”). In other studies, however, the new option is being considered because it carries reduced risk. Psychologically, such options are often seen not as a “gain” but instead as simply a reduced potential for “loss.” Indeed, there are plenty of data to show that people often treat things they perceive as losses differently from those they perceive as gains.12-16
This study was designed to explore the effect of different types of risk/benefit trade-offs on parents’ understanding of a trial of an investigational drug for postoperative pain in children. To do so, we created 4 distinct scenarios, 3 of which included various risk/benefit trade-offs and a fourth in which all the attributes were improved (no trade-off). This latter scenario provided a “control” condition to determine parental understanding of a clinical trial that only offered improved outcomes. We also explored how individual differences in numeracy and sociodemographics affected parents’ knowledge, attitudes, and choices.
METHODS
Population
This internet-survey study was deemed exempt by the University of Michigan's Institutional Review Board. Participants were drawn from a panel of individuals supplied by Survey Sampling International (SSI, Fairfield CT) who provided a stratified random sample of demographically diverse participants. Eligible participants included parents aged 25-55 years who had at least one child under the age of 18 years. The process by which SSI contacted and randomized participants has been reported in detail elsewhere.8, 9
Procedures
Participants received information about one hypothetical clinical study comparing two drugs (A and B) for the treatment of postoperative pain in children. A more detailed description of the survey is described elsewhere9 but, in summary, Drug A represented the standard treatment and drug B an investigational new drug yet to be tested in children. The risks and benefits of the two drugs were presented in absolute terms e.g., “60 out of 100 (60%) of children who take drug A will experience good pain relief after surgery.” Comparison of the risks and benefits between the two drugs was expressed as the incremental change e.g., “Compared with children who take drug A, 2 fewer children out of 100 (2%) who take drug B will experience slowed breathing.” Our choice of absolute and incremental descriptors of risks and benefits was based on research showing that use of incremental risk formats increases understanding by focusing attention on how the intervention changes personal risk.10, 17, 18
a) Risk / Benefit Trade-offs
Parents were randomized (computer-generated) to receive information about the study in one of the 4 scenarios that varied in terms of the incremental risk/benefit differences between the two drugs. The risks and benefit of drug A were identical across all scenarios, while those of drug B were varied to provide different incremental changes to the benefit (pain relief), a minor but common risk (itching), and a serious but rare risk (slowed breathing) [Table 1]. In all scenarios, drug B reduced the risk of both side effects. However, in one scenario labeled here as “No Trade-off”, drug B also increased the likelihood of pain relief and thus an improvement in all dimensions. The remaining 3 scenarios (labeled “Risk 1,” “Risk 2,” and “Risk 3”), provided different risk benefit trade-offs wherein drug B offered a reduced likelihood of pain relief and varying degrees of risk reductions.
Table 1.
Risk/Benefit Trade-offs in the Four Hypothetical Scenarios
| Scenario | Risk/Benefit | Drug A (%) | Drug B (%) | Incremental change (%) |
|---|---|---|---|---|
| No trade-off | Pain relief | 60 | 75 | +15 |
| Itching | 25 | 20 | −5 | |
| Slowed breathing | 7 | 5 | −2 | |
| Risk 1 | Pain relief | 60 | 55 | −5 |
| Itching | 25 | 20 | −5 | |
| Slowed breathing | 7 | 5 | −2 | |
| Risk 2 | Pain relief | 60 | 55 | −5 |
| Itching | 25 | 12 | −13 | |
| Slowed breathing | 7 | 5 | −2 | |
| Risk 3 | Pain relief | 60 | 55 | −5 |
| Itching | 25 | 20 | −5 | |
| Slowed breathing | 7 | 3 | −4 |
b) Individual Characteristics
Several individual characteristics were measured to examine their effects on parents’ understanding:
Numeracy (quantitative literacy) was measured using the Subjective Numeracy Scale which measures numerical adeptness.19, 20 Numeracy was presented as a dichotomous variable of low and high numeracy based on the median split.
Need for Cognition (NFC) refers to an individual's tendency to engage in effortful thinking and was measured using a previously validated scale.21 Subjects were categorized as having low or high NFC based on the median split.
Socio-Demographic characteristics included age, gender, race/ethnicity, education, English speaking, income, number of children, and whether or not the subject or their child had participated in a prior research study.
Measures of Understanding
i) Gist knowledge
Gist knowledge is a measure of the ability to understand the essential meaning of the information presented. Four questions in the survey measured gist knowledge (see appendix). The number of correct answers was totaled to provide an overall estimate of gist (0-4). For the purposes of this study, we defined “adequate” understanding as ≥ 3 correct answers out of a possible 4. This definition was based on previous studies8, 9 and the premise that since 100% understanding is neither likely nor necessary for participants to be fully informed, a metric for “adequate” understanding would be more pragmatic.
ii) Verbatim knowledge
Verbatim knowledge is the ability to correctly identify the numerical risk and benefit statistics. Seven items in the survey addressed verbatim understanding (see appendix). A sensitivity analysis using different cut-off points (e.g., 5/7 vs 6/7) to define “adequate” found qualitatively similar results (see appendix), therefore for the purposes of this study, adequate verbatim understanding was defined as ≥5 correct answers out of 7.
Measures of Parents’ Perceptions
Parents rated how likely they would have been to enroll their child in the study had it been real (1-11 interval scale where 1 = “not at all likely” and 11 = “extremely likely”). Furthermore, parents were asked to score their perceptions of the amount of risk and benefit that the study would pose to their child had they enrolled (1-11 interval scales where 11 = “extreme” benefit or risk, see appendix).
c) Statistical Analysis
Statistical analyses were performed using SPSS® statistical software (SPSS Inc., Chicago, IL). Sample size was based on previous data indicating the need for a minimum sample size of 4,800 (α = 0.05, β = 0.20, two-sided) to detect clinically important differences between groups.9 Parametric data were compared using analysis of variance with post-hoc analysis using either Tukey or Dunnett's C tests depending on the equality of variances. Nonparametric data were analyzed using Mann Whitney-U, chi-square, and Fisher's Exact test, as appropriate. Data are expressed as percentages and mean ± SD. Significance was accepted as P <0.016 (Bonferroni corrected).
RESULTS
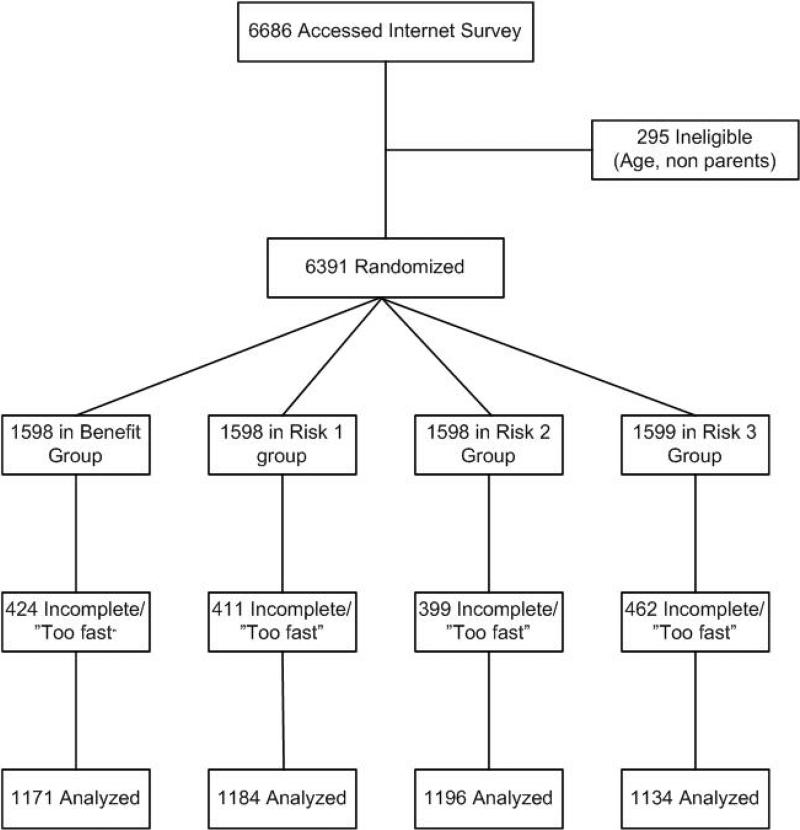
A total of 6,686 subjects accessed the Internet survey. Of these, 295 were excluded because they did not meet the parent eligibility criterion (Figure 1). Of the remaining 6,381 subjects, 1,424 were excluded because they did not complete the survey and 272 were excluded because they completed it “too quickly” (i.e., < 300 secs). Exclusion cut-off times were based on previous experience with the justification that those who appeared to “skim” through the material were unlikely to be giving it sufficient thought. There was no difference in the demographics of those who responded and those who did not. Data are therefore presented for a total of 4,685 subjects.
Figure 1.
Flow diagram of participant progress through the phases of the study
The demographics of the parents randomized to the various risk/benefit scenarios are described in Table 2. There were no significant differences in demographics between groups.
Table 2.
Demographics by Risk/Benefit Trade-off Scenario
| No Trade-off (n = 1171) | Risk 1 (n = 1184) | Risk 2 (n = 1196) | Risk 3 (n = 1134) | |
|---|---|---|---|---|
| Age (yrs, mean ± SD) | 38.9 ± 7.9 | 39.3 ± 8.1 | 38.9 ± 7.9 | 39.3 ± 7.7 |
| Gender (F/M)% | 56/44 | 57/43 | 59/41 | 58/41 |
| Race/ethnicity (%): | ||||
| Caucasian | 67.0 | 67.4 | 67.6 | 70.0 |
| African American | 12.2 | 13.3 | 11.8 | 10.7 |
| Hispanic | 13.1 | 12.0 | 13.0 | 12.7 |
| Asian | 4.9 | 3.7 | 4.2 | 3.5 |
| Other | 2.8 | 3.6 | 3.4 | 3.1 |
| Level of Education (%): | ||||
| ≤ High school graduate | 18.9 | 18.0 | 19.3 | 19.7 |
| Some college/trade school | 32.3 | 31.6 | 31.7 | 32.3 |
| Associate/Bachelor's | 37.9 | 39.3 | 37.3 | 36.6 |
| Degree | 10.9 | 11.1 | 11.7 | 11.4 |
| Graduate Degree | ||||
| Income Level (%): | ||||
| < $10,000 | 2.2 | 2.1 | 2.0 | 1.9 |
| $10,000-49,999 | 35.8 | 34.4 | 35.8 | 34.9 |
| $50,000-89,999 | 40.2 | 39.1 | 39.3 | 41.7 |
| ≥$90,000 | 21.8 | 24.4 | 22.9 | 21.5 |
| English as first language | 94.0 | 95.8 | 94.5 | 94.6 |
| Numeracy: | ||||
| High/Low (%) | 55/45 | 53/47 | 52/48 | 49/51 |
| NFC: | ||||
| High/Low (%) | 58/42 | 57/42 | 59/41 | 57/43 |
Data are expressed as Mean ± SD and %
Low numeracy = 0-35, High numeracy = 36-48 on the Subjective Numeracy Scale15
NFC = Need for Cognition: Low NFC= 0-21, High NFC = ≥ 22
a) Parents’ Understanding by Risk/Benefit Scenario
Parents’ understanding of items related to pain relief, itching, and slowed breathing for each of the 4 scenarios is described in Table 3. Parents who received the “no trade-off” scenario, which offered increased benefit of pain relief together with a concomitant reduction in complication risks, had greater gist and verbatim understanding compared with parents who received one of the other three scenarios that only conferred risk reductions. Of note, parents randomized to the no trade-off scenario had greater understanding not just of the benefits but also of the risks of itching and slowed breathing, despite the fact that these statistics were identical to those reported in the Risk 1 scenario.
Table 3.
Parents’ Gist and Verbatim Understanding by Trade-off Scenario
| Scenario |
Gist Understanding (%) |
Verbatim Understanding (%) |
||||
|---|---|---|---|---|---|---|
| Pain Relief | Itching | Slowed Breathing | Pain Relief | Itching | Slowed Breathing | |
| No trade-off | 70.4 | 78.2 | 76.6 | 54.7 | 71.2 | 72.9 |
| Risk 1 | 57.3* | 70.9* | 70.5* | 29.5* | 69.2 | 69.8 |
| Risk 2 | 57.5* | 76.7† | 73.3 | 27.4* | 63.7*† | 69.1 |
| Risk 3 | 57.5* | 70.2*‡ | 69.6* | 27.5* | 67.5 | 63.1*†‡ |
P< 0.001 vs No trade-off
P< 0.01 vs Risk 1
P<0.01 vs Risk 2
b) Parents’ Perceptions of the Risks and Benefits by Scenario
Parents who received the no trade-off scenario correctly perceived the potential benefits to the child as being significantly greater than those receiving either of Risk scenarios 1, 2, or 3 (6.8 ± 2.5 vs 6.1 ± 2.5, 6.1 ± 2.5, 5.9 ± 2.5, respectively, 1-11 scale, P<0.01). Furthermore, despite the fact that the no trade-off scenario and the Risk 1 and 3 scenarios offered essentially the same risk reductions, parents who received the no trade-off scenario viewed them differently, perceiving the risks to be significantly lower (5.6 ± 2.5 vs 6.0 ± 2.4 and 6.1 ± 2.4, respectively, P< 0.01). Parents who received the no trade-off scenario also reported that they would be more likely to enroll their child in the study had it been real compared to those receiving the other scenarios (6.7 ± 2.9 vs 5.9 ± 2.9, 6.3 ± 2.9, 5.9 ± 2.9, respectively, P< 0.01).
c) Numeracy and NFC
Parents with higher numeracy had greater gist and verbatim understanding of the risks and benefits of the research (Table 4). Additionally, for each scenario, parents with higher numeracy perceived the risks to be less and the benefits to be higher than those with low numeracy (5.6 ± 2.5 vs 6.1 ± 2.4, P< 0.001 and 6.4 ± 2.6 vs 6.0 ± 2.5, P< 0.001, respectively). Parents with higher numeracy were also more likely to report that they would have enrolled their child in the study had it been real (6.5 ± 3.0 vs 5.9 ± 2.9, P< 0.001, respectively). Higher parental NFC was associated with significantly improved verbatim understanding but had no apparent effect on gist understanding.
Table 4.
Adequate Gist and Verbatim Understanding by Numeracy and Need for Cognition (%)
| Adequate Gist Understanding‡ | OR (95% CI) | Adequate Verbatim Understanding§ | OR (95% CI) | |
|---|---|---|---|---|
| Numeracy: | ||||
| Low (R) | 56.8 | 1.75 (1.55, 1.98) | 41.4 | 2.75 (2.84, 3.21) |
| High | 69.7* | 66.7* | ||
| NFC: | ||||
| Low (R) | 61.8 | 1.12 (0.99, 1.27) | 50.6 | 1.29 (1.15, 1.45) |
| High | 64.5 | 56.9* |
(R) = Reference group. OR = Odds Ratio, CI = Confidence Interval
P< 0.001 vs Reference group
Adequate gist knowledge = ≥ 3 correct answers out of 4
Adequate verbatim knowledge = ≥ 5 correct answers out of 7
Low numeracy = 0-35, High numeracy = 36-48 on the Subjective Numeracy Scale15
Low NFC (Need for Cognition) = 0-21, High NFC = ≥ 22
d) Socio-demographic Variables
Table 5 compares parents’ understanding by socio-demographic variables. Results showed that gist and verbatim understanding were greater among subjects who were Caucasian and who had higher education. Hispanic participants also demonstrated significantly greater verbatim understanding than African Americans. Across all scenarios, Caucasian parents perceived both the risks and benefits to be lower compared with parents of other race/ethnicities (5.7 ± 2.4 vs 6.1 ± 2.5, P< 0.001 and 6.1 ± 2.5 vs 6.5 ± 2.6, P< 0.001, respectively). Males had similar gist understanding compared with females but significantly better verbatim understanding. Subjects whose first language was English had significantly greater gist understanding than those whose primary language was other than English.
Table 5.
Adequate Gist and Verbatim Understanding by Socio-Demographics (%)
| |
Adequate Gist Understanding‡ |
OR (95% CI) |
Adequate Verbatim Understanding§ |
OR (95% CI) |
|---|---|---|---|---|
| Age (yrs) | ||||
| 25-34 (R) | 64.3 | 53.1 | ||
| 35-44 | 63.6 | 0.97 (0.84, 1.12) | 54.5 | 1.06 (0.92, 1.21) |
| 45-55 |
61.6 |
0.89 (0.76, 1.05) |
54.3 |
1.05 (0.88, 1.22) |
| Gender: | ||||
| Male (R) | 62.8 | 58.8 | ||
| Female |
63.4 |
1.01 (0.97, 1.06) |
50.5* |
0.86 (0.81, 0.90) |
| Race/ethnicity: | ||||
| Caucasian (R) | 67.3 | 60.1 | ||
| African American | 52.4* | 0.54 (0.45, 0.64) | 34.2* | 0.35 (0.29, 0.42) |
| Hispanic |
56.5* |
0.63 (0.53, 0.76) |
41.0*‡ |
0.46 (0.39, 0.55) |
| Education: | ||||
| ≤ High school (R) | 54.7 | 40.7 | ||
| Some college | 62.7* | 1.39 (1.18, 1.64) | 51.6* | 1.49 (1.26, 1.77) |
| ≥Bachelor's Degree |
68.1*† |
1.77 (1.49, 2.09) |
63.4*† |
2.42 (2.05, 2.86) |
| Prior Research subject | ||||
| Yes (R) | 65.9 | 58.0 | ||
| No |
63.0 |
0.96 (0.89, 1.03) |
53.6 |
0.92 (0.85, 1.01) |
| English Language | ||||
| Yes (R) | 63.6 | 54.1 | ||
| No | 56.5* | 0.89 (0.79, 0.99) | 49.0 | 0.90 (0.79, 1.03) |
(R) = Reference group. OR = Odds Ratio, CI = Confidence Interval
P< 0.05 vs Reference group, ‡P< 0.01 vs African American
P< 0.01 vs some college/trade school
Adequate gist knowledge = ≥ 3 correct answers out of 4
Adequate verbatim knowledge = ≥ 5 correct answers out of 7
DISCUSSION
This study examined parents’ understanding of the risks and benefits of a hypothetical clinical drug trial, posed in various risk/benefit trade-off scenarios. Results suggest that the “No trade-off“ scenario, the only scenario that included both increased benefit and reduced risks, appeared to evoke a higher level of scrutiny among participants than did any of the three scenarios which only involved reductions in the risk of complications.
Waters et al. suggest that medical tradeoff decisions require patients to consider at least 4 probabilities related to the risks and benefits of accepting or rejecting a treatment.11 Our illustrative study scenarios were even more complex, reflecting the reality that most clinical interventions have more than 1 benefit and/or 1 risk. In our hypothetical study, parents were required to consider the relative likelihoods of the primary drug benefit (pain relief) and two attendant side-effects (itching and slowed breathing) between two drugs. Faced with this complex interpretative task, only a quarter of the participants had adequate understanding of the benefits (pain relief) of the study. Yet, participants who viewed the no trade-off scenario showing increased pain relief were not only more knowledgeable about benefits (as one might expect), but were also more so of the magnitude of the risk reductions associated with the experimental treatment. Such knowledge gains could imply that learning that the trial intervention might increase pain relief heightened participants’ attention to the details of the risk/benefit information being shown. Alternatively, it is possible that the 3 trade-off scenarios posed additional cognitive tasks that may have interfered with the participants’ ability to concentrate on the required calculations.
Also, of interest was the observation that subjects randomized to the no tradeoff scenario not only accurately perceived the benefit of drug B to be greater but also perceived the risks of itching and slowed breathing to be significantly less than did participants who viewed the other scenarios that offered the same probability of risk but with less benefit. This pattern is consistent with the so-called “affect heuristic,” which is the tendency to rely on the affective or emotional valence of option characteristics (perceived “goodness” or “badness”) in decision making.22-24 Because of the affect heuristic, most people expect things which have high benefits (a “good”) also to have low risks, even though benefits and risks are often positively, rather than negatively, correlated in the real world.
This affect heuristic is particularly relevant in decision making about clinical research. Because parents and patients tend to react to treatment alternatives (in research or otherwise) based on the feelings that each alternative evokes in them, certain types of research – those that evoke feelings of being “good” – will likely engender higher levels of participation than others. While most parents would agree that reducing the side-effects of analgesia is valuable, such risk reductions do not have the emotional power of relieving more pain. The different levels of knowledge, risk perceptions, and willingness to participate among parents who received the no trade-off scenario versus the other conditions probably reflect this difference in affect.
The comprehension of probability information requires a certain degree of cognitive effort and, as such, may pose a challenge to many lay individuals. In our study, cognitive effort was lessened somewhat by presenting incremental risk which simultaneously provides information regarding the baseline risk and the actual change in risk, and thus does not require an arithmetic calculation.10 Indeed, Waters et al. have shown that decreasing the amount of cognitive effort results in greater accuracy in trade-off decisions.11 However, although the use of incremental changes in risks and benefits may have reduced the cognitive effort required by the participants in this study, the ability to understand the information was, nevertheless, strongly associated with their numeracy. This is important given that approximately 22% of Americans demonstrate “below basic” numeracy25 and this has may be a barrier to the communication and understanding of basic medical data.19, 20, 26-28 Low numeracy has also been shown to affect risk and benefit perception, compliance with treatment, utility elicitation, and decision-making.29, 30 In one study, Fagerlin et al. showed that innumerate women were more likely to overestimate their risk of breast cancer30 and in another, low numeracy patients had significantly higher expectations of treatment benefit than numerate individuals.31 Similarly, our findings demonstrate that parents with poor numeracy have less understanding of research risks and benefits and when presented with identical risks, tended to overestimate them compared to their more numerate counterparts.
Results also showed that understanding was moderated by the education level and race of the participants. Caucasian parents were shown to have significantly greater gist and verbatim understanding of the information compared with participants of different race/ethnicities. Recently, Rajakumar et al. showed that compared with white parents, African Americans had significantly greater distrust regarding their child's participation in research and perceived the risk of research to be higher.32
Results of this study should be interpreted in the context of the potential limitations. First, this study involved an Internet survey and, as such, may have been biased towards those with the benefit of Internet access. Our use of an Internet survey was based on previous experience and the fact that this approach allowed us to conduct a randomized controlled experiment using a large diverse number of individuals quickly. Second, responses to the survey were based on a hypothetical clinical study and therefore may not represent “real life” attitudes and perceptions. Nevertheless, there is compelling evidence to show that hypothetical methodologies are integral to emotional theory construction and that behaviors based on real versus hypothetical situations are highly correlated.33, 34
This study identified several factors that moderate parents’ understanding and perceived salience of the risks and benefits of a clinical study. In particular, it draws clinicians’ and researchers’ attention to the reality that people do not evaluate whether or not to participate in research in the abstract. Specific details about the potential interventions, such as whether the study arms offer the possibility of increased benefit versus risk reduction, appear to affect not only parent attitudes but also their ability to process the study information in the first place. Identification of such factors is important in that it reminds us that medical and research information presented in a “one size fits all format” denies both the heterogeneity of clinical situations and the ability of many individuals to assimilate and fully comprehend the material. These data, therefore, inform and motivate further study to examine ways in which research information can be “tailored” to the study design and the characteristics and learning abilities of the individual parent or research participant and thus better ensure their informed decision-making.
Sensitivity Analysis.
A sensitivity analysis using different cut-points for the definition of “adequate” resulted in qualitatively similar results. For example, adequate gist measured as ≥ 2 correct answers out of 4 revealed that 79.8, 76.3, 77.5 and 73.7% of parents in the no trade-off, risk1, risk 2, and risk 3 scenarios, respectively fulfilled this criterion compared with 65.0, 57.8, 59.8, and 56.0% using a criterion of ≥ 3 correct answers out of 4. Similarly, adequate verbatim measured as ≥ 5 correct answers out of 7 revealed that 62.3, 54.3, 50.0, and 49.0% of parents in the no trade-off, risk1, risk 2 and risk3 scenarios, respectively fulfilled this criterion compared with 48.7, 41.5, 36.0, and 34.4% using a criterion of ≥ 6 correct answers out of 7.
ACKNOWLEDGMENTS
The authors wish to thank Bob Burbach and Aaron Pearlman for their help with the design and development of the Internet survey. We also thank Julie Parow, Rosemarie Pitsch, and Nicole Exe for their help in testing the survey instrument. Finally, we thank Dr. Peter Ubel for his insightful comments.
Supported in part by a grant to Dr. Tait from The National Institutes of Health, NICHD (R01 HD053594). Dr. Zikmund-Fisher is supported by a career development award from the American Cancer Society (MRSG-06-130-01-CPPB), and Dr. Fagerlin was supported by an MREP early career award from the U. S. Department of Veteran's Affairs. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors have no financial or commercial interests pertaining to this study. This study has not been registered as a clinical trial.
APPENDIX
GIST QUESTIONS:
Based on all the information that the researcher gave you about Drug A and Drug B, please answer the following questions:
Who is less likely to experience pain after surgery: A child who was randomized to receive Drug A, or a child who was randomized to receive Drug B?
-
□
Child who received Drug A
-
□
Child who received Drug B
-
□
They are equally likely
Who is more likely to experience itching: A child who received Drug A or a child who received Drug B?
-
□
Child who received Drug A
-
□
Child who received Drug B
-
□
They are equally likely
Who is more likely to experience slowed breathing: A child who received Drug A or a child who received Drug B?
-
□
Child who received Drug A
-
□
Child who received Drug B
-
□
They are equally likely
If a child was randomized to Drug B, which of the following is most likely?
-
□
Experiencing pain after surgery
-
□
Experiencing itching
-
□
Experiencing slowed breathing
VERBATIM QUESTIONS
Based on the information given to you by the researcher, please answer the following questions:
If 100 children took Drug B, approximately how many would experience pain after surgery?
_______children
If 100 children took Drug B, approximately how many would experience itching?
________children
If 100 children took Drug B, approximately how many would experience slowed breathing?
________children
Compared to children who take Drug A, approximately how many fewer (more) children would experience pain relief after surgery if they took Drug B?
_______children
Compared to children who took Drug A, approximately how many fewer children would experience itching if they took Drug B?
________children
Compared to children who took Drug A, approximately how many more (fewer) children would remain pain free if they took Drug B?
________children
Compared to children who took Drug A, approximately how many fewer children would experience slowed breathing if they took Drug B?
________children
RISK/BENEFIT QUESTIONS
Based on the information that the researcher gave you with respect to the risks and benefits of the study:
If this was a real study, how likely would you be to allow your child to participate?
In making a decision about your child's participation in the study, how do the risks and benefits of the study compare?
The risks outweigh the benefits
The benefits outweigh the risks
The risks and benefits are equal
How much risk do you think your child would face if s/he participated in the study?
How much risk do you think that this study poses to other children who participate?
How much benefit do you think your child would get from participating in the study?
How much do you think this study will benefit society (i.e., children undergoing surgery).
REFERENCES
- 1.Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research. JAMA. 2004202(13):1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 2.Tait AR, Voepel-Lewis T, Malviya S. Factors that influence parents’ assessments of the risks and benefits of research involving their children. Pediatrics. 2004;113:727–732. doi: 10.1542/peds.113.4.727. [DOI] [PubMed] [Google Scholar]
- 3.Vallance J, Ahmed M, Dhillon B. Cataract surgery and consent ; recall, anxiety, and attitude toward trainee surgeons preoperatively and postoperatively. J Cataract Refract Surg. 2004;30:1479–1485. doi: 10.1016/j.jcrs.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 4.Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk-benefit information. Hlth Affairs. 2007;26:741–748. doi: 10.1377/hlthaff.26.3.741. [DOI] [PubMed] [Google Scholar]
- 5.Zikmund-Fisher B, Sarr B, Fagerlin A, Ubel P. A matter of perspective: Choosing for others differs from choosing for yourself in making treatment decisions. J Gen Intern Med. 2006;21:618–622. doi: 10.1111/j.1525-1497.2006.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonas E, Schultz-Hardt S, Frey D. Giving advice or making decisions in someone else's place: the influence of impression, defense, and accuracy motivation on the search for new information. Pers Soc Psychol Bull. 2005;31:977–990. doi: 10.1177/0146167204274095. [DOI] [PubMed] [Google Scholar]
- 7.Kray L, Gonzalez R. Differential weighting in choice versus advice: I'll do this, you do that. J Behav Decis Making. 1999;12:207–217. [Google Scholar]
- 8.Hawley S, Zikmund-Fisher B, Ubel P, Jancovic M, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Counsel. 2008;73:448–455. doi: 10.1016/j.pec.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Tait AR, Voepel-Lewis T, Zikmund-Fisher B, Fagerlin A. The effect of format on parents’ understanding of the risks and benefits of clinical research: A comparison between text, tables, and graphics. J Hlth Comm. 2010 doi: 10.1080/10810730.2010.492560. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zikmund-Fisher B, Fagerlin A, Roberts T, Derry H, Ubel P. Alternate methods of framing information about medication side effects: Incremental risk versus total risk difference. J Hlth Comm. 2008;13:107–124. doi: 10.1080/10810730701854011. [DOI] [PubMed] [Google Scholar]
- 11.Waters E, Weinstein N, Colditz G, Emmons K. Formats for improving risk communication in medical tradeoff decision. J Hlth Comm. 2006;11:167–182. doi: 10.1080/10810730500526695. [DOI] [PubMed] [Google Scholar]
- 12.Edwards A, Elwyn G, Covey J, Matthews E, Pill R. Presenting risk information-A review of the effects of “framing” and other manipulations on patient outcomes. J Hlth Comm. 2001;6:61–82. doi: 10.1080/10810730150501413. [DOI] [PubMed] [Google Scholar]
- 13.Kahneman D, Slovic P, Tversky A. Judgment under uncertainty: Heuristics and biases. Cambridge University Press; Cambridge: 1982. [DOI] [PubMed] [Google Scholar]
- 14.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- 15.McNeil B, Pauker S, Sox H, Jr., Tversky A. On the elicitation of preferences for alternative therapies. NEJM. 1982;306:1259–1262. doi: 10.1056/NEJM198205273062103. [DOI] [PubMed] [Google Scholar]
- 16.Rothman A, Martino S, Bedell B, Detweiler J, Salovey P. The systematic influence of gain- and loss-framed messages on interest in and use of different types of health behavior. Personal Social Psychol Bull. 1999;25:1355–1369. [Google Scholar]
- 17.Forrow L, Taylor W, Arnold R. Absolutely relative: how research results are summarized can affect treatment decisions. Am J Med. 1992;92:121–124. doi: 10.1016/0002-9343(92)90100-p. [DOI] [PubMed] [Google Scholar]
- 18.Malenka D, Baron J, Johansen S, Wahrenberger J, Ross J. The framing effect of relative and absolute risk. J Gen Intern Med. 1993;8:543–548. doi: 10.1007/BF02599636. [DOI] [PubMed] [Google Scholar]
- 19.Fagerlin A, Zikmund-Fisher B, Ubel P, Jankovic A, Derry H, Smith D. Measuring numeracy without a math test: Development of the subjective numeracy scale (SNS). Med Dec Making. 2007;27:672–680. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 20.Zikmund-Fisher B, Smith D, Ubel P, Fagerlin A. A validation of the subjective numeracy scale: effects of low numeracy on comprehension of risk communication and utility elicitations. Med Dec Making. 2007;27:663–671. doi: 10.1177/0272989X07303824. [DOI] [PubMed] [Google Scholar]
- 21.Cacioppo J, Petty R, Kao C. The efficient assessment of need for cognition. J Personality Assess. 1984;48:306–307. doi: 10.1207/s15327752jpa4803_13. [DOI] [PubMed] [Google Scholar]
- 22.Finucane M, Alhakami A, Slovic P, Johnson S. The affect heuristic in judgments of risks and benefits. J Behav Decis Making. 2000;13:1–17. [Google Scholar]
- 23.Peters E, Lipkus I, Diefenbach M. The functions of affect in health communications and the construction of health preferences. J Commun. 2006;56(Suppl 1):S40–S62. [Google Scholar]
- 24.Slovic P, Peters E. Risk perception and affect. Curr Dir Psychol Sci. 2006;15:322–325. [Google Scholar]
- 25.Kirsch I, Jungelblut A, Jenkins L, Kalstad A. Adult literacy in America: a first look at the results of the adult national adult literacy survey. National Center for Education Statistics; Washington, DC: 1993. [Google Scholar]
- 26.Schwartz L, Woloshin S, Black W, Welch H. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127:966–972. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 27.Lipkus I, Samsa G, Rimer B. General performance on a numeracy scale among highly educated samples. Med Dec Making. 2001;21:37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 28.Woloshin S, Schwartz L. How can we help people make sense of medical data. Effective Clin Pract. 1999;2:176–183. [PubMed] [Google Scholar]
- 29.Davids S, Schapira M, McAuliffe T, Nattinger A. Predictors of pessimistic breast cancer risk perceptions in a primary care population. J Gen Intern Med. 2004;19:310–315. doi: 10.1111/j.1525-1497.2004.20801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fagerlin A, Zikmund-Fisher B, Ubel P. How making a risk estimate can change the feel of that risk: Shifting attitudes towards breast cancer risk in a general public survey. Patient Educ & Counsel. 2005;57:294–299. doi: 10.1016/j.pec.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Weinfurt K, Castel L, Li Y. The correlation between patient characteristics and expectations of benefit from phase I clinical trials. Lancet. 2003;359:1124–1125. doi: 10.1002/cncr.11483. [DOI] [PubMed] [Google Scholar]
- 32.Rajakumar K, Thomas S, Musa D, Alamario D, Garza M. Racial differences in parents’ distrust of medicine and research. Arch Pediatr Adolesc Hlth. 2009;163:108–114. doi: 10.1001/archpediatrics.2008.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jago A, Vroom V. Predicting leader behavior from a measure of behavioral intent. Acad Manage J. 1978;21:715–721. [Google Scholar]
- 34.Robinson M, Clore G. Simulation, scenarios, and emotional appraisal: Testing the convergence of real and imagined reactions to emotional stimuli. Personal Soc Psychol Bull. 2001;27:1520–1532. [Google Scholar]