Abstract
The outbreak of chikungunya fever that surfaced in India during late 2005 has affected more than 1.56 million people, spread to more than 17 states/union territories, and is still ongoing. Many of these areas are dengue- and leptospirosis-endemic settings. We carried out a cross-sectional survey in one such chikungunya-affected location in Dakshina Kannada District of Karnataka State to estimate the magnitude of the epidemic and the proportion of chikungunya virus (CHIKV) infections that remained clinically inapparent. The seropositivity for CHIKV infection was 62.2%, and the attack rate of confirmed CHIK fever was 58.3%. The proportion of inapparent CHIKV infection was 6.3%. The increasing trend in the seropositivity and attack rate of CHIKV infection with age group was statistically significant. The present study is an indicator of the magnitude of the ongoing outbreak of CHIKV infection in India that started during 2005–2006.
Chikungunya (CHIK) fever surfaced in India during October 2005 in the state of Andhra Pradesh, and by July 2009, it spread to 17 states/union territories.1–3 The epidemic affected all the states in south India. These states are also dengue- and leptospirosis-endemic areas.4,5 By July 2009, a total of 1,568,630 suspected cases were reported throughout India.2,3 However, this statistic is considered a gross underestimate.6 More than one-half of these cases were reported from the southern Indian state of Karnataka.2,3 We carried out a cross-sectional survey among the people residing in the jurisdictional area of a primary health center (PHC) in Dakshina Kannada District of Karnataka State to estimate the magnitude of the epidemic and the proportion of CHIK virus (CHIKV) infections that remained clinically inapparent.7 Institutional Ethics Committee approval for the study and informed consent from subjects were obtained.
In Dakshina Kannada District, the outbreak started in January 2008. As per the statistics of Health Services, by August 2008, around 40,000 people were suspected to have suffered CHIK fever based on a surveillance case definition laid down by the National Vector Borne Disease Control Program (NVBDCP), India. The surveillance case definition stipulated that any patient reporting with fever and arthralgia/arthritis be considered as a suspected case of CHIK fever. The district is a dengue- and leptospirosis-endemic area.8,9 The number of laboratory-confirmed cases of CHIK fever, dengue, and leptospirosis until mid-August 2008 was 173, 29, and 28, respectively. Laboratory-confirmed dengue cases reported in 2003, 2004, 2005, 2006, and 2007 were 100, 1, 3, 7, and 14, respectively, and the leptospirosis cases reported for the same years were 1, 15, 37, 48, and 23, respectively. CHIK fever was not reported before 2008.
The study was carried out from August to September 2008 in Adyanadka PHC jurisdiction area containing two villages. The PHC area has a population of 13,861 people living in 3,000 households. Based on PHC statistics, around 2,000 people suffered from suspected CHIK fever by mid-August.
The number of suspected cases of CHIK fever reported to the PHC during the period of January to September 2008 by month of reporting was obtained from the records of PHC. A cross-sectional survey was carried out among a sample population of 1,174 living in 300 households drawn from all the four subcenter areas (75 households in each subcenter area) of the PHC. In each subcenter area, a systematic sampling method was used. The sampling frame was the household enumeration list maintained in PHC. The first house to be surveyed in each subcenter area was selected at random from the sampling frame and then, every 10th house was included in the sample. Those that suffered from fever, joint pain, or both during the epidemic period were considered as suspected cases of CHIK fever. The study population was stratified into five age strata [i.e., < 13 years (children), 13–19 years (teenagers), 20–29 years, 30–44 years, and ≥ 45 years (elderly)]. The proportion of persons suspected to have suffered CHIK fever (age- and sex-specific attack rate of suspected CHIK fever) was calculated for each age and sex strata.
Serological testing was carried out in a subsample of 360 individuals selected from the study population at random after stratifying for age group and sex (excluding children aged less than 5 years). This sample size was sufficient to estimate a prevalence of CHIKV infection similar to the observed prevalence of suspected CHIK fever with an absolute precision of 5%. Brief histories of all the subjects were taken, and any other symptoms suffered during epidemic period were recorded. A blood sample was collected from these subjects. The serum was separated, and the presence of anti-CHIKV immunoglobulin M (IgM) antibody was tested by the IgM-capture enzyme-linked immunosorbent assay (ELISA) method using the kit developed by the National Institute of Virology (NIV, Pune, India). A test was considered positive when the test optical density (OD) was ≥ 2.1 times the negative control OD. The in-house validation of this assay carried out at NIV has shown a high specificity of 96.5% (A. Sudeep, personal communication).
Figure 1 shows the number of cases of suspected CHIK fever reported to the PHC by month of reporting. The epidemic started in February, peaked in June, and tapered off during the next few months.
Figure 1.
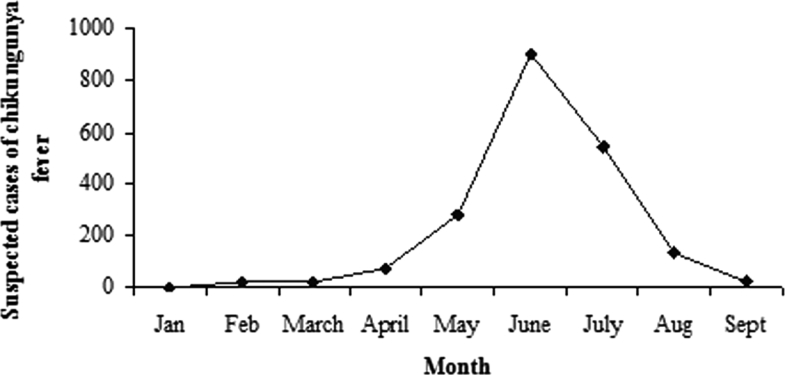
The number of cases of suspected CHIK fever reported to the PHC by month of reporting, January to September 2008.
The age and sex structure of the study population was similar to that of the census population in the PHC area. The overall attack rate of suspected CHIK fever based on case definition (i.e., fever, joint pain, or both) among the surveyed population was 66.4% [95% confidence interval (CI) = 63.7–69.1].
Of the 360 subjects tested for the presence of anti-CHIKV IgM antibody, 237 had suffered symptoms specified in the case definition between March 2008 and September 2008. Of the 237 subjects, 210 tested positive for anti-CHIKV IgM antibody. Fourteen subjects who did not suffer from the symptoms specified in the case definition also tested positive for anti-CHIKV IgM antibody.
The distribution of the OD to cut-off ratios among the 360 study subjects (data not shown) was bimodal, with the first mode at 0.5 and the anti-mode at 1.0, which coincided with the cut-off OD used. Values below the anti-mode were closely clustered around the first mode, indicating that the test effectively separated infected and non-infected persons. The mean of the OD to cut-off ratios among subjects with laboratory-confirmed CHIKV infection was 4.3 ± 2.3, and the mean among the others was 0.8 ± 1.0; this difference was statistically significant (t = 19.8, P < 0.001). However, among the laboratory-confirmed patients, the distribution did not differ between those who fulfilled the case definition and those who did not (mean OD/cut-off = 4.3 ± 2.2 versus 3.8 ± 2.4, t = 0.72, P = 0.485).
Of the 210 laboratory-confirmed patients who tested positive for anti-CHIKV IgM antibodies and suffered one or more symptoms specified in the case definition, 2.0% suffered only fever, 15.0% had only joint pain, and 83.0% had both. The 27 IgM-negative symptomatic patients included 5 (18.5%) persons with fever alone, 3 (11.1%) with joint pain alone, and 19 (70.4%) with both.
For comparing the distribution of OD to cut-off ratios among symptom groups, the study subjects were grouped into two groups based on the presence of symptoms. Three such comparisons were done: the first with fever, the second with joint pain, and the third with fever and/or joint pain as the categorizing symptom. In all these comparisons, the means of the OD to cut-off ratios were higher among those with the symptom compared with those without, and the differences were statistically significant (3.8 ± 2.4 versus 1.9 ± 1.3, t = 7.8, P < 0.001 for fever; 4.0 ± 2.4 versus 1.3 ± 1.8, t = 10.7, P < 0.001 for joint pain; 3.9 ± 2.4 versus, t = 13.5, P < 0.001 for fever and/or joint pain).
Table 1 summarizes the survey results. The overall seropositivity for CHIKV infection was 62.2% (95% CI = 57.0–67.3). In 6.3%, the infection was inapparent, because they did not suffer from any of the symptoms specified in the case definition. Although the proportion of inapparent infection among school-aged children was high [14.3% (4/28)] compared with the other groups [5.1% (10/196)], the difference was not statistically significant (χ2 = 3.53, P = 0.06). The attack rate of confirmed cases of CHIK fever observed was 58.3% (95% CI = 53.0–63.5). The increasing trend in CHIKV infection with age group was statistically significant (χ2 for linear trend = 47.631, P < 0.01). A similar trend is also observed in the attack rate of confirmed CHIK fever. CHIKV seroprevalence among women (66.3%) was higher than that among men (58.5%). However, the difference was not statistically significant (χ2 = 2.31, P = 0.12). The difference in the attack rate of confirmed CHIK fever among males (54.8%) and females (62.2%) was also not statistically significant.
Table 1.
Household survey based on case definition and serological survey for the presence of anti-CHIK IgM antibody
| Age group (years) | Disease survey based on case definition | Serosurvey | Inapparent infection | Attack rate of confirmed CHIK fever (%) | |||
|---|---|---|---|---|---|---|---|
| Surveyed | Positive (%) | No. | Positive (%) | No. | Proportion (%) | ||
| < 13 | 241 | 98 (40.7) | 75 | 28 (37.3) | 4 | 14.3 | 32.0 |
| 13–19 | 84 | 53 (63.1) | 27 | 14 (51.9) | 1 | 7.1 | 48.1 |
| 20–29 | 306 | 188 (61.4) | 94 | 53 (56.4) | 4 | 7.5 | 52.1 |
| 30–44 | 265 | 200 (75.5) | 83 | 57 (68.7) | 1 | 1.8 | 67.5 |
| ≥45 | 278 | 241 (86.7) | 81 | 72 (88.9) | 4 | 5.6 | 84.0 |
| Overall | 1,174 | 780 (66.4) | 360 | 224 (62.2) | 14 | 6.3 | 58.3 |
Dengue IgM testing was performed on all serum samples and was detected in only 14 patients, 9 of whom were also CHIKV IgM positive.10 Leptospira IgM antibody was found in only five patients.
The present study is an indicator of the magnitude of the ongoing outbreak of CHIK fever in India, which started during 2005 and 2006.1,11 The high seroprevalence of anti-CHIKV IgM antibodies (62.2%) and attack rate (58.3%) observed in the present study is comparable with the figures documented during earlier outbreaks in Africa, Grande Comore Island, and other parts of India.12–14 However, the seroprevalence studies done in these territories have shown large variations between regions in the prevalence rate.15 It was 38.2% in La Reunion Island and 63% in Grande Comore Island.13,15 The current serosurvey was conducted at the end of outbreak, avoiding the risk of underestimating the number of cases that might occur if serosurveys are conducted during epidemic.
The present data indicate higher risks of CHIKV infection and development of clinical illness among older populations. This was observed earlier on Reunion Island (74% of the victims were over 30 years of age) and in India.12,16,17
The study could estimate the proportion of inapparent CHIKV infection during CHIK fever outbreak, and this was found to be low (6.3%). The serosurvey done on Reunion Island has documented a 16.0% prevalence of inapparent infection.15
There seems to be an association between the overall seroprevalence in a study population and the proportion of inapparent infection. When there is widespread infection, as indicated by high seroprevalence, the proportion of inapparent infection seems to be low, as in the case of the present study (62.2% and 6.3%), whereas in study populations with lower seroprevalence, as in the case of the study population on La Reunion Island (38.2%), the proportion of inapparent infection was found to be higher (16.0%).15 When the exposure is widespread and frequent, a large proportion of the population is likely to be infected, resulting in high seroprevalence. In such situations, the dose of infection is also likely to be high because of frequent exposure to the bites of carrier mosquitoes, possibly resulting in more severe infection than when the dose of infection is low. This could be one explanation for a higher proportion of symptomatic infection in communities where the exposure and seroprevalence is widespread. However, unlike dengue fever and leptospirosis, the proportion of inapparent infection seems to be much less in CHIKV infection.18–20 The age-group differences in the proportion of inapparent infection are more likely explained by age-dependent effects on disease symptoms.21
We documented the simultaneous presence of CHIKV, dengue virus, and leptospirosis infection in the community as well as the concurrent presence of CHIKV and dengue antibodies in same subjects.22,23 However, the contribution of leptospiral and dengue viral infection in the present outbreak is low, which is evidenced by low seropositivity to these infections among the patients affected during the outbreak.
There were a few limitations to the study. The seroprevalence survey did not include children below 5 years of age, who might have had a lower seroprevalence than the older population. This might have resulted in some overestimation of the overall seroprevalence. A diagnosis of CHIKV infection was based on the presence of anti-CHIKV IgM antibodies. IgM antibodies in some of the persons infected early during the course of the outbreak might have declined to undetectable levels by the time testing was done, thus leading to misclassification of such persons as non-infected. This might have resulted in an underestimation of the seroprevalence and attack rate. The survey depended on self-reported symptoms during the previous 5 months, and there could be some recall bias, although it is unlikely to be substantial enough to grossly distort the overall finding of the study.
In summary, the present study confirms previous observations—the widespread nature of the CHIKV infection during an outbreak resulting in high attack rate. Only one study before this documented the proportion of inapparent infection in CHIK outbreak, and it was very low compared with other arboviral diseases. Our study further shows that it still could be low when the overall attack rate is high. The increase in attack rate with increasing age needs explanation and further research.
Acknowledgments
We are thankful to District Health and Family Welfare Officer, Mangalore, Dr. Jagannath, MBBS, MS, for his support and to the field and other supporting staff of Adyanadka Primary Health Centre. We are also thankful to Mr. N. T. Raja, Mr. Krishna Upadhyaya, Mrs. Laxmi Upadhyaya, Mr. Bhaskara Upadhyaya, and Mrs. Parameshwari for providing logistical help for our field team during the entire period of stay in the remote village.
Footnotes
Financial support: The study was supported by the internal funds of Regional Medical Research Centre, Port Blair (Indian Council of Medical Research).
Authors' addresses: Sathya P. Manimunda, Attayur P. Sugunan, Paluru Vijayachari, Ananganallur N. Shriram, Sameer Sharma, Nagarajan Muruganandam, Itta K. Chaitanya, and Dev R. Guruprasad, Regional Medical Research Centre (RMRC-ICMR), Port Blair 744 101, Andaman and Nicobar Islands, India, E-mails: sathyamanimunda@rediffmail.com, apsugunan@gmail.com, vijayacharip@yahoo.com, shriraman@icmr.org, meabhisharma@rediffmail.com, n.muruganandam@gmail.com, chaitanyaitta@gmail.com, and guruprasad.prasad1983@gmail.com. Subhodh K. Rai, Department of District Health and Family Welfare, Adyanadka 574260, Karnataka, Dakshina Kannada, India, E-mail: sathya_pblair@yahoo.co.in. Anakkathil B. Sudeep, National Institute of Virology, Pune 411 021, Maharashtra, India, E-mail: sudeepmcc@yahoo.co.in.
References
- 1.Ravi V. Reemergence of chikungunya virus in India. Indian J Med Microbiol. 2006;24:83–84. doi: 10.4103/0255-0857.25175. [DOI] [PubMed] [Google Scholar]
- 2.Krishnamoorthy K, Harichandrakumar KT, Kumari AK, Das LK. Burden of chikungunya in India: estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J Vector Borne Dis. 2009;46:26–35. [PubMed] [Google Scholar]
- 3.National Vector Borne Disease Control Programme Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. Chikungunya Situation in India. 2009. http://nvbdcp.gov.in/Doc/chikun-update07.pdf Available at. Accessed July 18, 2009.
- 4.World Health Organization . Dengue Haemorrhagic Fever. Diagnosis, Treatment, Prevention and Control. 2nd ed. Geneva, Switzerland: World Health Organization; 1997. pp. 1–3. [Google Scholar]
- 5.Sambsiava RR, Naveen G, Bhalla P, Agarwal SK. Leptospirosis in India and the rest of the world. Braz J Infect Dis. 2003;7:179–193. doi: 10.1590/s1413-86702003000300003. [DOI] [PubMed] [Google Scholar]
- 6.Mavalankar D, Shastri F, Raman P. Chikungunya epidemic in India: a major public health disaster. Lancet Infect Dis. 2007;7:306–307. doi: 10.1016/S1473-3099(07)70091-9. [DOI] [PubMed] [Google Scholar]
- 7.National Informatics Centre, Karnataka State Government of India. Ministry of Communication and Information Technology, Department of Information Technology. District Profile. Dakshina Kannada (Mangalore) 2009. http://www.kar.nic.in/zpdk/district_profile.htm Available at. Accessed July 18, 2009.
- 8.Padbidri VS, Adhikari P, Thakare JP, Ilkal MA, Josho GD, Pereira P, Guttikar SN, Walhekar BD, Chowla N, Hegde BM. The 1993 epidemic of dengue fever in Mangalore, Karnataka State, India. Southeast Asian J Trop Med Public Health. 1995;26:699–704. [PubMed] [Google Scholar]
- 9.Baruah J, Ananda S, Arun Kumar G. Incidence of dengue in a tertiary care centre, Kasturba Hospital Manipal. Indian J Pathol Microbiol. 2006;49:462–463. [PubMed] [Google Scholar]
- 10.Gadkari DA, Shaikh BH. IgM antibody captures ELISA in the diagnosis of Japanese encephalitis, West Nile and dengue virus infections. Indian J Med Res. 1984;80:613–619. [PubMed] [Google Scholar]
- 11.Kaur P, Ponniah M, Murhekar MV, Ramachandran V, Ramachandran R, Raju HK, Perumal V, Mishra AC, Gupte MD. Chikungunya outbreak, South India, 2006. Emerg Infect Dis. 2008;14:1623–1625. doi: 10.3201/eid1410.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pialoux G, Gauzere BA, Jaureguibery S, Stobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;5:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 13.Sergon K, Yahaya AA, Brown J, Bedja SA, Mlindasse M, Agata N, Allaranger Y, Ball MD, Powers AM, Ofula V, Onyango C, Konongoi LS, Sang R, Njenga MK, Breiman RF. Seroprevalence of chikungunya virus infection on Grande Comore Island, Union of the Comoros, 2005. Am J Trop Med Hyg. 2007;76:1189–1193. [PubMed] [Google Scholar]
- 14.Kalantri SP, Joshi R, Riley LW. Chikungunya epidemic: an Indian perspective. Natl Med J India. 2006;19:315–322. [PubMed] [Google Scholar]
- 15.Geradin P, Guernier V, Perrau J, Fianu A, Roux KL, Grivard P, Michault A, Lamballerie XD, Flahault A, Favier F. Estimating chikungunya prevalence in La Reunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis. 2008;8:99. doi: 10.1186/1471-2334-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quatresous I. E-alert 27 January: chikungunya outbreak in Reunion, a French overseas department. Euro Surveill. 2006;11:E060202.1. [PubMed] [Google Scholar]
- 17.Yergolkar PN, Tandale BV, Arankalle VA, Sathe PS, Sudeep AB, Gandhe SS, Gokhle MD, Jacob GP, Hundekar SL, Mishra AC. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580–1583. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 19.Sehgal SC, Sugunan AP, Murhekar MV, Sharma S, Vijayachari P. Randomized controlled trial of doxycycline prophylaxis against leptospirosis in an endemic area. Int J Antimicrob Agents. 2000;13:249–255. doi: 10.1016/s0924-8579(99)00134-x. [DOI] [PubMed] [Google Scholar]
- 20.Speelman P. In: Harrison's Principles of Internal Medicine. Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. New York: McGraw-Hill; 2005. pp. 988–991. (Leptospirosis). [Google Scholar]
- 21.Tsai TF, Weaver SC, Monath TP. In: Clinical Virology. 2nd ed. Richman DD, Whitley RJ, Hayden FG, editors. Washington, DC: ASM Press; 2002. pp. 1199–1201. (Alphaviruses). [Google Scholar]
- 22.Chahar HS, Bharaj P, Dar L, Guleria R, Kabra SK, Broor S. Co- infections with chikungunya virus and dengue virus in Delhi, India. Emerg Infect Dis. 2009;15:1077–1080. doi: 10.3201/eid1507.080638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leroy EM, Nkoghe D, Ollomo B, Nze-Nkogue C, Becquart P, Grard G, Pourrut X, Charrel R, Moureau G, Ndjoyi-Mbiguino A, Lamballerie XD. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg Infect Dis. 2009;15:591–593. doi: 10.3201/eid1504.080664. [DOI] [PMC free article] [PubMed] [Google Scholar]


