Abstract
Background
South African guidelines recommend protease-inhibitor (PI)-based antiretroviral therapy (ART) with lopinavir/ritonavir (LPV/r) for HIV-infected children <36 months of age. We investigated factors associated with viral suppression and mortality among young children initiating ART.
Methods
Treatment-naive, ART-eligible, HIV-infected children, ages 6-104 weeks, were enrolled in an ART strategies trial in South Africa and initiated PI-based ART. Mortality and the probability of viral suppression (HIV RNA <400 copies/ml) by 39 weeks after ART initiation, were investigated.
Results
254 children initiated ART of whom 39% were co-treated for TB during follow up. The mortality rate was 14%. Factors predicting mortality were lower pre-ART weight-for-age z-sores and higher HIV RNA. 84% of surviving children suppressed by 39 weeks. Children not co-treated for TB were more likely to suppress (94.8%) than children co-treated at ART initiation (74.2%) or who started TB co-treatment after ART initiation (51.6%), (p<0.0001). Other factors predicting lower probability of viral suppression were lower pre-ART weight- and length-for-age z-scores, higher HIV RNA and WHO stage.
Conclusions
High rates of viral suppression can be achieved among infants and young children initiating PI-based ART. Co-treatment for TB reduced viral suppression. How best to treat HIV-infected children who require TB treatment warrants urgent investigation.
Keywords: virologic suppression, pediatric HIV Infection, TB treatment, antiretroviral therapy, age
Introduction
In 2007 an estimated 33.2 million people had HIV, including 2.5 million children <15 years of age.[1] In infants and young children, immune system immaturity and high viral loads lead to a high risk of rapid disease progression [2, 3] and, in sub-Saharan Africa, without access to treatment, ~53% of infected children die by 2 years of age.[4] In past years, increased availability of highly active antiretroviral therapy (ART) has resulted in significant health improvements for HIV-infected children in high prevalence, resource-constrained settings.[5]
Recently, a ground-breaking trial demonstrated a significant reduction in mortality in children initiating ART during the first months of life.[6] Accordingly, the World Health Organization revised pediatric treatment guidelines to recommend ART initiation for all infected infants <12 months of age regardless of clinical or immunologic status.[7] Guidelines also advise that infants previously exposed to nevirapine for prevention of mother-to-child transmission (PMTCT) receive ART with two nucleoside reverse transcriptase inhibitors (NRTI) and a boosted protease inhibitor (PI).[7] While good viral responses have been reported among older children receiving PI-based ART [8, 9], data are limited among infants and young children.
In this study we examined mortality and viral response to PI-based ART in a cohort of children <2 years of age enrolled in the pre-randomization phase of an ART strategies trial in Johannesburg, South Africa. Specifically, we evaluated the effectiveness of PI-based treatment by adherence, tuberculosis (TB) co-treatment, age and pretreatment characteristics, including CD4 count and viral load.
Methods
Study design
We report results from the pre-randomization phase of an ART strategies trial.[10] HIV-infected children aged 6-104 weeks, exposed to nevirapine for PMTCT and eligible for ART based on immunologic or clinical criteria but who were otherwise treatmentnaive, were enrolled between April 2005 and July 2007 at one site in Johannesburg, South Africa. All children were treated with PI-based ART until they became eligible for randomization (earliest 24 weeks on therapy) or until 52 weeks. The study was approved by the Institutional Review Boards of Columbia University and the University of the Witwatersrand and the child’s guardian signed informed consent. For this analysis, we included only follow-up time accrued prior to randomization.
Drug regimens
All children ≥6 months of age were treated with lopinavir/ritonavir (LPV/r) (250mg/m2), stavudine (d4T) (1mg/kg) and lamivudine (3TC) (4mg/kg) every 12 hours following South African guidelines.[11] Children <6 months of age or those receiving TB treatment received ritonavir (RTV) (400-450 mg/m2), d4T and 3TC every 12 hours. When children passed age 6 months, or after completing TB treatment, RTV was switched to LPV/r. At the time of study, “super-RTV-boosted” LPV or doubling the LPV/r dose had not yet been included in South African guidelines; RTV was the PI recommended for young (<6mo) and TB co-treated children [11]. Due to poor pharmacokinetic data, double-dose LPV/r is no longer recommended [12]. At each visit, doses were adjusted according to body surface area. All medications were administered as syrups.
TB diagnosis was made on clinical grounds; diagnostic tests were performed when available. Data on the diagnostic tests were not systematically collected. If clinicians felt that TB treatment was indicated, it was initiated and the children’s ART regimens were changed accordingly. TB treatment was prescribed according to South African guidelines[13]: rifampin and isoniazid for 6 months with pyrazinamide during the initial 2 months. With concomitant Bacillus Calmette-Guérin (BCG)-disease ethionamide was added and treatment duration extended to 9 months. TB treatment was also prescribed for some children with BCG-disease only: treatment consisted of rifampin, isoniazid and ethionamide for 9 months. BCG vaccination is given routinely at birth in South Africa.
Study measurements
Blood samples drawn prior to ART initiation were tested for CD4 count and HIV RNA quantity using the standard assay (quantification range 400-750,000 copies/ml Roche Amplicor, Branchburg, NJ). Blood samples were repeated at weeks 4, 8, 16, 24, 36 and 52 post-ART (the latter two time points only if not yet randomized) and tested for HIV RNA. The ultra-sensitive test (quantification range of 50-150,000 copies/ml (Roche)) was usually used but occasionally due to errors or clinical expectations that HIV RNA may be high, the standard test was used instead. CD4 cell counts were repeated at 4, 16, 24, 36 and 52 weeks post-ART initiation. At each visit, weight and length were measured and concomitant medications were recorded. Children were examined by a physician monthly and whenever medically warranted. Counseling regarding medication administration, social needs and adherence, was provided for the children’s caretakers.
Adherence assessments
At each visit, caretakers were asked to return all medication bottles and were queried about adherence.. The pharmacists weighed the bottles and reconciled the contents with the expected usage of each drug since the previous visit. Caretakers’ reports included missed doses for time intervals: 1-2 weeks, 2-4 weeks, 5-12 and >12 weeks prior to the visit. For this analysis, we utilized week 4, 12, 24 and 39 data.
Statistical methods
We compared demographic and clinical variables between different subgroups pre-treatment using Wilcoxon test for continuous and Chi-squared or Fisher’s exact tests for categorical variables. Main outcome measures were mortality and viral suppression (<400copies/ml). Less than 400 copies/ml was selected as this measurement was available on all children. Independent variables were age, weight-for-age Z (WAZ)-scores, height-for-age Z (HAZ)-scores, CD4%, CD4 cell count, WHO stage, adherence, TB treatment. WAZ-scores and HAZ-scores were calculated using WHO software.[14] Using Kaplan-Meier methods we calculated for each independent variable the probability of death, and of viral suppression by 39 weeks of ART. Follow-up time was censored at randomization or at time of last study assessment and was truncated at 39 weeks after ART initiation. Cox proportional hazards regression was used for multivariable analyses. TB co-treatment was investigated as a time-dependent variable and as a fixed covariate. Variables were retained in final models if significantly associated with the main outcome (p<0.05) or if their inclusion led to changes in the estimated Hazard Ratio (HR) of other covariates by more than 10%.
To categorize adherence assessed via unused medication we assigned the child at each visit to one of four categories: a) returning 10% more than expected, b) returning 10% less than expected, c) returning −10% to +10% of the anticipated drug volume, and d) not returning the bottles. Adherence over the entire follow-up period was defined hierarchically (a>b>c>d), meaning that once a child was classified as “returning more” he/she stayed in this category throughout the remaining follow-up period. This classification was used for each drug independently and for all drugs combined. Based on the observed relationship between viral suppression and these categories, we combined b and c into an ‘adherent’ category “adherent” and a and d a “non-adherent” category in terms of medication return. For caretaker reports, patients were classified as non-adherent if the caregiver ever reported ≥1missed dose at any visit. All analyses were performed using SAS 9.1.3 (Cary, NC).
Results
Study population
Of 272 treatment-naive children enrolled, 9 (3.3%) died and 9 (3.3%) children withdrew before ART initiation. Characteristics of the 254 children who initiated ART are shown in Table 1. A RTV-based regimen was initiated because of young age in 54/254 (21.3%) and because of TB co-treatment in 62/254 (24.4%) children. By 39 weeks, 27/254 (10.6%) children were lost to-follow-up and 32/254 (14%) children had died. The median duration of follow-up on therapy was 36 weeks [IQR: 26-47 weeks].
Table 1.
Characteristics of 254 HIV-infected infants and young children (6-104 weeks of age) at initiation of protease inhibitor-based antiretroviral therapy
| Total initiated ART | Ritonavir | Lopinavir/Ritonavir | Ritonavir vs. Lopinavir/r |
|
|---|---|---|---|---|
| N | 254 | 116 | 138 | |
| Male n (%) | 132(52) | 56(48) | 76(55) | |
| Median age in months [IQR] |
8.75 (5.2-13.8) | 5.65(4.1-13.4) | 9.44(6.9-14.2) | <0.0001 |
| Median HIV RNA in copies/ml [IQR] |
750,000 (642,000-750,000) |
750,000 (668,000-750,000) |
750,000 (442,000-750,000) |
0.133 |
| Median CD4 cell count in cells/mm3 [IQR] |
869.0 (466-1392) |
868 (446-1404) |
870.5 (473-1390) |
0.781 |
| Median CD4% [IQR] | 18.95(12.8-24.5) | 17.9(12.8-25.6) | 19.2(13.1-24.1) | 0.973 |
| Mean weight-for-age z-score in Standard deviation (SD) |
−2.38(1.72) | −2.49(1.71) | −2.29(1.72) | 0.293 |
| Mean height-for-age z-score in Standard deviation (SD) |
−3.45(1.72) | −3.53(1.83) | −3.38(1.61) | 0.558 |
| WHO stage n (%) | ||||
| I | 39(15.4%) | 16(13.8%) | 23(16.7%) | |
| II | 11(4.3%) | 1(0.9%) | 10(7.3%) | |
| III | 130(51.2%) | 62(53.5%) | 68(49.3%) | |
| IV | 74(29.1%) | 37(31.9%) | 37(26.8%) | 0.071 |
TB treatment
At ART initiation, 24.4% were receiving TB treatment and through 39 weeks an additional 37 (14.6%) children started TB co-treatment. Children who initiated TB treatment before ART had lower median pre-treatment CD4%, were more wasted and growth retarded and were older than children never co-treated (Table 2). For those on TB treatment at ART initiation, TB treatment continued on average for 30 weeks [interquartile range (IQR): 25-37 weeks]. Children co-treated for TB after ART initiation had higher HIV RNA viral loads than those never co-treated (Table 2). For those who initiated TB co-treatment after ART, the median time between ART start and TB co-treatment initiation was 4 weeks [IQR: 3-9 weeks] and the median duration of TB treatment was 24 weeks [IQR: 13-29 weeks].
Table 2.
Characteristics of 254 HIV-infected children (6-104 weeks of age) initiating protease inhibitor-based antiretroviral treatment stratified by TB co-treatment status
| never TB co-treatment |
TB treatment before ART initiation |
TB co-treatment after ART initiation |
before vs. never |
after vs. never |
|
|---|---|---|---|---|---|
| N | 155 | 62 | 37 | ||
| Median age in months [IQR] |
7.8 (4.8-12.6) | 13.2 (9.1-17.7) | 6.84 (4.7-8.8) | <0.0001 | 0.111 |
| Median HIV RNA in copies/ml [IQR] |
750,000 (593,000- 750,000) |
750,000 (367,000-750,000) |
750,000 (750,000- 750,000) |
0.526 | 0.039 |
| Median CD4% [IQR] | 20.7 (13.7- 26.9) |
13.9 (9.9-19.7) | 17.2 (13.4-25.5) | <0.0001 | 0.110 |
| Mean weight-for-age z- score in Standard deviation (SD) |
−2.23 (1.8) | −2.67 (1.7) | −2.55 (1.6) | 0.024 | 0.116 |
| Mean height-for-age z- score in Standard deviation (SD) |
−3.28 (1.7) | −3.90 (1.6) | −3.44 (2.0) | 0.019 | 0.642 |
| WHO stage n (%) | |||||
| I | 30 (19.4) | 3 (4.8) | 6 (16.2) | ||
| II | 9 (5.8) | 1 (1.6) | 1 (2.7) | ||
| III | 79 (51.0) | 33 (53.2) | 18 (48.7) | ||
| IV | 37 (23.9) | 25 (40.3) | 12 (32.4) | 0.007 | 0.660 |
| Start regimen includes Lopinavir/Ritonavir, n (%) |
112 (72.3) | 0 (0.0) | 26 (70.3) | ||
| Start regimen includes Ritonavir, n (%) |
43 (27.7) | 62 (100.0) | 11 (29.7) | <0.0001 | 0.840 |
Mortality
Mortality in the first 39 weeks of ART was associated with a lower WAZ-score and higher HIV RNA viral load at treatment initiation (Table 3). The mortality rate was 31% among children with a WAZ-score <−4 vs. 8% among those with WAZ-scores >−2 (HR=1.6 [95%CI 1.2-2.2]; p=0.002). Children with HIV RNA ≥750,000 copies/ml had a 19% probability of dying compared to 4% among those with lower viral loads. The association between death and HIV RNA was slightly attenuated after adjustment for pre-treatment CD4%, WAZ-score and age at ART initiation (HR=3.2 [95%CI: 0.970-10.8]; p=0.06). Children who initiated TB co-treatment after ART had a 22% probability of dying by the end of 39 weeks compared to 11% among those never treated for TB and 14% among those who initiated TB co-treatment before ART start, but these differences were not significant. If TB co-treatment was included as a time-dependent variable there was still no significant association between TB co-treatment and mortality (HR 1.65; 95%CI 0.80-3.42).
Table 3.
Factors at time of ART initiation associated with mortality during the first 39 weeks of treatment
| Kaplan-Meier results | Cox Proportional Hazards results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| died | alive | probability of dying |
log rank p- value |
crude Hazard Ratio |
95% CI | p-value | adjust ed Hazard Ratio |
95%CI | p-value | |
| Total | 32 | 222 | 14.0 | |||||||
| Sex | ||||||||||
| male | 18 | 114 | 14.7 | ref | ref | |||||
| female | 14 | 108 | 13.2 | 0.547 | 0.81 | 0.40-1.62 | ||||
| Age in months | ||||||||||
| < 12 | 27 | 148 | 17.6 | 2.54 | 0.98-6.61 | 0.055 | 2.44 | 0.92-6.45 | 0.073 | |
| ≥ 12 | 5 | 74 | 6.4 | 0.047 | ref | ref | ref | ref | ||
| Weight-for-age z-score a | ||||||||||
| > −2 | 8 | 103 | 8.1 | |||||||
| > −3 to −2 | 3 | 53 | 6.5 | |||||||
| > −4 to −3 | 8 | 34 | 20.3 | |||||||
| ≤ −4 | 13 | 32 | 30.8 | 0.001 | 1.75 | 1.30-2.36 | 0.0002 | 1.61 | 1.19-2.19 | 0.002 |
| HIV RNA in copies/ml * | ||||||||||
| < 750,000 | 3 | 76 | 3.8 | ref | ref | ref | ref | |||
| ≥ 750,000 | 29 | 137 | 19.3 | 0.005 | 4.71 | 1.44- 15.48 |
0.011 | 3.22 | 0.97- 10.78 |
0.057 |
| Pretreatment CD4% | ||||||||||
| < 15 | 17 | 78 | 19.1 | 1.97 | 0.99-3.95 | 0.055 | 1.72 | 0.84-3.50 | 0.136 | |
| ≥ 15 | 15 | 144 | 11.1 | 0.051 | ref | ref | ref | ref | ||
| TB treatment | ||||||||||
| never | 16 | 139 | 11.2 | ref | ref | |||||
| Start before ART | 8 | 54 | 13.9 | 1.10 | 0.47-2.56 | |||||
| Start after ART | 8 | 29 | 22.4 | 0.321 | 1.88 | 0.80-4.41 | ||||
in Cox proportional hazard analyses included as continues variable
9 children did not have a pre-treatment viral load
Virologic, clinical and immunological response
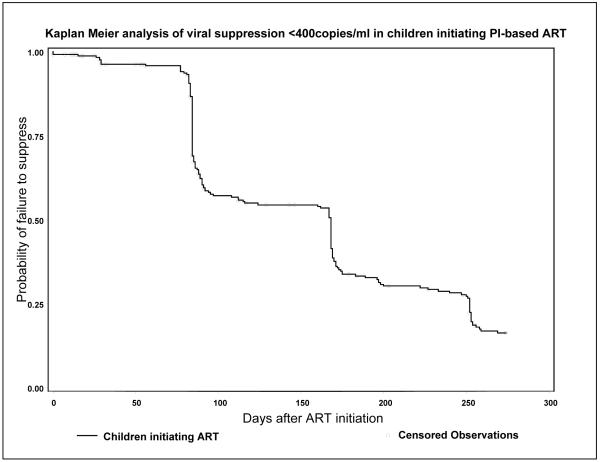
Initiation of ART was associated with improvements in clinical, immunological and virologic characteristics. The probability of viral suppression was 45.6% by the end of 12 weeks, 70.8% by the end of 26 weeks and 83.7% by the end of 39 weeks after ART initiation (Figure 1). Among the 179 children who suppressed, 170 (95%) had an ultra-sensitive assay, of whom 87(51.2%) and 111(65.3%) respectively had ≥1 measurement <50 copies/ml by 24 and 39 weeks. The WAZ-score increased from - 2.38±1.8 at ART initiation to 0.82±1.3 39 weeks later and the median increase in CD4% was 9.4 [IQR: 4.3-15.7]. CD4% changes were similar regardless of TB co-treatment. The median increase in CD4% during the first 39 weeks of ART was 10.4% [IQR: 4.3-17.5] among children never co-treated, compared to 8.3% [IQR: 5.2-11.4, p=0.16] among those with TB treatment at ART initiation and 9.4% [IQR: 3.1-13.4, p=0.24] among children co-treated for TB after ART initiation.
Figure 1.
Predictors of viral suppression
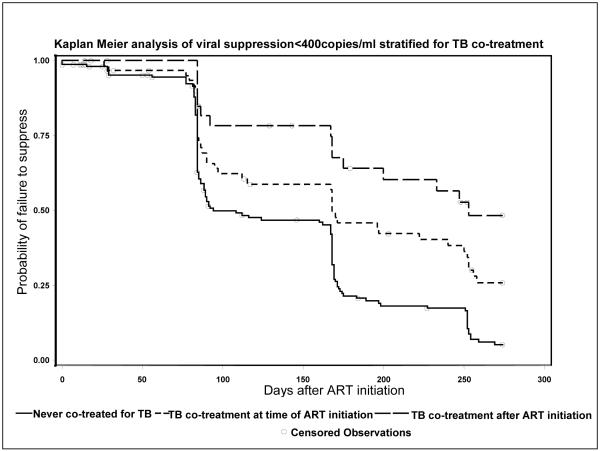
In univariate analysis, pre-treatment WHO stage, CD4%, HAZ-score, WAZ-score, HIV RNA and TB co-treatment were associated with viral suppression (Table 4, Figure 2). In multivariate analysis, pre-treatment WAZ-score and HIV RNA quantity, and TB co-treatment remained associated with viral suppression after adjustment (Table 4). If TB co-treatment was entered as a time-dependent covariate the association with viral suppression was similar (HR=0.54[95%CI 0.37-0.79]; p=0.002) after adjusting for WAZ and viral load. There were no differences in suppression probabilities among children never co-treated for TB whether they initiated RTV-based (91% probability of suppression) or LPV/r-based ART (96.3% probability of suppression) (logrank p=0.19).
Table 4.
Factors associated with viral suppression to HIV RNA < 400copies/ml
| Kaplan-Meier results | Cox Proportional Hazards results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| suppressed | not suppressed | probability of suppressing |
log rank p- value |
crude Hazard Ratio |
95%CI | p-value | adjusted Hazard Ratio |
95%CI | p-value | |
| Total | 179 | 75 | 83.7 | |||||||
| Sex | ||||||||||
| male | 91 | 41 | 82.6 | ref | ref | |||||
| Female | 88 | 34 | 85.1 | 0.860 | 0.98 | 0.73-1.31 | 0.870 | |||
| Age in months | ||||||||||
| < 6 | 52 | 27 | 84.6 | ref | ref | |||||
| ≥ 6 - < 12 | 67 | 29 | 84.0 | 1.0 | 0.71-1.47 | 0.910 | ||||
| ≥ 12 - ≤ 24 | 60 | 19 | 83.3 | 0.990 | 1.0 | 0.71-1.50 | 0.872 | |||
| WHO stage | ||||||||||
| I + II | 44 | 6 | 94.9 | ref | ref | |||||
| III+IV | 135 | 69 | 80.8 | 0.006 | 0.63 | 0.45-0.89 | 0.008 | |||
| Weight-for-age z-score a | ||||||||||
| > −2 | 93 | 18 | 95 | |||||||
| > −3 to −2 | 39 | 17 | 76.7 | |||||||
| > −4 to −3 | 25 | 17 | 78.7 | |||||||
| ≤ − 4 | 22 | 23 | 67.7 | 0.001 | 0.77 | 0.66-0.88 | 0.0002 | 0.78 | 0.68-0.91 | 0.001 |
| Height-for-age z-score a | ||||||||||
| > −2 | 39 | 6 | 93.4 | |||||||
| > −3 to −2 | 45 | 12 | 88.9 | |||||||
| > −4 to −3 | 44 | 21 | 84.7 | |||||||
| ≤ −4 | 51 | 36 | 73.3 | 0.019 | 0.82 | 0.72-0.93 | 0.003 | |||
| CD4% | ||||||||||
| < 15 | 59 | 36 | 77.9 | 0.67 | 0.50-0.92 | 0.013 | ||||
| ≥ 15 | 120 | 39 | 87 | 0.009 | ref | ref | ||||
| HIV RNA in copies/ml * | ||||||||||
| < 750,000 | 68 | 11 | 93.1 | ref | ref | ref | ref | |||
| ≥ 750,000 | 103 | 63 | 78.4 | <0.0001 | 0.47 | 0.35-0.64 | <0.0001 | 0.52 | 0.38-0.71 | <0.0001 |
| TB treatment | ||||||||||
| never | 123 | 32 | 94.8 | ref | ref | ref | ref | |||
| Start before ART | 41 | 21 | 74.2 | 0.55 | 0.38-0.78 | 0.0001 | 0.54 | 0.37-0.79 | 0.001 | |
| Start after ART | 15 | 22 | 51.6 | <0.0001 | 0.30 | 0.17-0.51 | <0.0001 | 0.36 | 0.21-0.61 | 0.0002 |
in Cox proportional hazard analyses included as continues variable
9 children did not have a treatment naïve viral load
Figure 2.
Of 41 children who started TB treatment before ART and suppressed, 22 (53.6%) suppressed while being co-treated and 19 (46.4%) after completing TB therapy. The median time to suppression was 14 weeks [IQR: 12-24.weeks], which did not differ from time to suppression among 123 children never co-treated (median 13 weeks, [IQR 12-24]; p=0.47). Among 15 children starting TB co-treatment after ART initiation who suppressed, one (6.7%) was suppressed before TB co-treatment initiation, 12 (80%) suppressed while being co-treated and two (13.3%) after completing TB therapy. The average time from ART initiation until the first viral load <400 copies/ml was 24 weeks [IQR 12-29 weeks], which did not differ from those never co-treated (p=0.21).
Viral rebound
The probability of viral rebound (>400 copies/ml) within 16 weeks of viral suppression was 17.6%. Among all factors investigated, only TB co-treatment was associated with the risk of viral rebound. Of 15 children who initiated TB co-treatment after ART and suppressed, 8 (53.3%) had viral rebound (>400 copies/ml) compared to 12% among those without TB and 2.8% among those who started TB co-treatment before ART initiation (HR=5.2 [95%CI: 2.1-12.9]; p<0.0001).
Adherence
There was no association between caretakers’ reports of adherence and viral suppression. Cumulatively by the end of 39 weeks, the caretakers of 23.5% of the children who suppressed and 21.2% of those who did not reported ever having missed a drug dose (p=0.80). In contrast, non-adherence based on medication return was significantly associated with less viral suppression. Among the children classified as non-adherent via medication return (n=91), 78.3% suppressed after 39 weeks of ART compared to 86.5% among those classified as adherent (n=154) (HR=0.66 [95%CI: 0.47-0.93]; p=0.02).
There was a non-significant trend towards worse adherence based on medication reconciliations by TB co-treatment status (40.3%, 43.2% and 34.3% of children were classified as non-adherent among those co-treated for TB at ART initiation, after ART initiation and never treated for TB, respectively). Among children who were never treated for TB (n=155), the probability of viral suppression was high regardless of adherence measured via medication return (92.1% and 96.1% suppressed among non-adherent vs. adherent respectively; p=0.14). The association between adherence and suppression in the never TB co-treated sub-group became significant in the multivariate analysis after adjusting for pre-treatment viral load and WAZ-score (HR 0.64 [95%CI: 0.42-0.98], p=0.04). Among children ever on TB treatment (n=99), viral suppression occurred among 58.9% of non-adherent children compared to 70.5% of adherent children (crude HR 0.81 [95%CI: 0.47-1.4]; p=0.44; adj HR 0.77 [95%CI: 0.43-1.4]; p=0.38). After adjusting for adherence, the association between TB co-treatment and viral suppression remained significant (HR=0.54 [95% CI 0.37-0.78] among children co-treated for TB at ART initiation and HR 0.36 [95%CI 0.21-0.62] among children co-treated after ART initiation).
Discussion
In this study of infants and young children with advanced HIV disease, high rates of viral suppression were achieved with PI-based ART. Overall 83.7% achieved a viral load <400 copies/ml by 39 weeks. Of those who suppressed, 17.6% had viral rebound within 16 weeks after suppression. These high rates of viral suppression are comparable to reports of other cohorts of children receiving PI-based ART.[8, 9, 15] A multicenter study, including data from the United States, Canada, Argentina, Bahamas, Panama and South Africa[9], reported good clinical response with robust weight gain and immunologic improvements, consistent with findings in other studies evaluating immunological response.[5, 8, 16] At the same time, although virologic response was excellent, mortality rates in our cohort were high in the first weeks of treatment most likely due to advanced disease at ART initiation.[17]
Children with higher pre-treatment viral loads and lower WAZ-scores were less likely to suppress, consistent with previous studies.[18-21] However, children receiving TB co-treatment at time of ART initiation and, in particular, those who initiated TB co-treatment after ART initiation, were less likely to suppress and more likely to rebound than children who were not co-treated. Given the relatively short follow up, it is possible that this rebound rate is a minimum estimate. This finding is important given the magnitude of the TB epidemic in high HIV burden countries. A study from the Western Cape estimated 23.4 cases of active TB per 100 HIV-infected children per year[22] and TB is the most common HIV-associated infection.[23] In sub-Saharan Africa, the high rate of concomitant TB[24] and BCG-related disease after ART initiation[10, 25] further complicate ART in children.
Rifampin is part of standard TB treatment in South Africa and is also used as part of treatment of BCG disease. Rifampin, along with the NRTIs and PIs, is a strong inducer of cytochrome P450 enzymes. Drug-drug interactions may result in sub-therapeutic antiretroviral plasma concentrations.[26-28] In adults co-administration of rifampin with ritonavir-boosted LPV and ritonavir-boosted atazanavir regimens result in sub-therapeutic PI concentrations.[26, 27] A recent pediatric pharmacokinetic study reported decreased LPV plasma concentrations in children on TB co-treatment who received LPV/r at double the current therapeutic dose.[12] Such interactions may partially account for our findings of lower rates of viral suppression with TB co-treatment.
Studies in adults have shown complex associations between TB co-treatment and ART.[29-34] Those studies reporting no associations between suppression rates and TB co-treatment[30-34] mostly did not distinguish between different ART regimens and, in one study, rifabutin was used.[30] Drug-drug interactions may also result in inadequate levels of TB medications, particularly rifampicin, and possible under-treatment of TB. Two recent pediatric studies reported considerably lower serum values for TB drugs than the suggested lower limits in adults [35, 36].
RTV was recommended for children on ART requiring TB treatment at the time this study was initiated. RTV is no longer recommended, but optimal ART for children requiring TB co-treatment is still controversial. In contrast to the findings of studies with standard PI-regimens, and the pediatric study of double-dose LPV/r, [12] two recent studies suggest that “super-boosted” LPV treatment i.e. LPV/r with additional ritonavir, provides adequate LPV plasma concentrations when administered in combination with rifampin.[27, 37] However super-boosted dosing has been associated with high rates of hepatotoxicity in adults[27] raising challenges for appropriate treatment recommendations.
Another possible explanation for our findings might be differences in adherence between TB co-treated and untreated groups due to the poor palatability of RTV,[38] or the increased medication burdens associated with treating two diseases. There was a suggestion in our data that the high burden of multiple medications influenced adherence, as those who were co-treated for TB were slightly less likely to be adherent. These results are difficult to interpret since caretakers’ reports may be biased towards socially-acceptable responses and, given the necessity of repeat dosing in young children through spitting etc., medicine reconciliations are difficult to interpret.
RTV may be less potent than RTV-boosted LPV and, in adults, PI-resistance occurs more often with unboosted than boosted PI-based therapy.[39] Chadwick and colleges reported good virological response in children on RTV-based treatment at 36 weeks, but at 104 weeks only 36% had viral loads <400 copies/ml. [15] Children in our study who received RTV because of their young age, and not because of TB co-treatment, had similar outcomes to children who started on LPV/r–based regimen. However, children were switched to LPV/r once they reached 6 months of age, which may have masked differences.
Rather than direct effects, the observed association may be due to confounding by the severity of disease between those who did and did not receive TB co-treatment. Children who initiated TB treatment before ART had lower pre-treatment CD4% and children co-treated for TB after ART had higher pre-treatment viral loads than children never co-treated. Such children may also have advanced and rapidly progressing HIV disease which might explain the lower suppression probabilities. We adjusted for these baseline factors but may not have been able to fully account for all differences.
Another possible mechanism to explain differences in suppression probabilities relates to inter-individual differences in immunological and viral responses to therapy. An insufficient immunological response after ART initiation might lead to a higher risk of developing TB, or conditions that might be confused with TB, and these processed, rather than exposure to the drugs, could be related to failure to suppress resulting in reverse causality. A study among South African adults[33] observed a smaller median CD4 cell-count increase after ART initiation among those who developed TB compared to those without TB. As a consequence they concluded an association between suboptimal immunological response to ART and development of TB. However, in our study there was no difference in the median CD4% change between children in the different TB treatment exposure categories. Overall, the median CD4% increase in our study was comparable with other pediatric studies.[8, 9, 16, 40] Immune reconstitution inflammatory syndrome (IRIS) might be another explanation. Many of the children initiating TB co-treatment after ART meet criteria for IRIS. We have previously observed BCG and TB-related IRIS to be common (21%) and to adversely affect viral suppression rates.[10] However, this would not explain the poor virologic response among children who initiated TB treatment before ART.
There are several limitations to our study. We stratified into TB co-treatment groups rather than TB-disease groups, given the difficulties of diagnosing TB in young children. The diagnosis of TB was made based on clinical findings, chest x-rays and exposure history. Sputum cultures were rarely performed and gamma-interferon release assays are not used. Therefore, it is possible that some children on TB treatment did not have TB infection. Our findings highlight the urgent need to improve TB diagnostics for children as TB co-treatment in the ART-treated child may have adverse consequences. Another limitation of our study is that no further viral load quantification ≥750,000 copies/ml was done pre-treatment limiting our ability to adjust for pre-treatment viral load and potentially contributing to residual confounding.
In conclusion, high rates of viral suppression can be achieved among infants and young children initiating PI-based ART. Our study suggests that RTV-based ART in children <2 years who are co-treated for TB is associated with lower rates of viral suppression. How best to diagnose TB in infants and young children remains an ongoing challenge. The ideal treatment strategy for young children who require co-treatment with antiretroviral and antituberculous therapy remains elusive. Given that many children will be co-treated for TB, there is an urgentneed to determine optimal drug regimens and dosing to ensure successful outcomes for HIV-infected children initiating ART.
Acknowledgments
The authors would like to acknowledge and thank all participating patients and their parents as well as all the staff members at the study site, past and present. Renate Strehlau, Leigh Maartens, Eloise Malan, Gillian Barry, Belinda Marais, Dafni Zisis, Judy Rothberg, Komeela Naidoo Salome Madumo,Lucia Thomas, Sunet Potgieter, Gift Ngwenya, Khumbuzile Mncube, Lois Nakan, Lindie Plaatjies, Amanda Smith, Vanessa Kok, Sally Dalene Maclear, Audrey Mosima and Maria Moshoeshoe.
Sources of funding: The study was supported in part by grants from the National Institutes of Child Health and Human Development (NICHD) HD 47177 and Bristol Myers-Squibb Secure the Future Foundation RES 219.
Part of the data were presented at the 16th Conference on Retroviruses and Opportunistic Infections (CROI 2009), February 8-11 2009, Montreal, Canada, Abstract 910
Footnotes
Conflict of interest statement: The authors do not have a commercial or other association that might pose a conflict of interest.
References
- 1.World Health Organization (WHO) AIDS epidemic update. WHO UNAIDS; 2007. Vol. UNAIDS/07.27E / JC1322E. [Google Scholar]
- 2.Obimbo EM, Wamalwa D, Richardson B, et al. Pediatric HIV-1 in Kenya: pattern and correlates of viral load and association with mortality. J Acquir Immune Defic Syndr. 2009;51:209–15. doi: 10.1097/qai.0b013e31819c16d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palumbo PE, Raskino C, Fiscus S, et al. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA. 1998;279:756–61. doi: 10.1001/jama.279.10.756. [DOI] [PubMed] [Google Scholar]
- 4.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 5.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–89. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 6.Violari A, Cotton MF, Gibb DM, et al. Early Antiretroviral Therapy and Mortality among HIV-Infected Infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization W . Revised treatment recommendations for infants. WHO; Apr 10-11, 2008. pp. 1–10. 2008 ed. [Google Scholar]
- 8.Jaspan HB, Berrisford AE, Boulle AM. Two-Year Outcomes of Children on Non-Nucleoside Reverse Transcriptase Inhibitor and Protease Inhibitor Regimens in a South African Pediatric Antiretroviral Program. Pediatr Infect Dis J. 2008 doi: 10.1097/INF.0b013e31817acf7b. [DOI] [PubMed] [Google Scholar]
- 9.Saez-Llorens X, Violari A, Deetz CO, et al. Forty-eight-week evaluation of lopinavir/ritonavir, a new protease inhibitor, in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2003;22:216–24. doi: 10.1097/01.inf.0000055061.97567.34. [DOI] [PubMed] [Google Scholar]
- 10.Smith K, Kuhn L, Coovadia A, et al. Immune Reconstitution Inflammatory Syndrome among HIV-infected South African Infants Initiating Antiretroviral Therap. AIDS. 2009 doi: 10.1097/QAD.0b013e32832afefc. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.South African Government . In: South African Guidelines, Factsheet Section 10, Antiretroviral. Government SA, editor. South African Government; 2007. pp. 76–95. [Google Scholar]
- 12.Helen McIlleron YR, Nuttall J, Riddick A, Kleynhans L, Rabie H, Cotton M, Eley B, Merry C, Maartens G. Double-dose Lopinavir/Ritonavir Provides Insufficient Lopinavir Exposure in Children Receiving Rifampicin-based Anti-TB Treatment; 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 13.Harry Hausler for the National Department of Health South Africa . In: Tuberculosis and HIV/AIDS, Clinical Guidelines. Africa NDoHS, editor. National Department of Health; Cape Town: 1999. [Google Scholar]
- 14.World Health Organization W . WHO Anthro (version 2) and macros. World Health Organization (WHO); 2005. 2005 ed. Vol. Version 2. [Google Scholar]
- 15.Chadwick EG, Rodman JH, Britto P, et al. Ritonavir-based highly active antiretroviral therapy in human immunodeficiency virus type 1-infected infants younger than 24 months of age. Pediatr Infect Dis J. 2005;24:793–800. doi: 10.1097/01.inf.0000177281.93658.df. [DOI] [PubMed] [Google Scholar]
- 16.Walker AS, Doerholt K, Sharland M, Gibb DM. Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV Paediatric Study. AIDS. 2004;18:1915–24. doi: 10.1097/00002030-200409240-00007. [DOI] [PubMed] [Google Scholar]
- 17.The KIDS-ART-LINC Collaboration Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49:523–31. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 18.Bekker V, Scherpbier HJ, Steingrover R, et al. Viral dynamics after starting first-line HAART in HIV-1-infected children. AIDS. 2006;20:517–23. doi: 10.1097/01.aids.0000210605.86009.5e. [DOI] [PubMed] [Google Scholar]
- 19.Nachman SA, Stanley K, Yogev R, et al. Pediatric AIDS Clinical Trials Group 338 Study Team Nucleoside analogs plus ritonavir in stable antiretroviral therapy-experienced HIV-infected children: a randomized controlled trial. JAMA. 2000;283:492–8. doi: 10.1001/jama.283.4.492. [DOI] [PubMed] [Google Scholar]
- 20.Starr SE, Fletcher CV, Spector SA, et al. Pediatric AIDS Clinical Trials Group 382 Team Combination therapy with efavirenz, nelfinavir, and nucleoside reverse-transcriptase inhibitors in children infected with human immunodeficiency virus type 1. N Engl J Med. 1999;341:1874–81. doi: 10.1056/NEJM199912163412502. [DOI] [PubMed] [Google Scholar]
- 21.Watson DC, Farley JJ. Efficacy of and adherence to highly active antiretroviral therapy in children infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1999;18:682–9. doi: 10.1097/00006454-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ. 2007;334:136. doi: 10.1136/bmj.39000.486400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization W . A research agenda for childhood tuberculosis. Improving the management of childhood tuberculosis within national tuberculosis programmes: research priorities based on a literature review. WHO; 2007. pp. 1–124. 2007 ed. Vol. WHO/HTM/TB/2007.381. [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) TB and HIV/AIDS CDC HIV/AIDS Facts. Centers for Disease Control and Prevention (CDC); Jan, 2008. 2008 ed. [Google Scholar]
- 25.Rabie HVA, Madhi S, Gibb DM, Steyn J, van Niekerk R, Josipovic D, Innes S, Dobbels E, Cotton MF. Complications of BCG vaccination in HIV infected and uninfected children; evidence from the Children with HIV Early Antiretroviral Therapy (CHER) Study; 15 Conference on Retroviruses and Opportunistic Infections CROI; Boston. 2008. [Google Scholar]
- 26.Burger D, Agarwala S, Child M, Been-Tiktak A, Wang Y, Bertz R. Effect of rifampin on steady-state pharmacokinetics of atazanavir with ritonavir in healthy volunteers. Antimicrob Agents Chemother. 2006;50:3336–3342. doi: 10.1128/AAC.00461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Porte C, Colbers E, Bertz RJ. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob Agents Chemother. 2004;48:1553–1560. doi: 10.1128/AAC.48.5.1553-1560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton ME, Shaw LM, Schentag JJ, Evans WE. Applied pharmacokinetics and pharmakodynamics, Principles of therapeutic drug monitoring. Fourth Edition ed. Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 29.Boulle A, Van Cutsem G, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–9. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- 30.Breen RA, Miller RF, Gorsuch T, et al. Virological response to highly active antiretroviral therapy is unaffected by antituberculosis therapy. J Infect Dis. 2006;193:1437–40. doi: 10.1086/503437. [DOI] [PubMed] [Google Scholar]
- 31.Edwards SDG, Matthews G, Fox E, Wood C, Navaratne L, Churchill D, Taylor G, Taylor C, De Ruiter A, Pozniak A. Does tuberculosis treatment alter immunological and virological response to HAART?; 7th Conference of retroviral and opportunistic infections; San Francisco. 2000; Vol. Abstract 256. [Google Scholar]
- 32.Hung CC, Chen MY, Hsiao CF, Hsieh SM, Sheng WH, Chang SC. Improved outcomes of HIV-1-infected adults with tuberculosis in the era of highly active antiretroviral therapy. AIDS. 2003;17:2615–22. doi: 10.1097/00002030-200312050-00008. [DOI] [PubMed] [Google Scholar]
- 33.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 34.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 35.Schaaf HS, Willemse M, Cilliers K, et al. Rifampin pharmacokinetics in children, with and without human immunodeficiency virus infection, hospitalized for the management of severe forms of tuberculosis. BMC Med. 2009;7:19. doi: 10.1186/1741-7015-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McIlleron H, Willemse M, Werely CJ, et al. Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis. 2009;48:1547–53. doi: 10.1086/598192. [DOI] [PubMed] [Google Scholar]
- 37.Ren Y, Nuttall JJ, Eley BS, et al. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr. 2009;50:439–43. doi: 10.1097/QAI.0b013e31819c33a3. [DOI] [PubMed] [Google Scholar]
- 38.Davies MA, Boulle A, Fakir T, Nuttall J, Eley B. Adherence to antiretroviral therapy in young children in Cape Town, South Africa, measured by medication return and caregiver self-report: a prospective cohort study. BMC Pediatr. 2008;8:34. doi: 10.1186/1471-2431-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kempf DJ, King MS, Bernstein B, et al. Incidence of resistance in a double-blind study comparing lopinavir/ritonavir plus stavudine and lamivudine to nelfinavir plus stavudine and lamivudine. J Infect Dis. 2004;189:51–60. doi: 10.1086/380509. [DOI] [PubMed] [Google Scholar]
- 40.Faye A, Bertone C, Teglas JP, et al. Early multitherapy including a protease inhibitor for human immunodeficiency virus type 1-infected infants. Pediatr Infect Dis J. 2002;21:518–25. doi: 10.1097/00006454-200206000-00008. [DOI] [PubMed] [Google Scholar]