Abstract
We performed a multicenter cross-sectional study of 134 sexually active systemic lupus erythematosus (SLE) patients to investigate the prevalence of and risk factors for high risk human papilloma virus (HPV) infection and cervical cytological abnormalities among Korean women with SLE. In this multicenter cross-sectional study, HPV testing and routine cervical cytologic examination was performed. HPV was typed using a hybrid method or the polymerase chain reaction. Data on 4,595 healthy women were used for comparison. SLE patients had greater prevalence of high-risk HPV infection (24.6% vs. 7.9%, P<0.001, odds ratio 3.8, 95% confidence interval 2.5-5.7) and of abnormal cervical cytology (16.4 vs. 2.8%, P<0.001, OR 4.4, 95% CI 2.5-7.8) compared with controls. SLE itself was identified as independent risk factors for high risk HPV infection among Korean women (OR 3.8, 95% CI 2.5-5.7) along with ≥2 sexual partners (OR 8.5, 95% CI 1.2-61.6), and Pap smear abnormalities (OR 97.3, 95% CI 6.5-1,456.7). High-risk HPV infection and cervical cytological abnormalities were more common among Korean women with SLE than controls. SLE itself may be a risk factor for HPV infection among Korean women, suggesting the importance of close monitoring of HPV infections and abnormal Pap smears in SLE patients.
Keywords: Systemic Lupus Erythematosus, Human Papilloma Virus; Cervical Cytological Abnormalities
INTRODUCTION
Human papillomavirus (HPV) infection is thought to be principle causal agent for cervical cancer worldwide (1). In particular, persistent infection with high-cancer-risk HPV type 16 and 18 is major risk factor for cervical cancer (2). In the case of immunocompromised host, the risk of HPV infection was reported to be much greater than the general population due to high-load, persistent infection with oncogenic HPV genotypes (3-6). The prevalence of HPV infection was shown to increase in organ transplant recipients (3, 7), human immunodeficiency virus (HIV)-infected women (4-6), and patients with systemic autoimmune diseases such as systemic lupus erythematosus (SLE) (8, 9).
Since SLE is a multi-system disease characterized by production of a vast array of autoantibodies against multiple tissues and organs, multiple factors including genetic and ethnic background, immunological abnormalities, extent of organ damage, and behavioral patterns might account for the increased susceptibility of SLE patients to HPV infection and cervical cancer. The socioeconomic impact of the increased risk of cervical cancer in Korean patients with SLE can be greater since it is known that there is an increase in prevalence of SLE in the Asian population (10). However, there is a paucity of data regarding the prevalence of high risk HPV infection and cervical cytological abnormalities in SLE patients of different ethnic background and regions. Moreover, disease related risk factors for HPV infections in SLE patients remain unclear. The purpose of our study was to investigate the prevalence of and risk factors for HPV infection, and cervical cytological abnormalities in Korean patients with SLE.
MATERIALS AND METHODS
Patients
All patients attending the rheumatology clinic at 7 tertiary hospitals in Korea who fulfilled the 1997 American College of Rheumatology (ACR) criteria for SLE were invited to participate in this cross-sectional study. Participating hospitals were located throughout Korea including Seoul, Gyeonggi-do, Gyeongsang-do, and Jeolla-do regions including both urban and rural areas. Patients were eligible for the study if they had been sexually active. Patients who never had sexual intercourse and patients younger than 20 yr old were excluded. One hundred thirty four SLE patients who signed the informed consent out of total 1525 SLE patients were recruited between February 2006 and March 2007 through a survey in clinic. The study was approved by the local ethics committee of each institution. For comparison, data from a cross-sectional study of women attending the National Cervical Cancer Screening Program carried out by Oh et al. (11) on prevalence of HPV infection of 4595 women in Busan and Suwon, Korea were used. Women attending the National Cervical Cancer Screening Program belonged to mostly low income social groups who visited government affiliated hospitals in both urban and rural areas. The data was supplemented by 113 women aged 20-29 yr who visited local hospital for routine gynecologic care, since the National Cervical Cancer Screening Program included mostly women over 30 yr of age.
Clinical data collection
Patient interviews and chart reviews were performed at the time of the enrollment to obtain information regarding sexual behaviors, obstetric and gynecological histories, and disease history related to SLE. We obtained demographic information including age, marital status, duration of education, and tobacco smoking, and reproductive and sexual history including number of gestations and parity, age at first intercourse, number of sexual partners, and history of sexually transmitted diseases. Disease history related to SLE included disease duration, extent of organ involvement, and type of treatment received. Major organ involvement was defined as involvement of one or more of the following organs: the central nervous system, lung, heart, kidney, intestine, and the hematologic system (hemolytic anemia, platelet <100,000/µL). Treatment with prednisolone >1 mg/kg/day, azathioprine, cyclosporine, mycophenolate mofetil and/or cyclophosphamide was defined as immunosuppressive therapy. Autoantibodies including antinuclear antibody (ANA), anti-double strand DNA (dsDNA), and systemic lupus erythematosus disease activity index (SLEDAI) were assessed at the time of gynecological evaluation.
Gynecological examination and specimen collection
All patients underwent gynecologic examinations, which included HPV testing and Pap smears, by gynecologists at each hospital. HPV DNA tests were performed on exfoliated cervical cells and examined by either Hybrid Capture II (HC-II) technology (The digene HPV test, Gaithersburg, MD, USA) which detected 13 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) or a polymerase chain reaction (PCR) based DNA microarray system (My HPV chip, Mygene, Seoul, Korea), which detect 16 types of high-risk HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68) and 8 types of low-risk HPV (6, 11, 34, 40, 42, 43, 44, 70). Five of the 7 participating centers utilize the Hybrid Capture II technology and 2 utilized the PCR based DNA microarray system. The Hybrid Capture II technology system was used for the community control. Both HC-II and microarrays were highly comparable in terms of sensitivity, specificity, and positive and negative predictive values for detecting HPV in cervical specimens (12). To perform hybrid capture technology, cervical specimens were combined with an extraction buffer to release and denature the HPV DNA. The released DNAs were combined with specific RNA probes to create the RNA-DNA hybrids, which were captured on a solid phase by an antibody specific for the hybrids. The captured RNA-DNA hybrids were tagged with antibody reagent, and measured using a luminometer. To perform PCR based DNA microarrays, target HPV DNA from cervical specimens were amplified by PCR using the primers of MY09/MY11 (5'-CGTCCMARRGGAWACTGATC-3'/5'-GCMCAGGGWCATAAYAATGG-3') and GP5+/GP6+(5'-TTTGTTACTGTGGTAGATACTAC-3'/5'-Cy3-GAAAAATAAACTGTAAATCATATTC-3'). Primers that amplify β-globin were used as controls (5'-Cy3-CAACTTCATCCACGTTCACC-3'/5'-GAAGAGCCAAGGACAGGTAC-3'). The PCR product was hybridized onto the chip at 42℃ for 4 hr and washed with buffer. Hybridized signals were visualized with a DNA chip scanner. If the binding of a high-risk HPV subtype was detected in hybrid capture and microarray tests, it was considered positive for HPV. In case of an abnormal Pap results, a histological examination was done. The interval between Pap smear and histological examination was less than 1 month.
Statistical analysis
Clinical parameters are presented as mean±SD. Comparisons between the SLE patients and the controls were analyzed using the χ2-test. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to estimate the association between each possible risk factor and HPV infection in SLE patients. Independent risk factors for HPV infection in SLE patients were determined using a multiple logistic regression equation. Statistical analysis was done using SPSS 12.0 (SPSS Inc, Chicago, IL, USA) software.
Ethics statement
The study was conducted according to the protocols approved by the institutional ethics committee of each institution in compliance with the Helsinki Declaration (IRB approval numbers: Ewha Womans University Mokdong Hospital; ECT 125-5, Daegu Catholic University Medical Center; CR-06-22-83, Chonnam National University Hospital; 06-nuh, Kyungpook National University Hospital; 7405-1929-2009.07.07, Inha University Hospital; 2006-580, Hanyang University Medical Center; 2006-29, Ajou University Hopital; AJIRB-CRO-06-109).
RESULTS
Characteristics of patients and controls
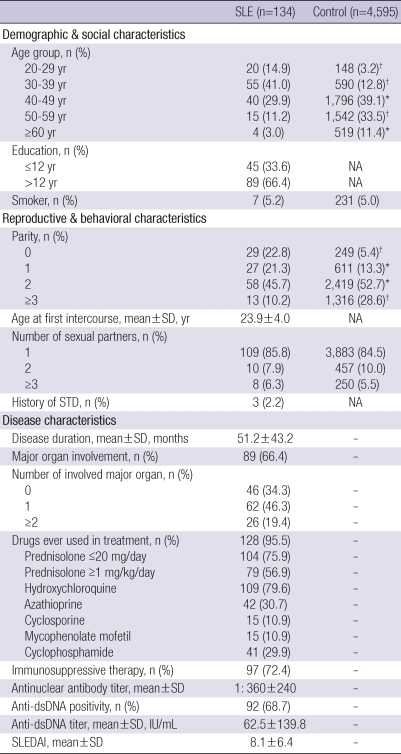
SLE patients and controls were all ethnically Koreans and demographically similar coming from both rural and urban areas, except that SLE patients included younger age group. The mean age of SLE patients was 39.5±9.4 (mean±SD) yr. Individuals younger than 39 yr of age accounted for 55.9% of the patient group, whereas 16.0% in the controls. Reproductive and behavioral characteristics between SLE patients and healthy controls were also similar with the exception of parity. SLE patients showed a significantly lower number of parity compared with the controls. The mean disease duration of SLE patients was 51.2±43.2 months. Major organs were involved in 89 (66.4%) patients, and in 26 (19.4%) patients, two or more multiple major organs were involved. Drug exposures ever during the course of lupus up to the present are as shown in Table 1. Immunosuppressive agents including prednisolone ≥1 mg/kg/day, azathioprine, cyclosporine, mycophenolate mofetil, and cyclophosphamide were prescribed for 97 (72.4%) patients. All patients were ANA positive and the mean titer was 1:360. Anti-dsDNA was observed in 92 (68.7%) of patients, and the mean titer was 62.5 IU/mL. SLEDAI at the time of gynecologic evaluation was 8.1±6.4 (Table 1).
Table 1.
Demographic, social, reproductive, behavioral, and disease characteristics of SLE patients and controls
*P<0.05; †P<0.001.
NA, not available; SLE, systemic lupus erythematosus; STD, sexually transmitted disease; SLEDAI, systemic lupus erythematosus disease activity index.
Prevalence of HPV infection and abnormal cytology in SLE patients
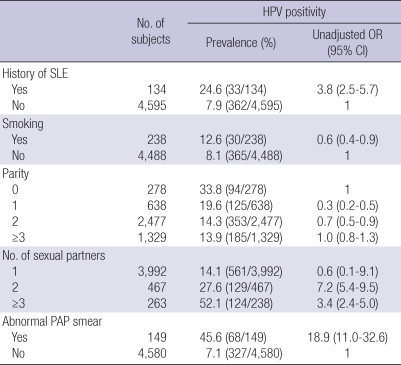
Infection with a high-risk type of HPV was detected in 33 (24.6%), and abnormal Pap smears were observed in 22 (16.4%) of SLE patients. Abnormal Pap smear results included epithelial dysplasia in 8 patients, atypical squamous cells of undetermined significance (ASCUS) in 7 patients, and carcinoma in situ (CIN) in 7 patients. In 15 patients, an abnormal Pap smear was confirmed by histologic evaluation, and CIN was found in 11 (61.1%) of patients. Compared with the controls, SLE patients had a significantly higher prevalence of high-risk HPV infections (24.6% vs. 7.9%, P<0.001, OR 3.8, 95% CI 2.5-5.7). The prevalence of abnormal cervical cytology was also increased in SLE patients compared with controls (16.4% vs. 2.8%, P<0.001, OR 4.4, 95% CI 2.5-7.8).
Risk factors for development of high risk HPV infection
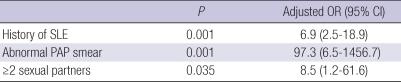
Using univariate analysis, high risk HPV infection was associated with history of SLE (OR 3.8, 95% CI 2.5-5.7), multiple sexual partners (2: OR 7.2, 95% CI 5.4-9.5; ≥3: OR 3.4, 95% CI 2.4-5.0), and abnormal Pap smear (OR 18.9, 95% CI 11.0-32.6). Smoking and parity were not associated with high risk HPV infection (Table 2). Potential risk factors identified by univariate analysis (history of SLE, multiple sexual partners, abnormal Pap smear) including age were put into a multivariate model to identify independent risk factors for high risk HPV infection. Independent risk factors for high risk HPV infection included a history of SLE (OR 6.9, 95% CI 2.5-18.9), ≥2 sexual partners (OR 8.5, 95% CI 1.2-61.6), and Pap smear abnormalities (OR 97.3, 95% CI 6.5-1456.7) (Table 3).
Table 2.
Unadjusted odds ratio for potential risk factors associated with high risk HPV infection
Table 3.
Odds ratios (95% confidence interval) determined by multiple logistic regression model using high risk HPV infection status as a variable
Disease related risk factors for high risk HPV infection in SLE patients
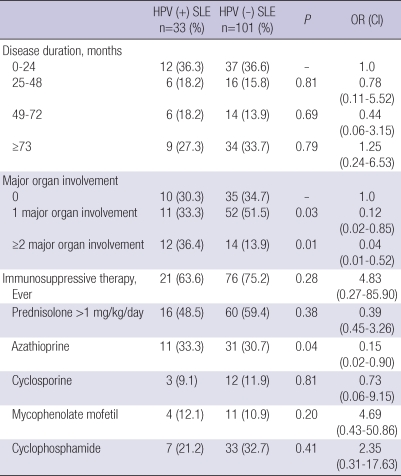
High risk HPV positive and negative SLE patients were compared to determine the possible disease related risk factors for high risk HPV infection in SLE patients (Table 4). Odds ratios adjusted for age, education, smoking, parity, number of sexual partners, age at first intercourse, and history of sexually transmitted diseases were determined by multiple logistic regression analysis. Significant increases in the number of patients with major organ involvement and the use of azathioprine were found in the high risk HPV positive SLE patients compared to the high risk SLE negative patients. However, adjusted odds ratios for these factors were not increased, thus not identified as a disease related risk factors for high risk HPV infection. No significant differences were found in the use of immunosuppressive agents ever, and the disease duration between patients with high risk HPV infection and those without (Table 4).
Table 4.
Disease related risk factors for high risk HPV infection in SLE patients*
*Values are the number (%) of patients.
HPV, human papillomavirus; SLE, systemic lupus erythematosus.
DISCUSSION
Our study suggests that Korean patients with SLE have a significantly increased prevalence of high-risk HPV infection and abnormal cervical cytology compared to controls. SLE itself appears to be the major risk factor for HPV infection.
Increased prevalence of HPV infection has been reported for small groups of SLE patients. In a study of 30 SLE patients by Nath et al. (8) from the United Kingdom, tests for high risk genotype HPV-16 was positive in 57% of SLE patients compared to 1% of controls. A study from of 85 SLE patients from China revealed intermediate- or high-risk HPV genotype infection in 10.6% of SLE patients versus 4.2% in controls (9). In a French study consisted of 11 women with SLE, HPV-positivity was observed in 37.5% of the patients compared to 14.7% of the controls (13). We observed that 24.6% of the 134 Korean SLE patients were infected with high-risk HPV. This represents 3.8 fold increases in the prevalence of high-risk HPV in Korean SLE patients compared to the general population. Our data confirmed the previous observation in other ethnic group that SLE patients were more prone to HPV infection.
The major risk factor for acquisition of HPV infection is high-risk sexual behavior (14). In this regard, the highest prevalence of HPV infection in the general population was noted in sexually active women of 20-24 yr of age, and those with multiple lifetime sexual partners (15). We also found that women with 2 or more sexual partners were 8.5 folds more at risk of acquiring high risk HPV infection. In turn, infection with high risk HPV increased the risk of developing abnormal cytology approximately 100 folds. However, other risk factor previously shown to be associated with HPV infection, cigarette smoking was not associated in our study. This may be due to much lower rate of cigarette smoking in our study population compared to other studies (9, 14). In addition to the external factors such as sexual behavior and cigarette smoking, host factor such as immunocompromised status as in SLE patients, can play a role in the perpetuation of HPV infection.
The possible mechanism for progression of HPV infection, from acquisition to development of cervical dysplasia, is incompletely understood in immunocompromised host such as patients with SLE. However, it can be speculated that multiple derangements of the immune system found in SLE can alter the natural course of the cervicovaginal infection to a more persistent and recurrent infection. Immune derangements in SLE patients include activated humoral immunity, ineffective cell-mediated immunity, and a shift in balance from Th1 to Th2 cytokines (16-18). In healthy individuals, T helper type 1 cells produce immunoregulatory cytokines that increase tumor immunity (19). Therefore, CD4+ cells infiltrating the cervical epithelium can induce regression of low-grade cervical lesions caused by low-risk HPV infection. In the general population, 70% of HPV infections are short-lived and spontaneously resolve in less than 1 yr due to effective viral clearance, especially in individuals under 30 yr of age (20). In cases of ineffective cytotoxicity performed by CD8+ T cells (17) or a shift in the balance of Th1 to Th2 cytokines (16) as seen in SLE, inefficient clearance of HPV occurs, resulting in the persistence of infection. Recurrent and persistent HPV infection has been shown to occur in patients with SLE (8, 9). Our observation that significantly increased number of patients with major organ involvement was found in the high risk HPV positive SLE patients compared to the HPV negative SLE patients suggests that the severity of the immune dysregulation of the host contributes to the increased susceptibility of SLE patients to HPV infection. However, due to small number of patients analyzed, risk for HPV infection was not increased in SLE patients with major organ involvement.
Immunosuppressive drugs used to treat the disease can be another risk factor for HPV infection in SLE patients. Immunosuppressive drugs can directly induce oncogenic cellular mutation or result in ineffective immune protection against oncogenic infection (7). Almost all studies to date reported increases in cervical dysplasia related to immunosuppressive drug use (21-23). In the one study reporting no differences, there was a failure to control concurrently for important clinical variables (9). We did not find a difference in HPV positivity between patients treated with or without immunosuppressive therapy, although use of azathioprine was more common in the high risk HPV positive SLE patients. We did not find increased risk for high risk HPV infection related to the immunosuppressive drug use in the SLE patients. This may be due to the small number of the patients analyzed, or may be related to low sexual activity in active severe SLE patients, which may decrease the chance of HPV infection by sexual intercourse.
The importance of high risk HPV infection is that it is the most important, and a requisite factor for development of cervical cancer, although other factors such as smoking, diet, cervical trauma, HIV co-infection and sexually transmitted infections may also contribute (24). Cervical cancer is still one of the leading causes of death worldwide and half a million of cervical cancers are attributable to HPV infection (25). The significance of HPV infection in SLE patients is greater since most SLE patients are women (10), and they are highly susceptible to become infected with high risk, oncogenic HPV types due to the immunocompromised state of the host, which in turn, lead to cervical cancer (8, 9). The prevalence of abnormal cervical cytology in SLE patients was reported to be much greater than that in the general population (9, 26). We found that cervical cytological abnormalities were increased to 16.4% in SLE patients compared to 2.8% in the general population.
The incidence of cervical cancer has decreased significantly because of the availability of screening tests for cervical cytology combined with HPV tests. The United States recommendations state that high-risk HPV test should be done every 3 yr for immuno-competent women over 30 yr of age with no past history of dysplasia (27). In Korea, current screening for cervical cancer in the general population only includes yearly Pap smears in females with a coitus history (28). However, development of guidelines including HPV testing in cervical cancer screening is currently under discussion. In addition, recent availability of HPV vaccines marked an important milestone in efforts to prevent cervical cancer (29, 30). With these efforts, cervical cancer has become one of the preventable cancers. Since result of our study in accordance with the others alerts the medical community of increased risk of cervical cancer in SLE patients, more stringent criteria should be applied to cervical cancer screening in SLE patients. To achieve this goal, studies examining cost effectiveness of the screening procedure, and efficacy of HPV vaccination in SLE patients should be conducted.
Our study is not without limitations. Because our comparison sample comes from a different baseline general population in regards to regional residence, sexual activity and volunteer bias for low socioeconomic group, these differences might have introduced somewhat biased results. Cross-sectional studies possess limitations compared to prospective studies in evaluating the incidence and the time course of development of HPV infections. However, the strength of this study includes the detailed evaluations of HPV status in a relatively large number of SLE patients. Age and region-matched prospective case-control study will provide more detailed information about risk factors for HPV infection in Korean SLE patients.
High-risk HPV infection and abnormal cervical cytology are more prevalent in Korean female patients with SLE compared with the controls. SLE itself appears to be a risk factor for HPV infection in Korean women. Based on our observations, close monitoring of HPV infections and abnormal PAP smears are recommended for immune-compromised individuals with SLE.
ACKNOWLEDGMENTS
The authors would like to thank the gynecologists who participated in this study. Without their dedication, the project would not have been possible.
Footnotes
This work was supported in part by the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, republic of Korea (A080588).
References
- 1.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roka S, Rasoul-Rockenschaub S, Roka J, Kirnbauer R, Muhlbacher F, Salat A. Prevalence of anal HPV infection in solid-organ transplant patients prior to immunosuppression. Transpl Int. 2004;17:366–369. doi: 10.1007/s00147-004-0738-z. [DOI] [PubMed] [Google Scholar]
- 4.Heard I, Tassie JM, Schmitz V, Mandelbrot L, Kazatchkine MD, Orth G. Increased risk of cervical disease among human immunodeficiency virus-infected women with severe immunosuppression and high human papillomavirus load(1) Obstet Gynecol. 2000;96:403–409. doi: 10.1016/s0029-7844(00)00948-0. [DOI] [PubMed] [Google Scholar]
- 5.Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC., Jr Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med. 1997;337:1343–1349. doi: 10.1056/NEJM199711063371903. [DOI] [PubMed] [Google Scholar]
- 6.Stricker H, Colucci G, Godio M, Mossi G, Mombelli G. The influence of a prolonged sitting position on the biochemical markers of coagulation activation in healthy subjects: evidence of reduced thrombin generation. J Thromb Haemost. 2003;1:380–381. doi: 10.1046/j.1538-7836.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 7.Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80(2 Suppl):S254–S264. doi: 10.1097/01.tp.0000186382.81130.ba. [DOI] [PubMed] [Google Scholar]
- 8.Nath R, Mant C, Luxton J, Hughes G, Raju KS, Shepherd P, Cason J. High risk of human papillomavirus type 16 infections and of development of cervical squamous intraepithelial lesions in systemic lupus erythematosus patients. Arthritis Rheum. 2007;57:619–625. doi: 10.1002/art.22667. [DOI] [PubMed] [Google Scholar]
- 9.Tam LS, Chan AY, Chan PK, Chang AR, Li EK. Increased prevalence of squamous intraepithelial lesions in systemic lupus erythematosus: association with human papillomavirus infection. Arthritis Rheum. 2004;50:3619–3625. doi: 10.1002/art.20616. [DOI] [PubMed] [Google Scholar]
- 10.Rus V ME, Hochberg MC. Wallace DJ, Hahn BH. Dubois' lupus erythematosus. Philadelphia: Lippincott Williams & Wilkins; 2007. The epidemiology of systemic lupus erythematosus; pp. 65–83. [Google Scholar]
- 11.Oh JK, Franceschi S, Kim BK, Kim JY, Ju YH, Hong EK, Cahang YC, Rha SH, Kim HH, Kim JH, Kim CY, Shin HR. Prevalence of human papillomavirus and Chlamydia trachomatis infection among women attending cervical cancer screening in the Republic of Korea. Eur J Cancer Prev. 2009;18:56–61. doi: 10.1097/CEJ.0b013e328305a0a6. [DOI] [PubMed] [Google Scholar]
- 12.Kim CJ, Jeong JK, Park M, Park TS, Park TC, Namkoong SE, Park JS. HPV oligonucleotide microarray-based detection of HPV genotypes in cervical neoplastic lesions. Gynecol Oncol. 2003;89:210–217. doi: 10.1016/s0090-8258(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 13.Berthier S, Mougin C, Vercherin P, Desmurs H, Gil H, de Wazieres B, Dupond JL. Does a particular risk associated with papillomavirus infections exist in women with lupus? Rev Med Interne. 1999;20:128–132. doi: 10.1016/s0248-8663(99)83029-x. [DOI] [PubMed] [Google Scholar]
- 14.Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, Miller S, Clayton L, Forhat S, Broering J, Darragh T, Palefsky J. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 15.Datta SD, Koutsky LA, Ratelle S, Unger ER, Shlay J, McClain T, Weaver B, Kerndt P, Zenilman J, Hagensece M, Suhr CJ, Weinstock H, Helmerhorst TJ. Human papillomavirus infection and cervical cytology in women screened for cervical cancer in the United States, 2003-2005. Ann Intern Med. 2008;148:493–500. doi: 10.7326/0003-4819-148-7-200804010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Bais AG, Beckmann I, Lindemans J, Ewing PC, Meijer CJ, Snijders PJ, Helmerhorst TJ. A shift to a peripheral Th2-type cytokine pattern during the carcinogenesis of cervical cancer becomes manifest in CIN III lesions. J Clin Pathol. 2005;58:1096–1100. doi: 10.1136/jcp.2004.025072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padula SJ, Clark RB, Korn JH. Cell-mediated immunity in rheumatic disease. Hum Pathol. 1986;17:254–263. doi: 10.1016/s0046-8177(83)80218-4. [DOI] [PubMed] [Google Scholar]
- 18.Pisetsky DS. The role of innate immunity in the induction of autoimmunity. Autoimmun Rev. 2008;8:69–72. doi: 10.1016/j.autrev.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Alcocer-Gonzalez JM, Berumen J, Tamez-Guerra R, Bermudez-Morales V, Peralta-Zaragoza O, Hernandez-Pando R, Moreno J, Gariglio P, Madrid-Marina V. In vivo expression of immunosuppressive cytokines in human papillomavirus-transformed cervical cancer cells. Viral Immunol. 2006;19:481–491. doi: 10.1089/vim.2006.19.481. [DOI] [PubMed] [Google Scholar]
- 20.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 21.Nyberg G, Eriksson O, Westberg NG. Increased incidence of cervical atypia in women with systemic lupus erythematosus treated with chemotherapy. Arthritis Rheum. 1981;24:648–650. doi: 10.1002/art.1780240503. [DOI] [PubMed] [Google Scholar]
- 22.Bateman H, Yazici Y, Leff L, Peterson M, Paget SA. Increased cervical dysplasia in intravenous cyclophosphamide-treated patients with SLE: a preliminary study. Lupus. 2000;9:542–544. doi: 10.1177/096120330000900711. [DOI] [PubMed] [Google Scholar]
- 23.Ognenovski VM, Marder W, Somers EC, Johnston CM, Farrehi JG, Selvaggi SM, McCune WJ. Increased incidence of cervical intraepithelial neoplasia in women with systemic lupus erythematosus treated with intravenous cyclophosphamide. J Rheumatol. 2004;31:1763–1767. [PubMed] [Google Scholar]
- 24.Castellsague X, Munoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis--role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003;31:20–28. [PubMed] [Google Scholar]
- 25.World Health Organization.int [website on the internet] World Health Organization; c2008. [accessed on Sep 5, 2008]. [updated 2007] Available from http://www.who.int/hpvcentre/en. [Google Scholar]
- 26.Bernatsky S, Boivin JF, Joseph L, Rajan R, Zoma A, Manzi S, Ginzler E, Urowitz M, Gladman D, Fortin PR, Petri M, Edworthy S, Barr S, Gordon C, Bae SC, Sibley J, Isenberg D, Rahman A, Aranow C, Dooley MA, Steinsson K, Nived O, Sturfelt G, Alarcon G, Senecal JL, Zummer M, Hanly J, Ensworth S, Pope J, El-Gabalawy H, McCarthy T, St Pierre Y, Ramsey-Goldman R, Clarke A. An international cohort study of cancer in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1481–1490. doi: 10.1002/art.21029. [DOI] [PubMed] [Google Scholar]
- 27.Wright TC, Jr, Schiffman M, Solomon D, Cox JT, Garcia F, Goldie S, Hatch K, Noller KL, Roach N, Runowicz C, Saslow D. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304–309. doi: 10.1097/01.AOG.0000109426.82624.f8. [DOI] [PubMed] [Google Scholar]
- 28.Cancer.go.kr [website on the internet] National Cancer Information Center; C2008. [accessed on Aug 28, 2008]. Available from http://www.cancer.go.kr. [Google Scholar]
- 29.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. Sustained efficacy up to 4.5 yr of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 30.Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, Brown DR, Ferenczy A, Harper DM, Koutsky LA, Kurman RJ, Lehtinen M, Malm C, Olsson SE, Ronnett BM, Skjeldestad FE, Steinwall M, Stoler MH, Wheeler CM, Taddeo FJ, Yu J, Lupinacci L, Railkar R, Marchese R, Esser MT, Bryan J, Jansen KU, Sings HL, Tamms GM, Saah AJ, Barr E. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24:5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]