Abstract
Transcranial direct current stimulation (tDCS) is associated with enhancement or weakening of the NMDA receptor activity and change of the cortical blood flow. Therefore, repeated tDCS of the brain with cerebrovascular injury will induce the functional and histologic changes. Sixty-one Sprague-Dawley rats with cerebrovascular injury were used. Twenty rats died during the experimental course. The 41 rats that survived were allocated to the exercise group, the anodal stimulation group, the cathodal stimulation group, or the control group according to the initial motor function. Two-week treatment schedules started from 2 days postoperatively. Garcia, modified foot fault, and rota-rod performance scores were checked at 2, 9, and 16 days postoperatively. After the experiments, rats were sacrificed for the evaluation of histologic changes (changes of the white matter axon and infarct volume). The anodal stimulation and exercise groups showed improvement of Garcia's and modified foot fault scores at 16 days postoperatively. No significant change of the infarct volume happened after exercise and tDCS. Neuronal axons at the internal capsule of infarct hemispheres showed better preserved axons in the anodal stimulation group. From these results, repeated tDCS might have a neuroprotective effect on neuronal axons in rat stroke model.
Keywords: Cerebrovascular Trauma, Electrical Stimulation, Neuroprotection, Exercise, White Matter
INTRODUCTION
Many trials have been performed to find potential stroke treatments, such as, intravenous tissue plasminogen activator, neuroprotective agents, and stem cell transplantation (1, 2). Recently, noninvasive brain stimulation techniques, such as, repetitive transcranial magnetic stimulation and transcranial direct current stimulation (tDCS) have been introduced and studied in many clinical settings (3-5).
Transcranial direct current stimulation (tDCS) is used to polarize local brain regions by the non-invasive application of weak direct currents. The procedure elicits focal reversible shifts in cortical excitability, modulates brain function (6), and can enhance motor function in stroke patients temporarily. Repeated tDCS has been associated with a significant motor function improvement in stroke patients and the effect lasted for 2 weeks after the treatment (7). However, the events made in the injured brain are unknown and the mechanism of the motor recovery is uncertain.
Recently, several mechanisms have been proposed. Ardolino et al. (8) suggested that after-effects of tDCS had a non-synaptic mechanism of action based upon changes in neural membrane function. Nitsche et al. (9) revealed that long-lasting after-effects of tDCS were associated with enhancement or weakening of the NMDA receptor activity depending on the polarity of the stimulation (modification of NMDA receptor efficacy). Direct current stimulation alters the resting membrane potential of receptors and therefore, repeated tDCS may affect the efficacy of the NMDA receptor agonist or antagonist. Recent studies (10, 11) have revealed that several channels including glutamate and NMDA receptors are related to the cell death and that NMDA receptor blockade attenuates white matter injury in rat stroke model. Considering that repeated tDCS may affect the efficacy of the NMDA receptor agonist or antagonist, tDCS may affect the white matter protection from the ischemic injury in the acute phase of stroke through the modulation of NMDA receptors.
Another mechanism of tDCS to modify brain function is the change of regional cerebral blood flow in many cortical and subcortical regions, which was proven by PET study (12). A recent study (13) also supported this change using the functional near-infrared spectroscopy. Therefore, repeated tDCS will affect the infarct size by changing blood flow in cortical and subcortical regions.
In view of the above, it appears that repeated tDCS may have an effect on functional and histologic changes from ischemic injury. However, no study has been conducted about these changes. The purpose of this study is to reveal the histologic changes of the injured brain and the changes of the motor function after repeated tDCS in rat stroke model.
MATERIALS AND METHODS
All experimental protocols were approved by the Seoul National University Animal Research Committee.
Experimental design
Sixty-one 5-week-old Sprague-Dawley rats were allowed to adapt for a week at our Clinical Research Institute. Permanent left ischemic middle cerebral artery occlusion (MCAO) rat models were produced using a Longa method (14). At postoperative day (POD) 2, Garcia's motor behavioral test (15), the modified foot fault test (16), and the rota rod performance test (16) were performed to evaluate motor function. Animals were then divided into 4 groups (Garcia's motor behavior scores were matched to within 2 points); the exercise group (n=15), the anodal stimulation group (n=15), the cathodal stimulation group (n=16), and the control group (n=15). Animals underwent tDCS, exercise, or no treatment daily for 2 weeks. We anesthetized rats with 1% ketamine (15 mL/kg) before the tDCS. The reason for the use of anesthetics was that the movement of an awakened rat would change the electrode position and this study focused on the neuroprotection and the change of the ischemic size after the repeated tDCS, not to investigate the movement during the tDCS. We also used the same dosage of anesthetics in the control and exercise groups to equalize anesthetic effect on motor recovery.
To examine the negative effect of repeated anesthesia on motor recovery, we included the exercise group, because exercise was found to improve motor function and reduce infarct volume in rat stroke model (17). Motor function tests, as described above, were repeated at POD 9 and 16. After completing the experiments, animals were euthanized, decapitated, and brains were extracted to determine the pathologic changes and infarct volumes. Twenty rats died during the experiment (control 4; anodal 5; cathodal 6; exercise 5) and 41 rats completed the study protocol.
Transcranial direct current stimulation (tDCS)
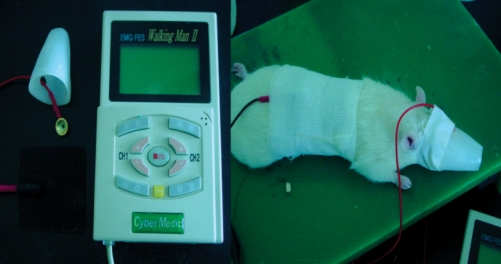
Cathodal and anodal tDCS were applied using a constant-current stimulator developed in cooperation with Cybermedic Corp. (Cybermedic Corp., Iksan, Korea) to deliver a current of 0.1 mA for 30 min. To simulate clinical studies in humans, the active electrode was attached transcranially to a rat and fixed with a molded plastic cup (Fig. 1). The active electrode was positioned 3 mm to the left and 2 mm in front of the interaural line. However, in contrast to human studies, the counter electrode was attached to the trunk and surrounded by gauze to avoid displacement. The active electrode was 1 cm diameter, cup-shaped and filled with gel. The contact area of active electrode was 0.785 cm2. The counter electrode was square with 3×3 cm2 rubber pad.
Fig. 1.
Transcranial direct current stimulation in rat stroke model. We used a cup electrode for the active and a rubber electrode for the reference. To reduce the skin impedance, the cup electrode was fixed by a molded plastic cup and the rubber electrode by gauzes.
Treadmill exercise
The exercise group was trained on a rat treadmill from POD 2 to 13 for a total of 10 days (except POD 8, 9) at 30 min per day. Treadmill velocity was set at 8 m/min on the first exercise day and 16 m/min on following days. The treadmill tilting angle was 0°. To encourage running, a heating bar was set at the end of the treadmill.
Evaluation of motor performance
All animals were evaluated for motor performance. Three evaluation tools were used to assess motor function (as detailed above). Testing was performed by a single experienced researcher (LJE) unaware of group allocations to eliminate the bias.
Garcia's motor behavior test (15), composed of 6 items, was performed at 10 a.m. to account for diurnal variation. Allocated scores ranged from 3 to 18, and higher scores indicate better motor performance.
In the modified foot fault test (16), a rat was placed on the grid and encouraged to traverse the grid surface for 1 min. The foot placing apparatus was made as the same size (10×110 cm2, with a square opening of 9 cm2) of that used in the previous study (18). When a rat traversed the grid, occasionally a forelimb was misplaced and fell through the grid. These mistakes were considered foot faults and their numbers per meter were counted and considered as the modified foot fault scores.
The rota rod performance test was performed using a 9 cm diameter rod. After 2 min of adaptation at 2 rpm, rotational speed was gradually increased by 1 rpm per minute. When a rat fell off the rod or rotated with the rod without moving, its performance score was determined to be the rotational speed set at the time. To reduce failures due to lack of familiarity, 3 trials were performed and the fastest rotational speed was taken.
Change of white matter axon from ischemic injury
Pathologic changes in ischemic models are visualized by loss of white matter axonal stainability using Bielschowskys silver impregnation. This technique demonstrates hypoxic injury, not at the cellular level, but at the white matter axonal level (19).
Twenty rats (control 5; anodal 5; cathodal 5; exercise 5) were anesthetized with sodium pentobarbital 100 mg intraperitoneally. Brains were removed, fixed in 10% neutral buffered formalin, dehydrated in a graded ethanol series, and embedded in paraffin. Coronal blocks were taken from both sides of the sensorimotor cortex in intact brains and from the cortex contralateral to infarcts in infarcted brains. Brain tissue was sectioned serially perpendicular to the pial surface using an Oxford vibratome at 200 µm. The staining procedure was performed in the same manner in the previous study (19).
To determine white matter axonal integrity, optical densities were measured in bilateral internal capsules. Optical densities were measured using images collected by a video-imaging microscope system running BIO-1D software (v. 11.05; Vilber Lourmat Imaging System). White matter axonal integrities were expressed as ratios of the density of ipsilesional areas to those of their contralateral counterparts. A decreased optical density value indirectly reflects destruction of white matter axon.
Infarct volume estimation
Twenty-one rats (control 6; anodal 4; cathodal 6; exercise 5) were euthanized to determine infarct volumes at POD 16. Brains were extracted and fixed by intracardiac perfusion with heparinized 0.9% saline followed by 10% formalin in a 0.1 M phosphate buffer (pH 7.4), removed and stored in fixative. They were then divided into 5 coronal sections (2 mm each), and immersed in 2% 2, 3, 5-triphenyl-tetrazolium chloride (TTC) at 37℃ for 30 min. Infarct volumes were measured using a Scion image program (Scion Corp., NIH, USA), and calculated as follows; infarct volume=(the sum of 5 unaffected hemispheric areas - the sum of 5 affected hemispheric areas)/the sum of 5 unaffected hemispheric areas ×50 (%).
Statistical analysis
Analysis of variance (ANOVA) was done to confirm motor performance score homogeneities (Garcia's, modified foot fault, and rota-rod performance scores) before treatment in the four groups. Improvement in motor performance scores were compared between groups using repeated measures analysis of covariance (ANCOVARM) using pre-treatment (POD 2) scores as covariates. The Kruskal-Wallis test was used to determine the differences of the optical density ratios and of infarct volumes between groups. SPSS ver. 12.0 was used for the statistical analyses and P values of <0.05 were considered statistically significant. Post-hoc Mann-Whitney U testing with Bonferroni correction was conducted to identify which group showed the difference from the other groups if the Kruskal-Wallis test revealed the significant difference.
RESULTS
Motor performance score
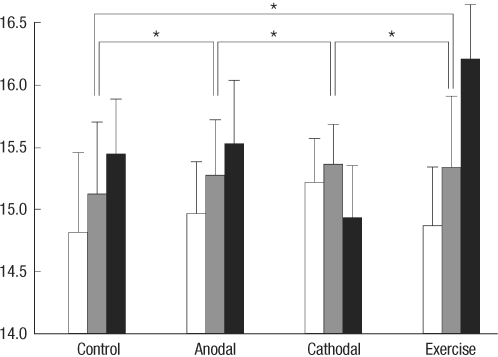
Motor performance scores before treatment were not significantly different between groups. Garcia's motor behavior scores are presented in Fig. 2. Repeated measures analysis of covariance revealed significant difference in terms of INTERVENTION [F(3, 36)=14.460, P<0.001] but no difference in terms of TIME [F(1, 36)=3.935, P=0.055] and TIME×INTERVENTION [F(3, 36)=1.755, P=0.173]. Post-hoc test showed that cathodal stimulation and control groups were significantly different from anodal stimulation and exercise groups. However, there was no significant difference between cathodal stimulation and control groups and between anodal and exercise groups.
Fig. 2.
Garcia's motor behavior scores between control, anodal stimulation, cathodal stimulation, and exercise groups at postoperative 2, 9, and 16 days (white, gray, and black bars). The values in the Y-axis represent the Garcia's scores (3-18). Asterisks represent P values less than 0.05.
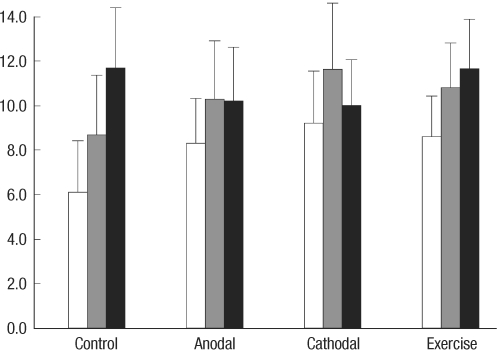
For rota-rod test results (Fig. 3), ANCOVARM revealed no significant effect in terms of INTERVENTION [F(3, 36)=0.570, P=0.638], TIME [F(1, 36)=0.769, P=0.386], and TIME×INTERVENTION [F(3, 36)=2.659, P=0.063].
Fig. 3.
Rota-rod performance scores between control, anodal stimulation, cathodal stimulation, and exercise groups at postoperative 2, 9, and 16 days (white, gray, and black bars). The values in the Y-axis represent the rotational speed (rpm) when a rat fell off the rod or rotated with the rod without moving.
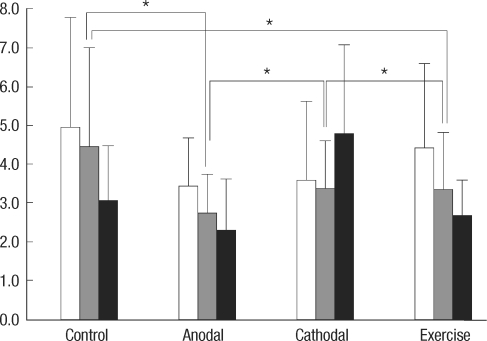
For modified foot fault test results (Fig. 4), a significant difference between groups was found [F(3, 36)=13.665, P<0.001] without any difference in the time factor [F(1, 376)=2.359, P=0.133]. No interaction between time and group factors was observed [F(1, 36)=4.108, P=0.050]. Cathodal stimulation and control groups were significantly different from the anodal stimulation and exercise groups by post-hoc test (all the corrected P values <0.001). Paradoxical deterioration of Garcia's and modified foot fault scores at POD 16 were exhibited by the cathodal stimulation group.
Fig. 4.
Modified foot fault scores between control, anodal stimulation, cathodal stimulation, and exercise groups at postoperative 2, 9, and 16 days (white, gray, and black bars). The values in the Y-axis represent the number of the forelimb misplacements over 1 minute period when a rat traversed the grid. Asterisks represent P values less than 0.05.
Infarct size
Mean infarct volume (±standard deviation) was 7.5±2.7% in the control group (n=6), 11.3±6.3% in the anodal stimulation group (n=4), 7.7±4.2% in the cathodal stimulation group (n=6), and 15.4±12.4% in the exercise group (n=5). No significant difference was found between the 4 groups [χ2=1.884, P=0.597].
Integrity of white matter axon
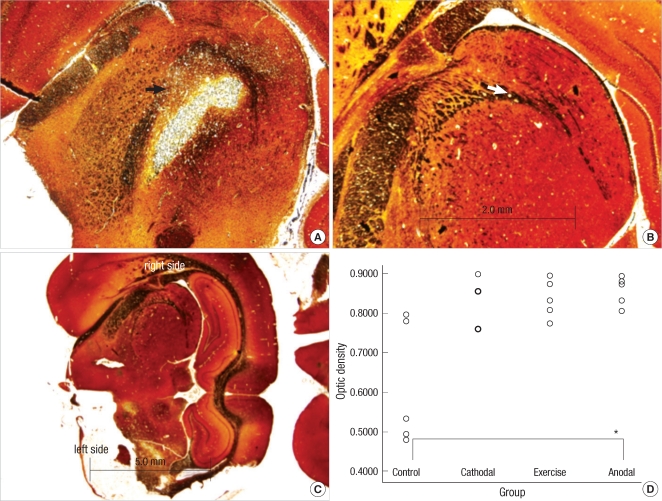
Neuronal axons at the internal capsule of infarct hemispheres in the control group were stained poorly (Fig. 5). However, neuronal axons in the same region in the anodal stimulation and exercise groups contained axons that were better preserved. Optical density ratios of ischemic areas versus contralateral hemisphere were significantly different between groups [χ2df=3=8.211, P=0.042]. The anodal stimulation group had well-stained axons in the infarct areas when compared to the control group (Bonferroni corrected P value=0.009).
Fig. 5.
Representative photomicrographs from bilateral internal capsules showing neural axons staining with Bielschowsky's method in rat stroke model (original magnification ×40 and ×12.5). (A) and (B) represent infarct and intact hemispheres at the level of internal capsules. The arrows in figures A and B represent the left and right internal capsules. (C) is the dorsal side of a rat brain section. (D) is a graph to show the optical density ratios in all groups. Optical density ratios of the infarct hemisphere to the intact one are measured and compared between them. Infarct areas in the anodal stimulation group show less neuronal axon and stain intensity changes than those in the control group.
DISCUSSION
This is the first study to reveal the functional and histological changes after tDCS using the rat stroke model. Repeated transcranial anodal stimulation improved motor function (according to Garcia's test and the modified foot fault test) in rat stroke model. Histologically, this had no effect on infarct size, but reduced neuronal axon deterioration.
Transcranial direct current stimulation has not been widely studied in rats, although it has been examined with respect to anticonvulsant effects (20) and the propagation velocity of cortical spreading depression, which represents cortical excitability (21). In these previous studies, a plastic body jacket and a coating of glass ionomer cement were used to attach the electrodes. In the present study, we used a molded plastic cup and gauze to attach them firmly, which reduced skin resistance to several hundred kilo ohms. Although current modeling in rats during tDCS has not been developed, separation between anodal and cathodal electrodes is known to prevent current shunting effects (22). We did not use implanted subcutaneous electrodes because they might have caused infection and stress, and thus, affected the results. Since the stimulation method was noninvasive, we did not include a sham stimulation group and instead used a group that received no stimulation as a control.
Since there was no significant difference in the time factor, this suggests that anodal stimulation for 2 weeks did not show superiority over stimulation for a week in terms of the motor recovery. These results are contrary to the findings of Boggio's study (7). On the other hand, cathodal stimulation for 2 weeks was associated with deterioration at POD 16, which is probably attributable to a cumulative effect caused by decreased excitability of the infarcted brain. Improvements in Garcia's test and the modified foot fault test, but not in the rota-rod test might be explained by the results of the previous study (18) which stated that improvement of simple movement did not always accompany coordinated and balanced movement.
Transcranial direct current stimulation (tDCS) and exercise failed to reduce infarct size in our model, which contradicts the findings of previous studies (23-25). We attribute this discrepancy to the repeated anesthetic insults, different injury types, and exercise intensities. As mentioned in the Methods, we could not rule out false negative results caused by repeated anesthetic insults of ketamine (26). In addition, we used a permanent infarct model and not a temporary model, as was used in previous studies. Moreover, in the present study, the treadmill speed was set at 16 m/min lower than those used in previous studies, because our rats could not endure a speed over 20 m/min. It could be said that this reduced level of exercise was insufficient to reduce infarct size as compared with forced exercise at 30 m/min (23).
White matter axons in the infarcted brain were well preserved after transcranial anodal stimulation compared to those in the control group. The results obtained are of importance because they demonstrate that white matter axonal damage after ischemia can be reduced by transcranial anodal stimulation. The mechanism of the protective effect of repeated transcranial anodal stimulation may involve modulation of the activities of calcium channel and NMDA receptor, activations of which cause white matter injury due to excessive glutamate release. However, Nitsche's study (27) showed that transcranial anodal stimulation might enhance NMDA receptor efficacy through the high-frequency presynaptic activity and postsynaptic subthreshold membrane depolarization. This is contradictory to our results. Some other factors might involve this phenomenon and further animal studies must be performed to clarify this.
We selected the stimulus intensity as 0.1 mA because this intensity and total charges would not injure the rat's brain according to the Liebetanz' study (28). The amounts of electrical charge delivered in this study could not be calculated because a mathematical model has not been developed in the rat brain. This makes it difficult to apply our results to clinical studies.
For the anesthesia, we used ketamine which was a non-competitive NMDA receptor antagonist and might affect the results in this experiment. However, ketamine is a weak glutamate antagonist and continuous infusion of ketamine (1.25 mg/kg/min during 55 min) failed to show the protective effect over the glutamate in the temporary middle cerebral artery occlusion rat model (29). Therefore, we do not think this induction dosage of ketamine would interfere with anodal and cathodal tDCS after-effects. Although ketamine has been found consistently to ameliorate neuronal death in vitro, results from in vivo studies have proved more conflicting (30).
During this experiment, many rats (20 rats) died. The causes of death could not be found but we hypothesize that they were due to the stressful condition after the ischemic insult. Although death rates were not different between the study groups by the Chi square test [Pearson χ2=0.542, P=0.910], they may have caused selection bias. As a consequence, type 2 error increased in this study because of small sample size. Further studies using larger sample sizes must be done to prove the negative results found in this study.
From our results, repeated transcranial anodal stimulation may have a neuroprotective effect of white matter in the cerebrovasular injured brain. However, we only observed histopathologic changes in axons of white matter, not in myelins using the Luxol fast blue-periodic acid Schiff stains. The addition of other stains would reveal the protection of white matter injury more accurately.
Transcranial anodal stimulation was found to make a functional improvement and well-preserved white matter axons in our rat stroke model. These findings may be useful for studies about the therapeutic mechanism of tDCS.
ACKNOWLEDGMENTS
We appreciate the Cybermedic Corporation (Iksan, Korea) for the support of the direct current stimulator. However, the content of this report is not related to the company's interest. We specially thank to Professor "Chung Chun Kee" for the guidance of this study. We thank to Dr. John Roberts and Shaina for assistance with the English and to our medical research collaborating center for assistance with statistics.
Footnotes
This study was supported by the research fund from the Clinical Research Institute of Seoul National University Hospital (Study Number: 06-2007-2259).
References
- 1.Ding DC, Shyu WC, Lin SZ, Li H. Current concepts in adult stem cell therapy for stroke. Curr Med Chem. 2006;13:3565–3574. doi: 10.2174/092986706779026237. [DOI] [PubMed] [Google Scholar]
- 2.Liu DD, Shyu WC, Lin SZ. Stem cell therapy in stroke: strategies in basic study and clinical application. Acta Neurochir Suppl. 2006;99:137–139. doi: 10.1007/978-3-211-35205-2_26. [DOI] [PubMed] [Google Scholar]
- 3.Boggio PS, Alonso-Alonso M, Mansur CG, Rigonatti SP, Schlaug G, Pascual-Leone A, Fregni F. Hand function improvement with low-frequency repetitive transcranial magnetic stimulation of the unaffected hemisphere in a severe case of stroke. Am J Phys Med Rehabil. 2006;85:927–930. doi: 10.1097/01.phm.0000242635.88129.38. [DOI] [PubMed] [Google Scholar]
- 4.Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, Rigonatti SP, Marcolin MA, Freedman SD, Nitsche MA, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- 5.Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disord. 2006;8:203–204. doi: 10.1111/j.1399-5618.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 6.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke. Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 7.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- 8.Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol. 2005;568:653–663. doi: 10.1113/jphysiol.2005.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 2008;29:268–275. doi: 10.1016/j.tips.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Manning SM, Talos DM, Zhou C, Selip DB, Park HK, Park CJ, Volpe JJ, Jensen FE. NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci. 2008;28:6670–6678. doi: 10.1523/JNEUROSCI.1702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merzagora AC, Foffani G, Panyavin I, Mordillo-Mateos L, Aguilar J, Onaral B, Oliviero A. Prefrontal hemodynamic changes produced by anodal direct current stimulation. Neuroimage. 2010;49:2304–2310. doi: 10.1016/j.neuroimage.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 14.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 15.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 16.Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- 17.Kim MW, Bang MS, Han TR, Ko YJ, Yoon BW, Kim JH, Kang LM, Lee KM, Kim MH. Exercise increased BDNF and trkB in the contralateral hemisphere of the ischemic rat brain. Brain Res. 2005;1052:16–21. doi: 10.1016/j.brainres.2005.05.070. [DOI] [PubMed] [Google Scholar]
- 18.Ding Y, Li J, Lai Q, Rafols JA, Luan X, Clark J, Diaz FG. Motor balance and coordination training enhances functional outcome in rat with transient middle cerebral artery occlusion. Neuroscience. 2004;123:667–674. doi: 10.1016/j.neuroscience.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Schabitz WR, Li F, Fisher M. The N-methyl-D-aspartate antagonist CNS 1102 protects cerebral gray and white matter from ischemic injury following temporary focal ischemia in rats. Stroke. 2000;31:1709–1714. doi: 10.1161/01.str.31.7.1709. [DOI] [PubMed] [Google Scholar]
- 20.Liebetanz D, Klinker F, Hering D, Koch R, Nitsche MA, Potschka H, Loscher W, Paulus W, Tergau F. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006;47:1216–1224. doi: 10.1111/j.1528-1167.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 21.Liebetanz D, Fregni F, Monte-Silva KK, Oliveira MB, Amancio-dos-Santos A, Nitsche MA, Guedes RC. After-effects of transcranial direct current stimulation (tDCS) on cortical spreading depression. Neurosci Lett. 2006;398:85–90. doi: 10.1016/j.neulet.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 22.Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol. 2006;117:1623–1629. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, Jimenez DF, Ding Y. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008;115:289–296. doi: 10.1007/s00401-008-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DY, Park SH, Lee SU, Choi DH, Park HW, Paek SH, Shin HY, Kim EY, Park SP, Lim JH. Effect of human embryonic stem cell-derived neuronal precursor cell transplantation into the cerebral infarct model of rat with exercise. Neurosci Res. 2007;58:164–175. doi: 10.1016/j.neures.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Wang RY, Yu SM, Yang YR. Treadmill training effects in different age groups following middle cerebral artery occlusion in rats. Gerontology. 2005;51:161–165. doi: 10.1159/000083987. [DOI] [PubMed] [Google Scholar]
- 26.Church J, Zeman S. Ketamine promotes hippocampal CA1 pyramidal neuron loss after a short-duration ischemic insult in rats. Neurosci Lett. 1991;123:65–68. doi: 10.1016/0304-3940(91)90159-q. [DOI] [PubMed] [Google Scholar]
- 27.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebetanz D, Koch R, Mayenfels S, Konig F, Paulus W, Nitsche MA. Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol. 2009;120:1161–1167. doi: 10.1016/j.clinph.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Ridenour TR, Warner DS, Todd MM, Baker MT. Effects of ketamine on outcome from temporary middle cerebral artery occlusion in the spontaneously hypertensive rat. Brain Res. 1991;565:116–122. doi: 10.1016/0006-8993(91)91742-j. [DOI] [PubMed] [Google Scholar]
- 30.Church J, Zeman S. Ketamine promotes hippocampal CA1 pyramidal neuron loss after a short-duration ischemic insult in rats. Neurosci Lett. 1991;123:65–68. doi: 10.1016/0304-3940(91)90159-q. [DOI] [PubMed] [Google Scholar]