Abstract
In developed countries, informed consent is based on the autonomy of the individual, a written description of the studies proposed, and previous experience of the participant with Western medicine. Consent is documented by the signature of the participant and supervised by institutional review boards (IRBs), which have conflicts of interest because they are also responsible for limiting institutional liability. In developing countries, the initial decision-making for informed consent is typically vested in the community rather than the individual, and illiteracy is common—limiting the value of written documents and signatures. The challenges in developing countries are exacerbated by the fact that persons at greatest risk of disease are often illiterate, have limited experience with Western medicine, and have limited understanding of the scientific rationale for the studies proposed. Given these differences, it is unrealistic to expect that consent strategies used in developed countries would be effective in such diverse settings.
Introduction
In developed countries, informed-consent procedures are so established and regulated1–4 that it is difficult for investigators to consider alternatives for obtaining and documenting informed consent. Although the current process works reasonably well, investigators often do not recognize the extent to which the informed consent transaction rests on culturally determined factors such as the autonomy of the individual, written documents that require signatures, and experience with Western medicine and legal disclaimers to limit liability—none of which are broadly acceptable in developing countries.5–10 For these reasons, the informed-consent process cannot simply be transferred from developed to developing countries without considering the cultural, socioeconomic, and educational factors that influence international research.
For example, a study of the pathogenesis of noma (cancrum oris, a bacterial infection of the face associated with malnutrition that is life-threatening in affected children) was controversial in Nigeria because of traditional beliefs that it was caused by evil spirits rather than bacterial flora of the oropharynx such as Fusobacterium necrophorum and Prevotella intermedia.11,12 Another example occurred recently when blood drawing was discussed for the study of anemia. Young mothers in Mali rejected the suggestion to sit in a chair if they felt faint after a venipuncture, because taking a chair is a statement of one's social importance (because most homes have only 1–2 chairs). These and other examples noted by Lavery and others12 in their book on ethical issues in international biomedical research suggest that fundamental social, cultural, and educational differences must be considered when examining informed consent in developing countries. Therefore, the objectives of this manuscript are to (1) examine the factors that make informed consent different in developed and developing countries and (2) recommend strategies to improve the quality and relevance of consent in both settings.
Factors that Differ Between Developing and Developed Countries
Basis of decision-making.
Autonomy of the individual and informed consent have their roots in the writings of Hippocrates, Socrates, Plato, Aristotle, Erasmus, Descartes, Rousseau, and Thoreau.4,13–17 As a result, they have been incorporated into English common law, the Napoleonic Code, the US Constitution and Bill of Rights, and the Helsinki Declaration.18 For these reasons, individual autonomy is accepted as a right by many developed countries and organizations such as the World Health Organization, Médecins sans Frontieres, and Amnesty International.
Communal decision-making.
In contrast, communal decision-making is frequent in developing countries.6–9,19,20 It has been described in societies as different as Eskimos, South Sea Islanders, Native Americans, tribes in the Amazon, and diverse ethnic groups in sub-Saharan Africa.4–9,19–22 In those societies, health is just one area in which less emphasis is placed on individual autonomy. Other examples include the roles of chiefs, elders, and the community in allocating land and water rights and determining social roles, occupations, marriages, and the roles of children.
Decision-Making from the Perspective of Informed Consent
Autonomy of the individual.
In developed countries, consent discussions typically involve the participants themselves—unless they are minors or legally incompetent (Figure 1). Thus, consent is an independent event for each participant. Except for studies that affect the entire population, representatives of the community are typically not involved in the consent process.
Figure 1.
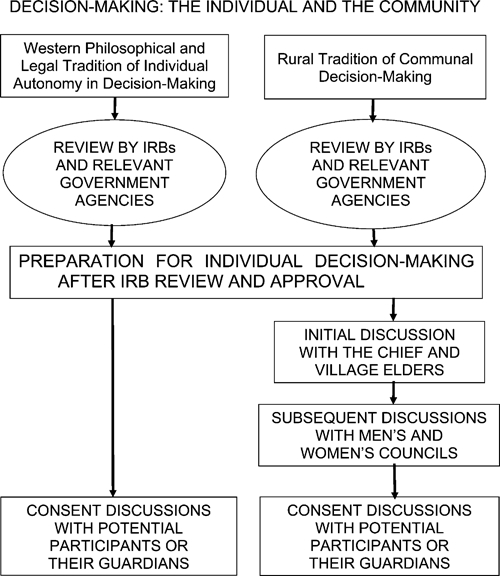
Decision-making: the individual and the community. The major effect of the communal nature of decision-making on informed consent in developing countries is the need to address the community as a whole—diagrammed here as additional discussions with the chief, village elders, and men's and women's councils before meeting with potential participants.
Communal decision-making.
Conversely, communal decision-making is common in many of the rural areas of sub-Saharan Africa where tropical diseases are prevalent. In these communities, the consent process typically begins with presentation of the study to the chief and village council (Figure 1).6–9,19,23 The proposal is then discussed with progressively broader audiences—councils of male and female elders, heads of household, other individuals, and parents. Meetings with potential research participants take place only after approval has been granted by these individuals and groups on behalf of the community. Thus, the major question raised by potential participants is typically whether their participation is appropriate rather than the goals or rationales for the study, the specific procedures involved, or the measures used to protect confidentiality.
Provision of Relevant Information to Potential Participants
Literacy and written documentation.
Literacy and written documentation are inextricably linked to informed consent in developed countries (Figure 2). In addition, consent forms are often burdened by clauses to limit liability and material required by the Health Insurance Portability and Accountability Act (HIPAA),24 which does not apply outside the United States. The result is that informed consent forms are longer and more complicated than necessary. This means that the information essential for informed consent may paradoxically be misunderstood or lost in the process intended to support it. The advantage of written documentation is it proves that participants provided their initials on each page and their signature on the final page. Its limitation is that it provides no information about the participant's understanding (i.e., whether the participant actually read the document or understood its content) (Figure 2), which is a necessary prerequisite for informed consent.25 In addition, the individuals at greatest risk of disease are often illiterate, and signatures (thumbprints or an X) on written documents are generally reserved for important business decisions. Thus, a request for a signature on a consent form may produce concern and distrust. Because it is unethical to ask a participant to sign a document that he/she cannot read, alternative strategies will be necessary to obtain and document informed consent with illiterate participants.26
Figure 2.
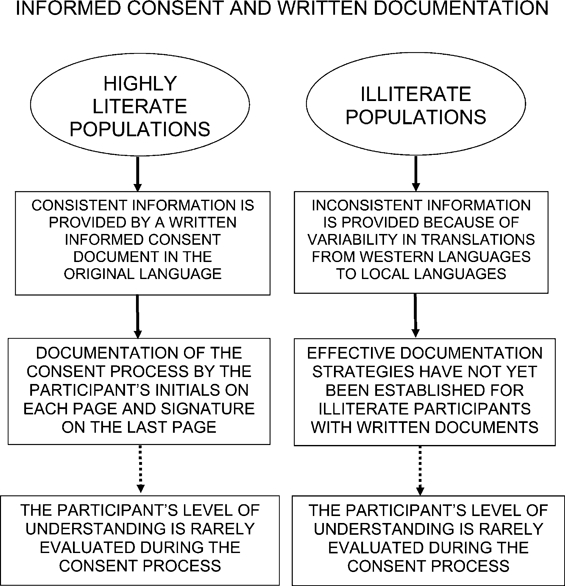
Informed consent and written documentation. Illiterate participants are at risk of misunderstanding from inadvertent changes in meaning during translation of consent documents by study staff. Note that the participant's understanding of the study is rarely assessed in either developed or developing countries.
Translation of written documents.
Translation of consent forms often changes both their meaning and content. Meaning and content are often changed again when consent forms are paraphrased in local languages during oral presentation to illiterate participants (Figure 2). Translation is an especially difficult challenge in developing countries, because (1) staff may not be fluent in the both languages involved and knowledgeable about the studies proposed and (2) there is often a need to paraphrase the consent form using local languages that have oral but not written forms.
Incentives for Participation and Access to Care
Financial incentives are used frequently, because participation in a study (with its implied access to medical care) is not an effective incentive if care is readily available. Note that many developed countries' institutional review boards (IRBs) require that investigators avoid other aspects of their participants' medical care to reduce both coercion and institutional liability. However, financial compensation is also important in areas with limited access to care. Without reimbursement for lost work time and transportation, the people at greatest risk of many tropical diseases may be unable to participate. In addition, the provision of care is frequently necessary for problems unrelated to the study and persons who are not participants.27,28 This is especially true in rural areas of sub-Saharan Africa where access to medical care may be limited.
Complex Issues
The right of refusal.
The extent to which the right of refusal is understood is unclear for both educated and illiterate individuals. The fact that there are acceptances and refusals does not by itself provide insight into those individuals' understanding of their right of refusal.29,30 The reasons given for refusal are usually related to practical issues such as work or time away from home. However, it is often not clear whether those were the actual reasons. This question should be examined separately in studies that include focus groups with persons who accepted and persons who refused to participate, because misunderstandings about the right of refusal may affect both types of decisions.
Randomization.
The term randomization is often mentioned by the media in developed countries, and people in both developing and developed countries can understand that they may (or may not) receive the investigational drug/procedure under study. However, understanding the rationale for randomization is more complex. Because its purpose is to protect a study against confounding by variables that are unknown at the time that the study is performed, it is not clear how well this concept can be understood without training in probability theory or statistics.31 In addition, both participants32 and investigators33 can be uncomfortable with the loss of control associated with randomization. Participants may wonder why they received one treatment rather than another (or placebo) or why their village received bed nets rather than indoor residual spraying; physicians may arrange for patients to receive what they believe is the treatment of choice (by admitting patients on odd versus even days of the month). In addition, the challenge of understanding complex issues is often exacerbated by more limited education in developing countries, where randomization is rarely mentioned and less frequently understood31—in part because there is no word for randomization in many local languages. Thus, requiring an in-depth understanding of complex issues such as randomization for study participation could bias study outcome by limiting the enrollment of participants from rural disease-endemic areas.
IRB Expertise and Challenges
IRBs are responsible for evaluating risks and benefits to human participants, study design, and the consent strategies proposed to protect the rights of study participants.1,8–10,34,35 In addition to the broad range of interests at academic centers (Figure 3), IRBs must cope with institutional demands to limit liability, federal demands for specific language (HIPAA), and investigators' demands for short turnaround times. To cope with these demands, IRBs must develop a keen sense of priorities (Table 1). This means that they typically do not have time to consider the additional challenges of consent in developing countries, each of which has its own cultural nuances. As a result, developed-country IRBs may inadvertently propose or impose requirements that make sense in developed countries but not in developing countries. In addition, IRBs in developing countries have (1) less experience (many have existed for less than 10 years), (2) fewer faculty and financial resources, and (3) less expertise in specialty areas such as molecular biology, bioinformatics, transplantation, genomics, and ethics.1–3,36–41 Conversely, developing-country IRBs often have expertise in the performance of field studies under challenging conditions, which is rare in developed-country IRBs, and an interdisciplinary perspective grounded in a keen awareness of the cultural and practical issues relevant to the protection of human participants.
Figure 3.
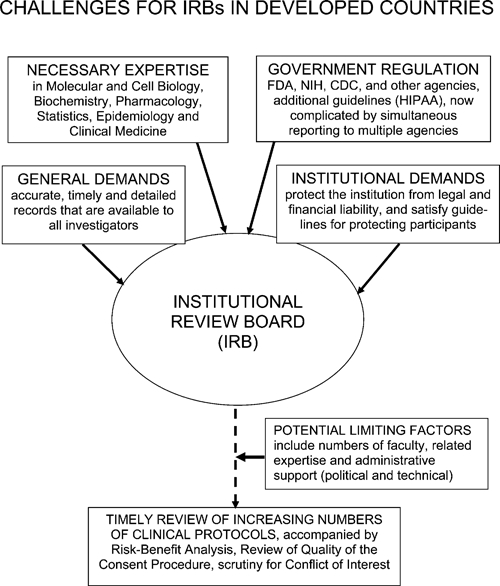
Challenges for IRBs in developed countries. IRBs in developed countries are pressured by both the government and the institutions that they represent to process increasing numbers of protocols more rapidly, while simultaneously protecting their institutions from legal and financial liability.
Table 1.
Priority list for improving informed consent
| Developed countries | Developing countries |
|---|---|
| Reduce the length of the written documentation used for informed consent | Develop strategies to present health studies and document informed consent without the use of written documents to eliminate the use of written documents with illiterate participants |
| Develop alternative strategies to protect the sponsoring institution from financial and legal liability | Obtain liability insurance for injury incurred from participating in health research when insurance is available |
| Obtain liability insurance for injury incurred from participating in health research when insurance is available | Develop locally relevant guidelines for recusal and conflict of interest |
| Restrict the addition of material by special interest groups, including government agencies | Develop strategies to assess a potential participant's understanding of the protocol before permitting their participation |
| Develop strategies to assess a potential participant's understanding of the protocol before permitting their participation |
Potential Conflicts of Interest in Research and During IRB Review
The most important conflicts of interest are financial.36–38 If a study is sponsored by industry, there is a clear obligation to inform potential participants. There is also a conflict of interest in the need for IRBs to protect both study participants and the sponsoring institution in developed countries (Table 1). Because the issue of research liability has not been resolved, this problem will not disappear in the foreseeable future. (Note that insurance to cover these risks is now available in some sub-Saharan African countries.) More subtle conflicts of interest include receiving income for testing candidate drugs or vaccines and opportunities for career advancement. Although conflicts of interest may arise over income from studies performed for industry in both developed and developing countries, they are potentially a greater concern in developing countries because those investigators often have very low salaries. Industry-sponsored studies pose a special challenge, because they vary widely. Some are as valuable as the best studies supported by competitive funding agencies; others are performed only to obtain approval for products that offer no health advantage.
An additional problem is a frequent lack of guidelines for recusal by IRB members. Because developing-country IRBs have fewer faculties to draw on, it can be difficult to find someone with the requisite expertise who has no conflict of interest with the investigators whose proposal is being reviewed (Table 1).
Conclusions and Summary
We conclude that (1) informed consent can be improved in both developed and developing countries and (2) there is no logical reason to insist that informed consent be identical in countries with markedly different cultures, social traditions, and literacy, as illustrated by community participation in the decision-making process and differences in literacy, access to medical care, and the understanding of complex issues such as the right of refusal and randomization. Insisting that the same strategy be used in such different environments is both illogical and inappropriate; it has the potential to severely reduce international collaboration on important health questions.
We make seven recommendations. (1) IRBs should be responsible only for the rights of participants and the integrity of the consent process (not for institutional liability). (2) IRBs should consider the customs and cultural nuances of the host country when making recommendations to investigators. (3) Medical expenses for injuries incurred during health research should be covered by liability insurance (when available) to protect study participants from potential financial hardship. (4) Community leaders should participate in the consent process when appropriate to ensure that participants understand the research proposed and its potential risks and benefits when they provide their consent. (5) Strategies consistent with the cultural practices and traditions of the communities in which studies are performed should be developed to present those studies to illiterate participants without using written documents (e.g., presentation of a study in the local language on videotape with demonstrations of the procedures involved in the study). (6) Informed consent should likewise be obtained without the use of written documents (e.g., by videotaping the consent transaction between the participant and a representative of the study [oral consent that is videotaped]). (7) The understanding of potential participants should be assessed orally before they are permitted to enroll or participate in clinical studies.25,42,43
Acknowledgments
The authors thank Ogobara K. Doumbo, Boubacar Cissé, Juan J. L. Lertora, Ina Friedman, Richard Mirabelli, David Mushatt, Mark James, and Brian Byrd for their thoughtful reviews of earlier versions of the manuscript, and the IRBs of the University of Bamako and Tulane University for their dedication in the face of these challenges. The authors thank Ruth Faden, Jeanette Magnus, Judith LaRosa, Erna Bauer, Gina Etheridge, Christine Grady, Elizabeth Higgs, Judith LaRosa, Melody Lin, Myron M. Levine, Louis H. Miller, William R. Robinson III, and Richard K. Sakai for their thoughtful discussions and constructive suggestions.
Footnotes
Financial support: These studies were supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID; Grants AI-074058 and AI 21536), Cooperative Agreement P50 AI 34969 from NIAID to the Mali-Tulane Tropical Medicine Research Center, grants from the United Nations Development Programme (UNDP)/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases (TDR; 94-0622 and 92-0751), and a New Initiatives in Malaria Research Award to D.J.K. from the Burroughs–Wellcome Fund.
Authors' addresses: Donald J. Krogstad, Department of Tropical Medicine and Center for Infectious Diseases, Tulane University, New Orleans, LA, E-mail: krogstad@tulane.edu. Samba Diop and Ousmane A. Koita, Department of Public Health, Faculties of Medicine, Pharmacy, and Dentistry, University of Bamako, Bamako, Mali, E-mails: saibd@icermali.org and okoita@icermali.org. Amadou Diallo, University of Bamako, Bamako, Mali, E-mail: amaddia2003@yahoo.fr. Fawaz Mzayek, Department of Epidemiology, University of Memphis, Memphis, TN, E-mail: fmzayek@memphis.edu. Joseph Keating, Department of International Health and Development, Tulane University, New Orleans, LA, E-mail: jkeating@tulane.edu. Yéya T. Touré, Faculty of Medicine, Pharmacy and Dentistry, University of Bamako, Bamako, Mali, E-mail: tourey@who.int.
References
- 1.Shalala D. Protecting research subjects—what must be done. N Engl J Med. 2000;343:808–810. doi: 10.1056/NEJM200009143431112. [DOI] [PubMed] [Google Scholar]
- 2.Spicer CM. Federal oversight and regulation of human subjects research—an update. Kennedy Inst Ethics J. 2000;10:261–264. doi: 10.1353/ken.2000.0023. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous Protection of human research subjects—HHS: notice of proposed rulemaking. Fed Regist. 1998;63:27794–27804. [PubMed] [Google Scholar]
- 4.Faden RR, Beauchamp TL. A History and Theory of Informed Consent. New York, NY: Oxford University Press; 1986. pp. 3–330. [Google Scholar]
- 5.Tangwa GB. Moral agency, moral worth and the question of double standards in medical research in developing countries. Dev World Bioeth. 2000;1:156–162. doi: 10.1111/1471-8847.00022. [DOI] [PubMed] [Google Scholar]
- 6.Tangwa GB. The traditional African perception of a person: some implications for bioethics. Hastings Cent Rep. 2000;30:39–43. [PubMed] [Google Scholar]
- 7.Barrett RJ, Parker DB. Rites of consent: negotiating research participation in diverse cultures. Monash Bioeth Rev. 2003;22:9–26. doi: 10.1007/BF03351389. [DOI] [PubMed] [Google Scholar]
- 8.Benatar SF. Reflections and recommendations on research ethics in developing countries. Soc Sci Med. 2002;54:1131–1141. doi: 10.1016/s0277-9536(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 9.Bhutta ZA. Ethics in international health research: a perspective from the developing world. Bull World Health Organ. 2002;80:114–120. [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez S, Salazar G, Tijero M, Diaz S. Informed consent procedures: responsibilities of researchers in developing countries. Bioethics. 2001;15:398–412. doi: 10.1111/1467-8519.00250. [DOI] [PubMed] [Google Scholar]
- 11.Enwonwu CO, Falkler WA, Jr, Idigbe EO, Afolabi BM, Ibrahim M, Onwujekwe D, Savage O, Meeks VI. Pathogenesis of cancrum oris (noma): confounding interactions of malnutrition with infection. Am J Trop Med Hyg. 1999;60:223–232. doi: 10.4269/ajtmh.1999.60.223. [DOI] [PubMed] [Google Scholar]
- 12.Lavery JV, Grady C, Wahl ER, Emanuel EJ. Ethical Issues in International Biomedical Research. New York, NY: Oxford University Press; 2007. pp. 263–294. [Google Scholar]
- 13.Haworth L. In: Autonomy: An Essay in Philosophical Psychology and Ethics. Haworth L, editor. New Haven, CT: Yale University Press; 1986. pp. 1–46. (The concept of autonomy). [Google Scholar]
- 14.Dworkin G. The Theory and Practice of Autonomy. Cambridge, UK: Cambridge University Press; 1988. pp. 3–120. [Google Scholar]
- 15.Dalla-Vorgia P, Lascaratos J, Skiadas P, Garanis-Papadatos T. Is consent in medicine a concept only of modern times? J Med Ethics. 2001;27:59–61. doi: 10.1136/jme.27.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horner J. Morality, ethics, and law: introductory concepts. Semin Speech Lang. 2003;24:263–274. doi: 10.1055/s-2004-815580. [DOI] [PubMed] [Google Scholar]
- 17.Majumdar SK. Aristotle's ethical theory and modern health care. Bull Indian Inst Hist Med Hyderabad. 1996;26:75–80. [PubMed] [Google Scholar]
- 18.Brema TA. Proposed revisions to the Declaration of Helsinki. N Engl J Med. 1999;341:527–531. doi: 10.1056/NEJM199908123410712. [DOI] [PubMed] [Google Scholar]
- 19.Knippenberg R, Alihonou E, Soucat A, Oyegbite K, Calivis M, Hopwood I, Niimi R, Diallo MP, Conde M, Ofosu-Amaah S. Implementation of the Bamako Initiative: strategies in Benin and Guinea. Int J Health Plann Manage. 1997;12((Suppl 1)):S29–S47. doi: 10.1002/(sici)1099-1751(199706)12:1+<s29::aid-hpm465>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Das PK. Community participation in vector borne disease control: facts and fancies. Ann Soc Belg Med Trop. 1991;71((Suppl 1)):233–242. [PubMed] [Google Scholar]
- 21.Uzochukwu BS, Onwujekwe OE, Akpala CO. Effect of the Bamako Initiative drug-revolving fund on the availability and rational use of essential drugs in primary care facilities in south-east Nigeria. Health Policy Plan. 2002;17:378–383. doi: 10.1093/heapol/17.4.378. [DOI] [PubMed] [Google Scholar]
- 22.Soucat A, Gandaho T, Levy-Bruhl D, de Bethune X, Alihonou E, Ortiz C, Gbedonou P, Adovohekpe P, Camara O, Ndiaye JM, Dieng B, Knippenberg R. Health seeking behaviour and household health expenditures in Benin and Guinea: the equity implications of the Bamako Initiative. Int J Health Plann Manage. 1997;12((Suppl 1)):S137–S163. doi: 10.1002/(sici)1099-1751(199706)12:1+<s137::aid-hpm469>3.3.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Oguz NY. Research ethics committees in developing countries and informed consent: with special reference to Turkey. J Lab Clin Med. 2003;141:292–296. doi: 10.1016/S0022-2143(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 24.Willerson JT, Kereiakes DJ. Clinical research and future improvement in clinical care: the Health Insurance Portability and Accountability Act (HIPAA) and future difficulties but optimism for the way forward. Circulation. 2003;108:919–920. doi: 10.1161/01.CIR.0000089331.82015.46. [DOI] [PubMed] [Google Scholar]
- 25.Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: a new measure of understanding among research subjects. J Natl Cancer Inst. 2001;93:139–147. doi: 10.1093/jnci/93.2.139. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald DW, Marotte C, Verdier RI, Johnson WD, Jr, Pape JW. Comprehension during informed consent in a less-developed country. Lancet. 2002;360:1301–1302. doi: 10.1016/S0140-6736(02)11338-9. [DOI] [PubMed] [Google Scholar]
- 27.Angell M. Investigators' responsibilities for human subjects in developing countries. N Engl J Med. 2000;342:967–969. doi: 10.1056/NEJM200003303421309. [DOI] [PubMed] [Google Scholar]
- 28.Macfarlane S, Racelis M, Muli-Musiime F. Public health in developing countries. Lancet. 2000;356:841–846. doi: 10.1016/s0140-6736(00)02664-7. [DOI] [PubMed] [Google Scholar]
- 29.Truog RD. Is “informed right of refusal” the same as “informed consent?”. J Clin Ethics. 1996;7:87–89. [PubMed] [Google Scholar]
- 30.van Bogaert L-J. Rights and duties to non-consenting patients: informed refusal in the developing world. Dev World Bioeth. 2006;6:13–22. doi: 10.1111/j.1471-8847.2006.00132.x. [DOI] [PubMed] [Google Scholar]
- 31.Featherstone K, Donovan JL. Random allocation or allocation at random: patients' perspectives of participation in a randomised controlled trial. BMJ. 1998;317:1177–1180. doi: 10.1136/bmj.317.7167.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llewellyn-Thomas HA, McGreal MJ, Thiel EC, Fine S, Erlichman C. Patients' willingness to enter clinical trials: measuring the association with perceived benefit and preference for decision participation. Soc Sci Med. 1991;32:35–42. doi: 10.1016/0277-9536(91)90124-u. [DOI] [PubMed] [Google Scholar]
- 33.Taylor KM, Kelner M. Informed consent: the physicians' perspective. Soc Sci Med. 1987;24:135–143. doi: 10.1016/0277-9536(87)90246-2. [DOI] [PubMed] [Google Scholar]
- 34.Macpherson CC. Ethics committees. Research ethics: beyond the guidelines. Dev World Bioeth. 2001;1:57–68. doi: 10.1111/1471-8847.00008. [DOI] [PubMed] [Google Scholar]
- 35.Arda B. Evaluation of research ethics committees in Turkey. J Med Ethics. 2000;26:459–461. doi: 10.1136/jme.26.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sollitto S, Hoffman S, Mehlman M, Lederman RJ, Youngner SJ, Lederman MM. Intrinsic conflicts of interest in clinical research: a need for disclosure. Kennedy Inst Ethics J. 2003;13:83–91. doi: 10.1353/ken.2003.0015. [DOI] [PubMed] [Google Scholar]
- 37.Morin K, Rakatansky H, Riddick FA, Jr, Morse LJ, O'Bannon JM, 3rd, Goldrich MS, Ray P, Weiss M, Sade RM, Spillman MA. Managing conflicts of interest in the conduct of clinical trials. J Am Med Assoc. 2002;287:78–84. doi: 10.1001/jama.287.1.78. [DOI] [PubMed] [Google Scholar]
- 38.LeVine RA, Dexter E, Velasco P, LeVine S, Joshi AR, Stuebing KW, Tapia-Uribe FM. Maternal literacy and health care in three countries: a preliminary report. Health Transit Rev. 1994;4:186–191. [PubMed] [Google Scholar]
- 39.Macklin R. Informed consent for research: international perspectives. J Am Med Womens Assoc. 2000;55:290–293. [PubMed] [Google Scholar]
- 40.Doumbo OK, Krogstad DJ. Doctoral training of African scientists. Am J Trop Med Hyg. 1998;58:127–132. doi: 10.4269/ajtmh.1998.58.127. [DOI] [PubMed] [Google Scholar]
- 41.McPherson CC. Research ethics: beyond the guidelines. Dev World Bioeth. 2001;1:57–68. doi: 10.1111/1471-8847.00008. [DOI] [PubMed] [Google Scholar]
- 42.Minnies D, Hawkridge T, Hannekom W, Ehrlich R, London L, Hussey G. Evaluation of the quality of informed consent in a vaccine field trial in a developing country. BMC Med Ethics. 2008;9:15. doi: 10.1186/1472-6939-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krosin MT, Klitzman R, Levin B, Cheng J, Ranney ML. Problems in comprehension of informed consent in rural and periurban Mali, West Africa. Clin Trials. 2006;3:306–313. doi: 10.1191/1740774506cn150oa. [DOI] [PubMed] [Google Scholar]


