Abstract
To evaluate the association of Toll-like receptors (TLRs), antimicrobial peptides (AMPs) and vitamin D receptors (VDRs) in psoriasis, lesional (PP) and perilesional skin (PN) from psoriasis, atopic dermatitis (AD) patients and healthy controls (NN) were studied by immunohistochemistry. Compared with PN, AD and NN skin, dysregulated expression of TLRs, AMPs and VDR was detected in PP skin. Noteworthy, our results showed altered correlation between TLR2 and VDR expression in PP and PN skin. Human beta defensin 2 (HBD2) and cathelicidin (LL-37) expressions in the PP skin were higher in serum vitamin D sufficient (VDS) groups than serum vitamin D deficient (VDD) groups. Negative correlation was found between TLR2 and VDR expression in the PP skin of VDD groups. However, positive correlation was noted in the PP skin of VDS groups. Based on the present results, therapies targeting the activity of TLRs, AMPs and vitamin D, including modulation of the TLR-VDR pathways, might provide new therapeutic approaches to the psoriasis and other inflammatory skin diseases.
Keywords: Psoriasis, Toll-Like Receptors, Antimicrobial Peptides, Vitamin D
INTRODUCTION
Psoriasis is a chronic relapsing skin disease mediated by elements of the innate and adaptive immune systems. Psoriatic skin is characterized by abnormal keratinocyte differentiation and proliferation. Signals derived from immune cells, such as proinflammatory cytokines, are able to stimulate keratinocyte proliferation, and the keratinocytes themselves may modulate immune cells through surface and secretory molecules. These molecules consist of toll-like receptors (TLRs), antimicrobial peptides (AMPs), and the active metabolite of vitamin D, 1,25(OH)2D3 (1).
Although the role of the TLRs in the pathogenesis of psoriasis remains to be clarified, TLR expression has been studied with conflicting results. Epidermal keratinocytes in normal skin constitutively expressed TLR1, TLR2 and TLR5, while TLR3 and TLR4 were barely detectable. TLR1 and TLR2 are expressed in entire epidermis, but more highly expressed in the basal keratinocytes. TLR5 is exclusively expressed in the basal cell layer (2). In contrast, in lesional psoriatic skin, strong TLR1 staining is observed in the keratinocytes of the upper epidermis. TLR2 is highly expressed in the keratinocytes of the upper epidermis, but not in the basal layer, whereas TLR5 is down-regulated in the basal keratinocytes of patients with psoriasis. TLR3 and TLR4 are weakly expressed in healthy and psoriatic skin (1, 2).
AMPs are involved in microbial infection, wound healing, and proinflammatory cytokine responses; they have been suggested to be involved in psoriasis (1). Inflammation, infection or injury may direct keratinocytes to enhance synthesis of AMP in the skin (3). Expression of human beta defensin (HBDs), such as HBD2 and HBD3, has been reported to be upregulated by TNF-α and IFN-γ in normal human keratinocytes (4). In addition, the HBD2 in keratinocytes can be induced by bacteria and vitamin D. However, HBD3 expression is stimulated by TLR2 interactions with bacterial components (1, 5). Cathelicidin LL-37, another AMP, is expressed by keratinocytes; its production can also be stimulated by vitamin D (6). Expression levels of these three AMPs are increased in psoriatic skin compared to the skin of patients with lesional atopic dermatitis (4, 7). However, the correlation of their expression with TLR expression as well as vitamin D levels has not been analyzed.
Vitamin D is produced by keratinocytes and regulates keratinocyte differentiation. The biological effects of 1,25(OH)2D3 are mediated through the vitamin D receptor (VDR) (8). The metabolite, 1,25(OH)2D3, inhibits keratinocyte proliferation and induces terminal differentiation of the keratinocytes. Vitamin D also modulates the immune system in a variety of ways (9-11). Although some suggest that serum vitamin D levels in psoriasis are inversely related to disease severity, others have shown no difference in the serum vitamin D levels between patients with psoriasis and in healthy subjects (12).
In this study, to gain insight into the association of TLRs, AMPs, and vitamin D in patients with psoriasis we compared the expression of TLRs, AMPs, and VDR in the epidermal keratinocytes of patients with psoriasis, atopic dermatitis and healthy controls. In addition, the correlation of the expression of these molecules, serum vitamin D concentrations and psoriasis disease severity were evaluated. We also compared the expression of these molecules between serum vitamin D sufficient (VDS) and vitamin D deficient (VDD) patients with psoriasis.
MATERIALS AND METHODS
Subjects
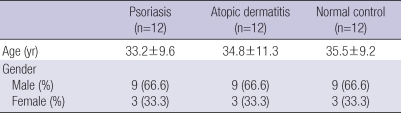
Twelve patients with newly diagnosed psoriasis (9 men and 3 women) were enrolled from the dermatology department of Ajou University Hospital from January to June, 2008 (Table 1). The controls consisted of 12 age, gender, and biopsy site matched patients with atopic dermatitis and no other evident dermatological disease (average disease duration, 86 months; 5-120 months), and 12 healthy volunteers. For the comparison between VDS and VDD patients with psoriasis, we added retrospectively, eight more psoriasis patients with decreased serum vitamin D levels to the VDD group due to the small number of patients in the VDD group.
Table 1.
Baseline demographics and characteristics of psoriasis patients, atopic dermatitis patients and healthy normal control group
Psoriasis disease severity index
Before the biopsy was performed, the psoriasis disease severity was evaluated. The clinical severity of psoriasis was graded according to the area affected by psoriasis area and severity index (PASI) scoring system. The PASI score of all patients was determined by one dermatologist.
Skin biopsy and immunohistochemical studies
For the patients with psoriasis, four to six-millimeter punch biopsies from the lesional (PP) and perilesional normal-appearing (usually 6 cm or more distant) skin (PN) were collected. The biopsies from the PP skin were taken within the lesion, 1 cm from the edge of the plaque border. The biopsies from the PN skin were taken 2 cm beyond the plaque border. Two to three-millimeter punch biopsies were taken from the lesional skin of patients with atopic dermatitis (AD) and from the normal skin of the healthy normal controls (NN). Paraffin-embedded tissue sections of 3-µm thickness were processed for light microscopic examination.
Immunohistochemistry with each primary antibody (Ab) was performed using standard techniques (13). The primary antibodies included TLR1 (ebioscience, Inc., San Diego, CA, USA), TLR2, TLR4 (Abcam, Inc., Cambridge, UK), HBD2 (R&D systems Inc., Minneapolis, MN, USA), HBD3 (Orbigen, Inc., San Diego, CA, USA), cathelicidin (Abcam, Inc., Cambridge, UK), and VDR (Affinity Bioreagents, Inc., Golden, CO, USA). The immunohistochemical findings were analyzed by two dermatologists.
Image analysis
Image signals were recorded on a personal computer and evaluated using Image Pro Plus Version 4.5 (Media Cybernetics Co., Silver Spring, MD, USA). The image analysis was performed on a representative area of each specimen. For each staining, we established a standard for Ab positivity and applied the same standards to the PP, PN, AD, and NN samples. The amount of positive Ab area was measured under the same magnification (×100). The ratio of positive Ab area to the measured epidermal area (PA/EA) was calculated for the PP, PN, AD, and NN skin samples (14-16). Careful examination was performed by manually tracing the borders of the epidermis and the rete ridges. The epidermal areas that contained hair follicles were excluded from the tracings.
Serum 25-hydroxyvitamin D
Venous blood samples were taken in the standard method. Serum concentrations of 25(OH)D were assayed with a radioimmunoassay kit (Dia-Sorin, Stillwater, MN, USA). Following extraction of 25(OH)D using a donkey anti-goat (DAG) precipitating complex, the treated sample was assayed according to the equilibrium RIA procedure. The reference range used for the assay was 9.0-37.6 µg/mL. Serum 25(OH)D concentrations of <9 µg/mL were considered vitamin D deficient (17, 18).
Statistical analysis
Data were expressed as the mean±standard deviation. To compare the PA/EA of TLRs, AMPs, and VDR, independent t-test was used for the PP, PN, AD, and NN skin. Spearman's rho correlation analysis was performed to determine the correlation among the TLRs, AMPs, VDRs, serum 25(OH)D levels and PASI score. Differences in the PA/EA of TLRs, AMPs, and VDR between the VDD and VDS groups were also analyzed using the independent t-test. Correlation between TLR2 and VDR expression in the VDD and VDS groups were analyzed using Spearman's rho correlation analysis. A probability of less than 0.05 was considered statistically significant. The SPSS v11.0 statistics program (SPSS, Inc., Chicago, IL, USA) was used for the analysis.
Ethics statement
All subjects provided informed consent to participate in this study. This study was approved by the institutional review board (IRB number: AJIRB-CRO-07-174) and was conducted in accordance with the declaration of Helsinki principles.
RESULTS
Quantitative analysis of expression of TLR1, TLR2, TLR4, HBD2, HBD3, LL-37 and VDR in psoriasis lesional and perilesional skin, atopic dermatitis lesional skin and healthy normal control skin
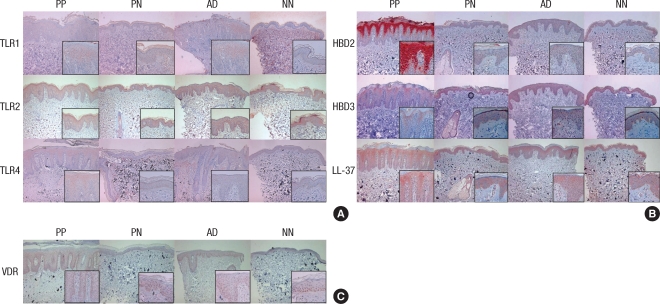
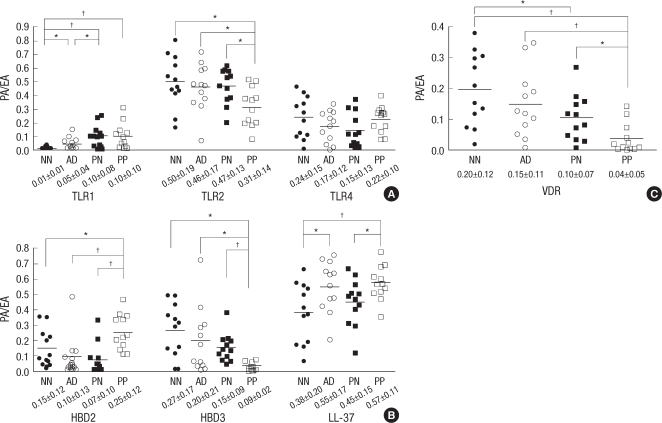
Expression of TLR1 in the PP skin was higher than in the NN skin (P=0.006). However, expression of TLR2 in the PP skin was lower than in the NN skin (P=0.012). The TLR4 expression was similar in the NN and PP skin samples. The expression level of HBD2 and LL-37 was elevated in the PP skin samples compared to the NN skin samples (P=0.044 and 0.007, respectively). However, the percentage of HBD3 positive keratinocytes was significantly decreased in the epidermal layers of the PP skin compared to the NN skin (P=0.001). The VDR expression levels declined from the NN skin samples to the AD, PN and PP skin samples resulting in a five fold reduction in the PP skin samples compared to the NN skin samples (P<0.001) (Figs. 1, 2).
Fig. 1.
The immunohistochemical studies of psoriasis lesional skin (PP) and perilesional normal skin (PN), atopic dermatitis lesional skin (AD), healthy normal control skin (NN). (A) Expression of TLR1, TLR2 and TLR4. (B) Expression of HBD2, HBD3 and LL-37. (C) Expression of VDR (×100, inset: ×400).
Fig. 2.
PA/EA of psoriasis lesional skin (PP) and perilesional normal skin (PN), atopic dermatitis lesional skin (AD), healthy normal control skin (NN) by quantitative analysis. (A) Expression of TLR1, TLR2 and TLR4. (B) Expression of HBD2, HBD3 and LL-37. (C) Expression of VDR.
*P<0.05; †P<0.01.
PA/EA, Ab positive area per measured epidermal area.
Correlation of the TLRs, AMPs, and VDR expression, serum 25(OH)D concentration and disease severity in patients with psoriasis
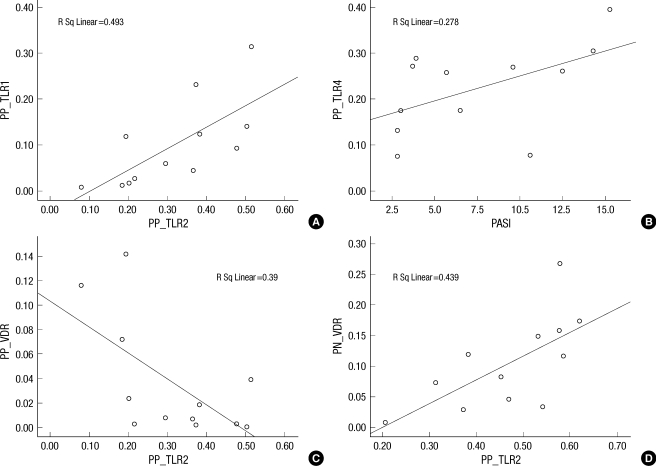
A positive correlation between the TLR2 and TLR1 expression, the PASI score and the TLR4 expression was found in the PP skin (Fig. 3A, B; TLR2 to TLR1, r=0.493, P=0.001 and PASI score to TLR4, r=0.278, P=0.041). Noteworthy, a significant negative correlation was found between TLR2 and VDR expression in the PP skin (Fig. 3C; TLR2 to VDR, r=0.390, P=0.045); while a positive correlation was noted in the PN skin (Fig. 3D; TLR2 to VDR, r=0.439, P=0.019). However, there was no significant correlation between serum vitamin D levels and the TLR and AMP expression in the patients with psoriasis.
Fig. 3.
Correlation between TLRs, AMPs and VDR expressions and PASI score in skin sample of psoriasis. (A) The expression of TLR2 shows a positive correlation with the expression of TLR1 in psoriasis lesional skin (r=0.493, P=0.001). (B) The PASI score shows a positive correlation with the expression of TLR4 in psoriasis lesional skin (r=0.278, P=0.041). (C) The expression of TLR2 showed a negative correlation with the expression of VDR in psoriasis lesional skin (r=0.390, P=0.045). (D) The expression of TLR2 shows a positive correlation with the expression of VDR in normal skin of psoriasis (r=0.439, P=0.019).
Different expression and correlation of TLRs, AMPs and VDR in psoriasis lesional skin in the serum vitamin D deficient group compared to the serum vitamin D sufficient group
Using a cut-point of 9 µg/mL, (17, 18) we divided the patients with psoriasis into two subgroups; serum vitamin D deficient (VDD) group (n=2) and serum vitamin D sufficient (VDS) group (n=10). None of the patients with critically low vitamin D levels had abnormal serum calcium or inorganic phosphate levels. In this preliminary study, HBD2, LL-37 and VDR expression had a tendency to be downregulated in the serum VDD group compared to the VDS group. Although the VDD group was a small sample, the HBD2 expression was significantly downregulated in this group (P=0.049) (data not shown).
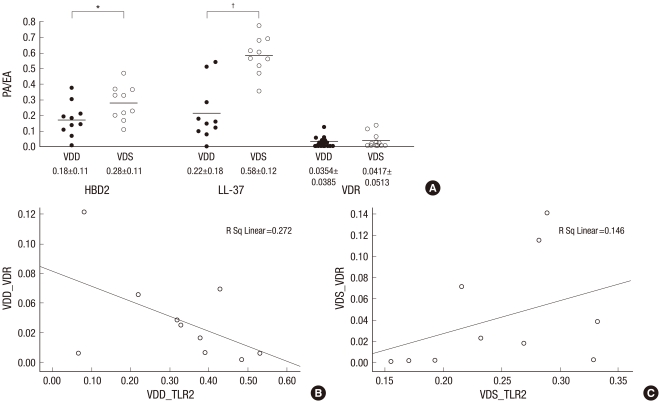
Because the sample size of the VDD group was small, we recruited additional eight patients that had decreased serum vitamin D levels into the VDD group retrospectively. As described in the above preliminary data, HBD2, LL-37 and VDR had a tendency to be downregulated in the VDD group; therefore, additional experiments were performed on the level of three antibodies. The final comparison of these molecules between the VDD (n=10) and VDS groups (n=10) is shown in Fig. 4A. HBD2 and LL-37 expression was significantly downregulated in the VDD group compared to the VDS group (Fig. 4A).
Fig. 4.
Different expression and correlation of TLRs, AMPs and VDR in psoriasis lesional skin in the serum vitamin D deficient (VDD) group compared to the serum vitamin D sufficient (VDS) group. (A) HBD2 and LL-37 expression are significantly downregulated in the VDD group compared to the VDS group. (B, C) Negative correlation is found between TLR2 and VDR expression in the VDD groups (B; r=0.272, P=0.174), however, significant positive correlation is noted in the VDS groups (C; r=0.146, P=0.048).
*P=0.041; †P=0.000. PA/EA, Ab positive area per measured epidermal area.
In Fig. 3C, we showed significant negative correlation between TLR2 and VDR expression in the PP skin. In the comparison study between the VDD (n=10) and VDS groups (n=10), although not significant, negative correlation was also found between TLR2 and VDR expression in the PP skin of VDD groups (Fig. 4B; r=0.272, P=0.174). However, significant positive correlation was noted in the PP skin of VDS groups (Fig. 4C; r=0.146, P=0.048).
DISCUSSION
In this study the epidermal expression levels of TLRs, AMPs, and VDR were examined in psoriatic skin samples. In addition, the correlation of the molecular expression of these compounds with serum vitamin D levels as well as with disease severity was analyzed. Unlike previous studies which evaluated TLR expression or AMP expression using a three to four point scale grading system, we analyzed the molecular expression levels by measuring the area stained by the relevant antibodies.
TLR2 expression showed an inverse relationship with VDR expression in the PP skin, but a positive correlation with the PN skin. A previous report also indicated a positive correlation between TLR2 and VDR in human macrophages of healthy donors (11, 19). They reported that TLR2 activation of normal human macrophages up-regulated expression of the vitamin D receptor and the vitamin D-1-hydroxylase genes. This altered correlation between TLR2 and VDR expression in the PP skin, might be associated with the development of psoriatic lesions. Liu et al. (20) reported that adequate levels of serum vitamin D were required for the induction of host-defense mechanisms via TLR2. Therefore this altered correlation between TLR2 and VDR could be due to a feedback mechanism to regulate further inflammation in psoriasis. Moreover, both TLR and VDR have recently been shown to be crucial in AMP expression of normal human monocytes (21). The decreased level of TLR2 and VDR expression in PP skin in this study also could be caused by a feedback mechanism to make inflammation in psoriasis. In this regard, the modulation of the TLR-VDR pathways might provide useful information for the development of new treatments.
A weak correlation was found between the expression levels of TLR4 and disease severity of the patients with psoriasis. In patients with spondyloarthropathy, a chronic inflammatory joint disease, down-modulation of the increased expression of TLR4 was found in peripheral monocytes and synovial fluid after tumor necrosis factor alpha blockade treatment (22). Based on previous reports and the results of this study, TLR4 might be associated with disease severity in chronic inflammatory diseases. However, because of low correlation coefficient (r=0.28), further study is needed to determine the biological relevance of the relationship between TLR4 and PASI score.
Expression of TLR1 in the PP skin was higher than in the NN skin. However, expression of TLR2 in the PP skin was lower than in the NN skin. The TLR4 expression was similar in the PP and NN skin samples. Consistent with our findings, Seung et al. (23) also reported that expression of TLR2 in psoriatic lesional skin samples was lower than in normal skin samples, and TLR4 expression showed no difference in comparisons between normal and psoriatic lesional skin samples. According to Wu et al. (24), incubated keratinocytes with mouse anti-K16 monoclonal antibodies showed increased TLR2 protein expression, but without change in the TLR4 protein expression. They suggested that anti-K16 autoantibodies promoted protein expression of TLR2 rather than TLR4 expression. Therefore, the modulation of TLR2 by anti-K16 autoantibodies, rather than TLR4, may be related to the chronic inflammation associated with psoriasis. Based on these findings and our results, TLR2 and TLR4 appeared to play different roles in the development of psoriatic lesions.
Consistent with previous reports (1, 25), the expression levels of HBD2 and LL-37 were elevated in the PP skin compared to NN, AD and PN skin samples. However, HBD3 expression levels in the PP skin were lower than in other skin samples. de Jongh et al. (25) also reported that expression level of HBD3 was decreased in PP skin, as compared to AD and NN skin. According to a recent report, HBD3 significantly attenuated the interleukin-6, IL-1, GM-CSF, and TNF-α responses induced by bacteria in human myeloid dendritic cells (26). It is possible that the activity of HBD3, as an attenuator of pro-inflammatory cytokine responses to microbial antigens, might be associated the pathogenesis of psoriasis.
In contrast to the psoriatic skin, AD skin has been shown to have decreased levels of AMP expression (4, 7). In contrast to the decreased expression of HBD2, HBD3 expression was increased in the AD skin compared to the PP skin, and the LL-37 expression was increased in the AD skin than NN skin, in this study. The disease duration of AD might be a possible cause of increased expression levels of HBD3 and LL-37. The immune responses in AD were Th2-mediated while those in psoriasis were Th1-mediated (4). However, in the chronic phase of AD, the immune responses showed Th1-dominant features rather than Th2-mediated features. In this study, we performed skin biopsies on those in chronic phase of AD, not in the acute exacerbation phase (average disease duration, 86 months; 5-120 months). This might explain the limited difference between the AD skin and the PP skin. Therefore, if skin samples from acute AD patients can be taken, the results may be different from present study.
VDR expression levels declined in the order of NN, AD, PN and PP skin samples resulting in five fold reductions in the PP skin compared to the NN skin. These results differed from those of Milde et al. (27), who reported on VDR expression in eight patients with psoriasis and 10 normal volunteers. They showed increased expression of VDR in the psoriatic lesional skin. However, they did not evaluate the serum vitamin D levels of patients (27). VDR has been shown to be upregulated by 1,25-(OH)2D3 in human keratinocytes (28). Although it was not significant, a decreased expression of VDR was detected in the decreased serum vitamin D group in our study. Therefore, this might account for the different expression levels of VDR in the patients with psoriasis in the present study compared to the levels reported in the previous study.
Expression of HBD2 as well as LL-37 in the PP skin was higher in serum vitamin D sufficient patients than serum vitamin D deficient patients. Previous reports have suggested that AMP expression in epidermal keratinocytes was inducible by high calcium concentrations, retinoic acid and 1,25(OH)2D3 (3, 11, 29). The vitamin D activation step occurred in monocytes and keratinocytes by CYP27B1 and was under the control of inflammatory stimuli combined with TLR2 (3, 6, 11). Liu et al. (21) also demonstrated that HBD2 and LL-37 could be upregulated by vitamin D since they had vitamin D response elements in their promoter region. Therefore, TLR activation upregulates the expression of VDR or vitamin D-1-hydroxylase genes, leading to the induction of the AMP (3, 11, 30).
As we mentioned before, significant negative correlation between TLR2 and VDR expression was noted in the PP skin (Fig. 3C). In the comparison between the VDD and VDS groups, negative correlation was also found between TLR2 and VDR expression in the VDD groups (not significant), while significant positive correlation was noted in the VDS groups. This altered correlation between TLR2 and VDR expression in the VDD and VDS group, might be associated with the role of vitamin D in the development of psoriatic lesions.
In conclusion, dysregulated expression of TLRs, AMPs, and VDR was detected in psoriatic skin. Our results showed altered correlation between TLR2 and VDR expression in psoriatic lesional and non-lesional skin. Our data also showed a correlation of the expression levels of HBD2 and LL-37 with serum vitamin D levels. Negative correlation was found between TLR2 and VDR expression in the PP skin of VDD groups. However, positive correlation is noted in the PP skin of VDS groups. Based on the present results, therapies targeting the activity of TLRs, AMPs and vitamin D, including modulation of the TLR-VDR pathways, might provide new approaches to the management of psoriasis and other inflammatory skin diseases. For example, patients with psoriasis could be divided into VDD and VDS groups and treated differently. More well designed studies are needed to clarify whether systemic vitamin D supplement can alter the disease severity or TLRs and AMPs expressions of psoriasis.
References
- 1.Buchau AS, Gallo RL. Innate immunity and antimicrobial defense systems in psoriasis. Clin Dermatol. 2007;25:616–624. doi: 10.1016/j.clindermatol.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003;148:670–679. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- 3.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, Leung DY. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 5.Sumikawa Y, Asada H, Hoshino K, Azukizawa H, Katayama I, Akira S, Itami S. Induction of beta-defensin 3 in keratinocytes stimulated by bacterial lipopeptides through toll-like receptor 2. Microbes Infect. 2006;8:1513–1521. doi: 10.1016/j.micinf.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122:261–266. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 8.Saeki H, Asano N, Tsunemi Y, Takekoshi T, Kishimoto M, Mitsui H, Tada Y, Torii H, Komine M, Asahina A, Tamaki K. Polymorphisms of vitamin D receptor gene in Japanese patients with psoriasis vulgaris. J Dermatol Sci. 2002;30:167–171. doi: 10.1016/s0923-1811(02)00073-7. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PF, Modlin RL, Bikle DD, Adams JS. Transpleural gradient of 1,25-dihydroxyvitamin D in tuberculous pleuritis. J Clin Invest. 1989;83:1527–1532. doi: 10.1172/JCI114048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002;8:174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 11.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 12.Morimoto S, Yoshikawa K, Fukuo K, Shiraishi T, Koh E, Imanaka S, Kitano S, Ogihara T. Inverse relation between severity of psoriasis and serum 1,25-dihydroxy-vitamin D level. J Dermatol Sci. 1990;1:277–282. doi: 10.1016/0923-1811(90)90120-3. [DOI] [PubMed] [Google Scholar]
- 13.Shi SR, Liu C, Taylor CR. Standardization of immunohistochemistry for formalin-fixed, paraffin-embedded tissue sections based on the antigen-retrieval technique: from experiments to hypothesis. J Histochem Cytochem. 2007;55:105–109. doi: 10.1369/jhc.6P7080.2006. [DOI] [PubMed] [Google Scholar]
- 14.Kim SK, Kang HY, Lee ES, Kim YC. Clinical and histopathologic characteristics of nevus depigmentosus. J Am Acad Dermatol. 2006;55:423–428. doi: 10.1016/j.jaad.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 15.Kim YC, Kim YJ, Kang HY, Sohn S, Lee ES. Histopathologic features in vitiligo. Am J Dermatopathol. 2008;30:112–116. doi: 10.1097/DAD.0b013e3181651511. [DOI] [PubMed] [Google Scholar]
- 16.Kim YJ, Han JH, Kang HY, Lee ES, Kim YC. Androgen receptor overexpression in Becker nevus: histopathologic and immunohistochemical analysis. J Cutan Pathol. 2008;35:1121–1126. doi: 10.1111/j.1600-0560.2008.00988.x. [DOI] [PubMed] [Google Scholar]
- 17.Holme SA, Anstey AV, Badminton MN, Elder GH. Serum 25-hydroxyvitamin D in erythropoietic protoporphyria. Br J Dermatol. 2008;159:211–213. doi: 10.1111/j.1365-2133.2008.08616.x. [DOI] [PubMed] [Google Scholar]
- 18.Lim HJ, Kim JI. Serum 25-hydroxyvitamin D status in wintertime in premenopausal working women. Korean J Nutr. 2006;39:649–660. [Google Scholar]
- 19.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–7120. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007;13:117–124. doi: 10.1016/j.molmed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zugel U, Hollis BW, Cheng G, Modlin RL. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Rycke L, Vandooren B, Kruithof E, De Keyser F, Veys EM, Baeten D. Tumor necrosis factor alpha blockade treatment down-modulates the increased systemic and local expression of Toll-like receptor 2 and Toll-like receptor 4 in spondylarthropathy. Arthritis Rheum. 2005;52:2146–2158. doi: 10.1002/art.21155. [DOI] [PubMed] [Google Scholar]
- 23.Seung NR, Park EJ, Kim CW, Kim KH, Kim KJ, Cho HJ, Park HR. Comparison of expression of heat-shock protein 60, Toll-like receptors 2 and 4, and T-cell receptor gammadelta in plaque and guttate psoriasis. J Cutan Pathol. 2007;34:903–911. doi: 10.1111/j.1600-0560.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu C, Luan Q, Li C, Zheng Z. Effects of antikeratin 16 antibodies on the expression of Toll-like receptors 2 and 4 in keratinocytes. Clin Exp Dermatol. 2009;34:236–239. doi: 10.1111/j.1365-2230.2008.02948.x. [DOI] [PubMed] [Google Scholar]
- 25.de Jongh GJ, Zeeuwen PL, Kucharekova M, Pfundt R, van der Valk PG, Blokx W, Dogan A, Hiemstra PS, van de Kerkhof PC, Schalkwijk J. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol. 2005;125:1163–1173. doi: 10.1111/j.0022-202X.2005.23935.x. [DOI] [PubMed] [Google Scholar]
- 26.Pingel LC, Kohlgraf KG, Hansen CJ, Eastman CG, Dietrich DE, Burnell KK, Srikantha RN, Xiao X, Belanger M, Progulske-Fox A, Cavanaugh JE, Guthmiller JM, Johnson GK, Joly S, Kurago ZB, Dawson DV, Brogden KA. Human beta-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. Immunol Cell Biol. 2008;86:643–649. doi: 10.1038/icb.2008.56. [DOI] [PubMed] [Google Scholar]
- 27.Milde P, Hauser U, Simon T, Mall G, Ernst V, Haussler MR, Frosch P, Rauterberg EW. Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J Invest Dermatol. 1991;97:230–239. doi: 10.1111/1523-1747.ep12480255. [DOI] [PubMed] [Google Scholar]
- 28.Reichrath J, Muller SM, Kerber A, Baum HP, Bahmer FA. Biologic effects of topical calcipotriol (MC 903) treatment in psoriatic skin. J Am Acad Dermatol. 1997;36:19–28. doi: 10.1016/s0190-9622(97)70320-7. [DOI] [PubMed] [Google Scholar]
- 29.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 30.Buchau AS, Schauber J, Hultsch T, Stuetz A, Gallo RL. Pimecrolimus enhances TLR2/6-induced expression of antimicrobial peptides in keratinocytes. J Invest Dermatol. 2008;128:2646–2654. doi: 10.1038/jid.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]