Abstract
To characterize the responses of raccoons to West Nile virus (WNV) infection, we subcutaneously exposed them to WNV. Moderately high viremia titers (≤ 104.6 plaque forming units [PFU]/mL of serum) were noted in select individuals; however, peak viremia titers were variable and viremia was detectable in some individuals as late as 10 days post-inoculation (DPI). In addition, fecal shedding was prolonged in some animals (e.g., between 6 and 13 DPI in one individual), with up to105.0 PFU/fecal swab detected. West Nile virus was not detected in tissues collected on 10 or 16 DPI, and no histologic lesions attributable to WNV infection were observed. Overall, viremia profiles suggest that raccoons are unlikely to be important WNV amplifying hosts. However, this species may occasionally shed significant quantities of virus in feces. Considering their behavioral ecology, including repeated use of same-site latrines, high levels of fecal shedding could potentially lead to interspecies fecal-oral WNV transmission.
Introduction
Over the past several years, numerous studies have indicated that many free-ranging wild mammalian species have been exposed to West Nile virus (WNV; genus Flavivirus; family Flaviviridae).1–9 Despite the apparent lack of mammalian involvement in WNV cycles and the general contention that they serve as dead-end hosts,10 researchers have established that various mammals serve as potential competent reservoir hosts. For example, viremia titers of ≥ 105.0 plaque forming units (PFU)/mL serum were observed in some experimentally infected golden hamsters (Mesocricetus auratus).11 In addition, fox squirrels (Sciurus niger),12–14 eastern gray squirrels (Sciurus carolinensis),15 eastern chipmunks (Tamias striatus),16 and eastern cottontail rabbits (Sylvilagus floridanus)17 developed viremia titers considered sufficient to infect some mosquitoes.18
Raccoons (Procyon lotor) are common throughout many urban and suburban communities in the United States. This species is highly adaptable, occupies a variety of habitats,19 and is an omnivorous opportunist, obtaining sustenance from a variety of both plants and animals.19,20 Raccoons are considered a public health threat for a variety of zoonotic diseases, such as rabies, larval migrans associated with Baylisascaris procyonis infection, and potentially avian and human influenza A viruses.21–23 Although it has been well established that raccoons are commonly exposed to WNV in at least several geographic regions of the United States,3–5,8,9 the reservoir competency and viral shedding profiles of this species have not been studied. This peridomestic species could be a potential public health threat if it is reservoir competent for WNV and/or sheds significant quantities of virus.
Although the WNV reservoir competence status has been established for many avian species,18 with few exceptions, wild mammals have been overlooked for their potential role in WNV transmission ecology.12–17 The potential importance of these animals should not be a priori discounted, as the apparent insignificance of wild mammals in WNV ecology may be from lack of scrutiny rather than from lack of significance.24
We conducted experimental infections of raccoons with WNV. Our objectives were to monitor morbidity and mortality rates, viremia profiles, viral shedding, tissue tropism, and to assess gross and histological lesions in WNV-infected raccoons.
Materials and Methods
Animal collection and holding.
Two groups (groups 1 and 2) of five raccoons each were used in this experiment during the spring and fall of 2009. All raccoons originated (the individuals or their offspring) from the greater Fort Collins area (Larimer County, Colorado) and all were < 1 year of age. Pre-experiment serum samples from these animals were tested by the plaque reduction neutralization test (PRNT) for antibodies to WNV. A second blood sample was drawn from each individual before the experiment as they were moved indoors to confirm their WNV serostatus. For the experimental infection, the raccoons were transferred to a Biosafety Level-3 (BSL-3) animal facility. During each of two separate experiments, four test raccoons were housed in individual isolator cages and a single control raccoon was housed in an open-air cage. Sustenance (omnivore diet; Mazuri, Purina Mills, LLC, St. Louis, MO) and water were provided ad libitum.
Experimental protocol.
On day 0 of each experiment, all test animals were subcutaneously inoculated with ~4,000 (103.6) PFU of WNV strain NY99-4132 (originally isolated from crow brain in New York) diluted in 0.1 mL BA1 medium (M199-Hank's salts, 1% bovine serum albumin, 350 mg/L sodium bicarbonate, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin B in 0.05 M Tris, pH 7.6). The control animals were sham-inoculated with a placebo (BA1 medium) by the same route and volume as WNV inoculates. Following inoculation, all raccoons were observed for signs of illness, bled, and swabbed (oral and fecal) each day. For inoculations and sampling, animals were anesthetized by a combination of ketamine and xylazine (5:1; e.g., ~10 mg/kg ketamine plus a 2 mg/kg xylazine) administered intramuscularly. Blood was placed in serum separator tubes and allowed to clot. Serum was extracted after centrifugation. Swabs were placed in 1 mL of BA1 medium and kept on wet ice until storage. All samples not immediately tested were stored at −80°C before testing. The first group of raccoons (group 1) was sampled daily from 1 to 10 days post-inoculation (DPI), after which they were anesthetized and humanely euthanized with an intracardiac injection of Euthasol.
Because of unexpected results from the experiment with group 1, a second group (i.e., group 2) of raccoons was sampled daily from 1 to 16 DPI. At the latter time point, it was known that viremia and shedding were undetectable or at the threshold of detection on 14 DPI; therefore, raccoons in group 2 were euthanized on 16 DPI.
Plaque assay.
The virus inoculum and post-inoculation sera, swabs, and tissue homogenates were tested for infectious WNV by Vero cell plaque assay as previously described.25 Briefly, Vero cell monolayers in 6-well plates were inoculated in duplicate with 0.1 mL of sample per well. After 1 hour of incubation at 37°C, the cells were overlaid with 3 mL/well of 0.5% agarose (in a yeast extract-lactalbumin overlay medium supplemented with 2,240 mg/L sodium bicarbonate, 292 mg/L L-glutamine, and antibiotics and amphotericin as with BA1). Two days later, cells were overlaid with 0.5% agarose in overlay medium, with 0.004% neutral red dye (Sigma, St. Louis, MO). Viral plaques were counted on the third and fourth days of incubation. The minimum threshold of WNV detection was 100.7 PFU per mL of serum, per mL of urine, per swab, and per mL of 10% tissue suspension.
Plaque reduction neutralization test.
Serum samples collected just before inoculation and upon euthanasia at 10 DPI (group 1), or from 5 to 15 DPI for those euthanized on 16 DPI (group 2), were screened for neutralizing antibodies to WNV using PRNT26 with the same WNV strain used for inoculation. In addition, all pre-inoculation sera were tested for anti-St. Louis encephalitis virus (SLEV) antibodies by PRNT using SLEV strain TBH-28. Before PRNT, serum samples were heat inactivated at 56°C for 30 minutes. For screening, sera were tested at serum dilution of 1:10 in BA1 medium against a challenge dose of 100 PFU of either WNV or SLEV. Sera that neutralized > 75% of viral PFU at a 1:10 dilution were considered positive for antibodies to the respective challenge virus. Sera collected on 16 DPI were serially diluted 2-fold and tested in duplicate to determine WNV reciprocal endpoint 80% neutralization (PRNT80) titers.
Necropsy and tissue processing.
Immediately after euthanasia of all raccoons, blood, oropharyngeal and rectal swabs, and tissues were collected. During necropsy of group 1 raccoons on 10 DPI, body condition and gross lesions were noted, and tissues were collected for both histological examination and for WNV plaque assay. For histological examination, the following tissues were preserved in 10% buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin: heart, spleen, liver, kidney, lung, brain (obex, cerebellum, pons, caudate nuclei and hypothalamus, superior calculi, thalamus), meninges, cervical and thoracic spinal cord, skeletal muscle, popliteal lymph node, small intestine, pancreas, large intestine, trachea, esophagus, stomach, skin, eye, and vertebral bone marrow. For plaque assay, skin, skeletal muscle, heart, lung, liver, spleen, kidney, gonad, small intestine, large intestine, cerebrum, spinal cord, pituitary gland, and eye were each collected into a vial containing 1 mL BA1 with 20% fetal bovine serum (for an approximate 10% tissue suspension) and homogenized and centrifuged as previously described.27 For group 2, heart, liver, spleen, kidney, small intestine, and urine (opportunistically) were collected for plaque assay. Following necropsy, all animal carcasses were incinerated.
Results
All raccoons survived to the end of their respective study period (i.e., 10 DPI for group 1 and 16 DPI for group 2). In addition, all animals appeared clinically normal and appeared to eat and drink normally throughout the experiment.
Of the four experimentally infected raccoons in group 1, two developed detectable viremia. In one of these, viremia was first detected on 4 DPI (102.3 PFU/mL serum) and was undetectable by 8 DPI (Table 1). The peak viremia titer for this animal occurred on 6 DPI (104.0 PFU/mL serum). Viremia was detected from 8 to 10 DPI in the second individual in this group (raccoon 5), and the highest titer detected (102.3 PFU/mL serum) was on 10 DPI, after which samples were not collected (Table 1). All four test animals in group 2 developed detectable viremia, and peak viremia titers ranged from 102.5–4.6 PFU/mL serum. For all individuals in group 2, peak viremia titers occurred on or after 6 DPI ( = 6.8, SE = 0.8; Table 1, Figure 1). No viremia was detected in the sham-inoculated control animal in either group.
= 6.8, SE = 0.8; Table 1, Figure 1). No viremia was detected in the sham-inoculated control animal in either group.
Table 1.
Viremia titers, oral and fecal shedding, and antibody status of raccoons (Procyon lotor) experimentally inoculated with West Nile virus
| Raccoon | Viremia* | Oral shedding* | Fecal shedding | Antibodies | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak titer | Peak DPI† | Range DPI | Peak titer | Peak DPI | Range DPI | Peak titer | Peak DPI | Range DPI | DPI > 75% neutralization | |
| 1‡ | < 0.7 | n/a§ | n/a | < 0.7 | n/a | n/a | < 0.7 | n/a | n/a | > 0 |
| 2 | < 0.7 | n/a | n/a | < 0.7 | n/a | n/a | < 0.7 | n/a | n/a | n/d§ |
| 3 | < 0.7 | n/a | n/a | < 0.7 | n/a | n/a | < 0.7 | n/a | n/a | n/d |
| 4 | 4.0 | 6 | 4–7 | 1.5 | 9 | 6–10¶ | 2.8 | 9 | 8–9 | 10 |
| 5 | 2.3 | 10 | 8–10⊥ | < 0.7 | n/a | n/a | < 0.7 | n/a | n/a | n/d |
| 6‡ | < 0.7 | n/a | n/a | < 0.7 | n/a | n/a | < 0.7 | n/a | n/a | n/d |
| 7 | 4.6 | 6 | 1–7 | 2.9 | 8 | 6–10 | 2.8 | 9 | 5–9¶ | 11 |
| 8 | 2.5 | 9 | 5–10 | 2.5 | 11 | 9–11¶ | 3.9 | 12 | 11–12 | 15 |
| 9 | 2.5 | 6 | 3–7 | 1.2 | 8 | 7–11¶ | 2.3 | 9 | 6–9 | 10 |
| 10 | 3.5 | 6–7 | 3–7 | 2.8 | 9 | 5–14¶ | 5.0 | 9 | 6–13¶ | 11 |
Viremia, oral shedding, and fecal shedding as determined by plaque assay (in log10 PFU/mL for serum and log10 PFU/swab for oral and fecal swabs).
DPI = day(s) post-infection.
Sham-inoculated negative controls.
n/a = not applicable; n/d = none determined.
Oral and fecal shedding was intermittent during these time frames.
This individual was above the minimum threshold of detection on 8 DPI, at the threshold of detection on 9 DPI, and above the threshold of detection on 10 DPI.
Figure 1.
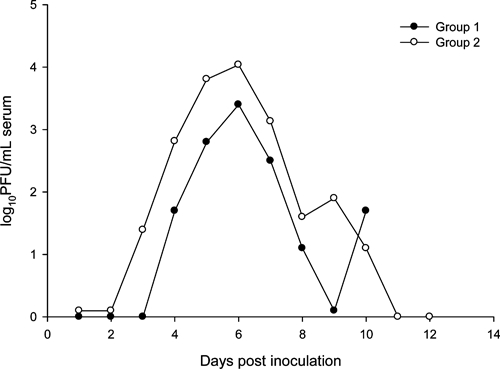
Mean viremia profiles of two groups of raccoons experimentally infected with West Nile virus.
West Nile virus was detected in oral swabs of one of four experimentally infected raccoons in group 1 and four of four experimentally infected raccoons in group 2. Shedding (oral or fecal) was not detected before 5 DPI, and typically initiated 3–5 days after the onset of viremia (Table 1). West Nile virus was shed orally at relatively low titers (e.g., < 103.0 PFU/swab), but lasted up to 7 days after the cessation of viremia. Positive fecal samples were detected in five of six raccoons that developed viremia. One raccoon yielded a high-titered fecal swab sample (105.0 PFU/swab). Because of the unusual nature of detecting such a high-titered fecal swab associated with low-level viremia, we corroborated that the plaques we observed during plaque assays were indeed caused by WNV by reverse transcription-polymerase chain reaction (RT-PCR). For all individuals in group 2, peak fecal shedding occurred on or after 9 DPI ( = 9.75, SE = 0.75; Figure 2). No viral shedding was detected in either of the sham-inoculated control animals (Table 1).
= 9.75, SE = 0.75; Figure 2). No viral shedding was detected in either of the sham-inoculated control animals (Table 1).
Figure 2.
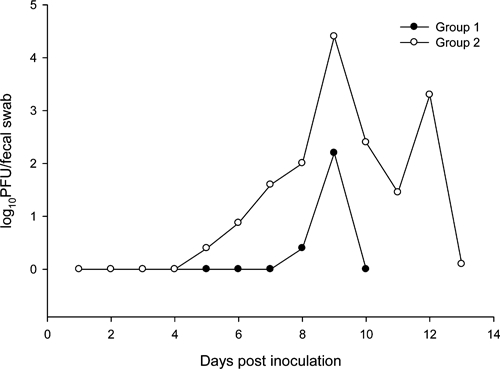
Mean fecal shedding profiles of two groups of raccoons experimentally infected with West Nile virus.
West Nile virus was not detected in tissues tested at either 10 (group 1) or 16 DPI (group 2), or in any opportunistically collected urine sample. No virus was detected in tissues or urine from either of the sham-inoculated control animals.
Serologic testing indicated that five of eight experimentally inoculated raccoons developed detectable anti-WNV antibodies (i.e., ≥ 75% neutralization) within the study period. These five raccoons had initial evidence of seroconversion between 10 and 15 DPI. All raccoons were negative for anti-SLEV antibodies upon inoculation and neither of the sham-inoculated control animals had evidence of seroconversion to WNV throughout the study.
All raccoons from group 1 were in good body condition and hydration upon necropsy. In addition, all had mild to moderate splenomegaly (2–4× normal size). The sham-inoculated control animal and one of the WNV-inoculated animals had hemorrhage at the midline dorsal cerebrum visible on the cerebral surface, of ~3/4 cm width from the vermis to the frontal lobe. Another WNV-inoculated raccoon had hemorrhage evident in the caudo-ventral aspect of the left kidney (~1 cm diameter) also on midline near the retroperitoneal cavity. In general, microscopic lesions were mild or absent. The sham-inoculated control animal had mild focal lymphocytic inflammation in the renal pelvis and multifocal mild infiltration of lymphocytes, plasma cells, and neutrophils within the alveolar spaces of the lungs as well as macrophages within the alveolar walls. In addition, there was a focus of fibroplasia and chronic hemorrhage in skeletal muscle (seimimembranosus muscle). Among inoculated animals, one raccoon (#3) had a mild lymphoplasmacytic accumulation within the alveolar walls and between the muscle layers of the urinary bladder, with occasional neutrophils in the latter. Another individual (#5) had mild spongiosis of the grey matter tracts of the spinal cord. Two WNV-inoculated raccoons had no significant microscopic lesions. All gross and microscopic lesions in this study were interpreted as incidental findings.
Discussion
Raccoons are one of the few free-ranging mammal species in North America for which a high prevalence of WNV exposure has been well documented.2–5,7,8 However, no information about the reservoir competence, shedding, or morbidity rates of this species is available. Our observation of high survival with little or no obvious signs of disease in raccoons is consistent with that reported for eastern cottontail rabbits and fox squirrels.12–14,17 The microscopic lesions within the pulmonary parenchyma observed in one negative control and one WNV-inoculated raccoon were likely incidental findings and may have been associated with previous parasite migration, whereas the fibrosis within the skeletal muscle was likely caused by an injury from injection with anesthetic drugs. The cause of the spinal cord grey matter spongiosis in an inoculated animal appeared to be early edema of unknown etiology.
The high variability in the timing, duration, and magnitude of viremia in the tested raccoons is noteworthy and is in contrast to that which has been previously reported for most other mammals. For example, one raccoon (#7) was viremic from 1 to 7 DPI, although its viremia was at the minimum threshold of detection on 1 and 2 DPI (Table 1). A second raccoon (#8) was viremic 5–10 DPI, whereas a third raccoon (#5) was only viremic on 8–10 DPI (Table 1). In other mammals such as fox squirrels, viremia was generally cleared by 5 DPI,12 although this may vary with exposure route and/or viral strain.14 It is atypical for WNV viremia to initiate as late as 8 DPI. However, delayed onset of viremia was documented for bobwhite quail (Colinus virginianus) experimentally infected with St. Louis encephalitis virus.28 On the other hand, WNV pathogenesis in raccoons following needle-inoculation may differ from mosquito (i.e., natural) inoculation. For example, chickens inoculated by mosquito had enhanced early infection when compared with those inoculated by subcutaneous injection.29
Along with apparently delayed pathogenesis in some experimentally WNV-inoculated raccoons in this study, some individuals were apparently refractory to infection. Two raccoons failed to show evidence of viremia, viral shedding, or seroconversion, thereby suggesting that they did not become infected or that their viremia and shedding levels were below the threshold of detection or delayed in onset beyond the sampling timeline. Although there are competing arguments, the high degree of variability in viremia profiles we observed suggests that the study endpoint for group 1 (i.e., 10 DPI) may have precluded either viremia or seroconversion in these two animals. However, the apparent failure of some test animals to become infected is consistent with another WNV experimental infection in mammals in which only five of eight fox squirrels became viremic after exposure to virus by the oral route.14
Unlike dogs, cats,30 and horses,25 (no close relatives of raccoons have been experimentally evaluated) several raccoons shed WNV by the oral cavity, though at relatively low titers (< 103.0 PFU/swab) (Table 1). Interestingly, Tiawsirisup and others14 recently showed that fox squirrels orally challenged with WNV at a titer similar to the present study (i.e., 102.3 or 103.4 PFU) subsequently developed viremia titers considered sufficient to infect some mosquito vectors. Considering this successful oral inoculation of a wild mammal species with WNV,14 aspects of raccoon behavior that may lead to oral transmission, such as sibling and temporary feeding groups (e.g., close contact),19 may contribute to the high WNV seroprevalence rates noted for this species.3–5
Similar to oral shedding, fecal shedding has implications for inter- and intraspecies transmission of WNV. In this study, fecal shedding was observed in the majority of inoculated individuals and typically began several days after the onset of viremia (e.g., similar to oral shedding). Fecal swabs occasionally contained relatively high WNV titers (mean peak titer of 104.3 PFU/swab among fecal shedders), suggesting that whole feces may contain much higher viral loads. This observation may have both intra- and interspecific transmission implications among wildlife species. For example, raccoons are well known to use latrine (defecation) sites. In Indiana, a total of 14 mammal and 15 bird species were documented to visit raccoon latrine sites, several of which fed on undigested seeds in raccoon feces.31 Importantly, several of the species documented at these sites (e.g., fox squirrels,12 eastern gray squirrels,15 eastern chipmunks,16 eastern cottontails,17 and blue jays [Cyanocitta cristata])18 are thought to produce viremia titers considered sufficient to infect some mosquito species with WNV, and ingestion of virus is a known transmission route.18 In addition, considering the social behavior of raccoons mentioned previously, and that raccoons often manipulate their food items with their hands, the oral-fecal route may provide a mechanism of intraspecific transmission of WNV among raccoons.
Surprisingly, WNV was not detected in raccoon urine samples, even though evidence of infectious virus and viral RNA has been detected in the urine of other mammalian species, including humans.11,12,32,33 Chronic shedding of WNV in urine has been documented in hamsters (initial detection occurred between 35 and 54 DPI),11 and more recently in humans.33 In addition, WNV was not detected in any raccoon tissues tested in the present study, and along with lack of microscopic lesions, this suggests that virus may have failed to reach high titers in tissues (contrary to WNV pathogenesis in some bird species),18 or was rapidly and efficiently cleared from tissues without causing tissue damage.
This study suggests raccoons are probably not an epidemiologically important host in WNV mosquito transmission cycles. Only 75% of test animals developed a detectable viremia and the peak viremia titers of this peridomestic mammal indicate that while they may occasionally infect mosquito vectors with WNV, this event is likely relatively rare. For example, Komar and others18 considered birds with peak viremia titers of < 105.0 PFU/mL serum to be incompetent reservoirs for WNV infection of mosquitoes; peak titers of raccoons in the present study were below this threshold (i.e., maximum of 104.6 PFU/mL serum). However, blood meals from lower viremia titers may be sufficient to infect some mosquito species, although at lower infection rates.30 For example, Tiawsirisup and others34 noted that some mosquito species became infected after feeding on chickens with only moderate viremia titers (e.g., 104.5 CID50/mL serum). Alternately, the role of nonviremic WNV transmission in nature is not well understood but the phenomenon has been demonstrated experimentally in mammals, and precludes virus replication in the vertebrate host.35 The relatively high WNV seroprevalence among raccoons suggests that they may be frequently fed upon by some mosquito species.2–5,7–9 In addition, significant fecal shedding was noted in some raccoons, which may have transmission implications among wildlife by the behavioral ecology of this mesopredator. Thus, although raccoons are well documented to be commonly exposed to WNV in nature,3,5 their role in WNV transmission, if any, may be more likely to be associated with fecal contamination of the environment rather than involvement in mosquito-host transmission cycles.
Acknowledgments
We thank Tricia Fry and Paul Oesterle (National Wildlife Research Center [NWRC]) for logistical assistance, NWRC animal care staff (especially Samantha Granum, Amber Strom, Adam Mitchell, Bryan Vogel, Daniel Gossett, and Gordon Gathright) for assistance with raccoons and excellent animal care, Kaci VanDalen (NWRC) for BSL-3 assistance, and Ginger Young and Nick Thomas for laboratory assistance.
Footnotes
Financial support: Funding for this work was provided by the U.S. Department of Agriculture and the U.S. Centers for Disease Control and Prevention (CDC IAA 03FED12031).
Author's addresses: J. Jeffrey Root, Kevin T. Bentler, and Alan B. Franklin, United States Department of Agriculture, Wildlife Services, National Wildlife Research Center, Fort Collins, CO, E-mails: jeff.root@aphis.usda.gov, kevin.t.bentler@aphis.usda.gov, and alan.b.franklin@aphis.usda.gov. Nicole M. Nemeth, Department of Biomedical Sciences, Colorado State University, Fort Collins, CO, E-mail: nicole.nemeth@colostate.edu. Thomas Gidlewski, United States Department of Agriculture, Wildlife Services, National Wildlife Disease Program, Fort Collins, CO, E-mail: thomas.gidlewski@aphis.usda.gov. Terry R. Spraker, Department of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, CO, E-mail: terry.spraker@colostate.edu.
Reprint requests: J. Jeffrey Root, National Wildlife Research Center, 4101 La Porte Ave., Fort Collins, CO 80521.
References
- 1.Kramer LD, Bernard KA. West Nile virus infection in birds and mammals. Ann N Y Acad Sci. 2001;951:84–93. doi: 10.1111/j.1749-6632.2001.tb02687.x. [DOI] [PubMed] [Google Scholar]
- 2.Dietrich G, Montenieri JA, Panella NA, Langevin S, Lasater SE, Klenk K, Kile JC, Komar N. Serologic evidence of West Nile virus infection in free-ranging mammals, Slidell, Louisiana, 2002. Vector Borne Zoonotic Dis. 2005;5:288–292. doi: 10.1089/vbz.2005.5.288. [DOI] [PubMed] [Google Scholar]
- 3.Root JJ, Hall JS, McLean RG, Marlenee NL, Beaty BJ, Gansowski J, Clark L. Serologic evidence of exposure of wild mammals to flaviviruses in the central and eastern United States. Am J Trop Med Hyg. 2005;72:622–630. [PubMed] [Google Scholar]
- 4.Docherty DE, Samuel MD, Nolden CA, Egstad KF, Griffin KM. West Nile virus antibody prevalence in wild mammals, southern Wisconsin. Emerg Infect Dis. 2006;12:1982–1984. doi: 10.3201/eid1212.060173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentler KT, Hall JS, Root JJ, Klenk K, Schmit B, Ramey P, Blackwell BF, Clark L. West Nile virus seroprevalence in meso-predators in North America. Am J Trop Med Hyg. 2007;76:173–179. [PubMed] [Google Scholar]
- 6.Root JJ, Oesterle PT, Sullivan HJ, Hall JS, Marlenee NL, McLean RG, Montenieri JA, Clark L. Fox squirrel (Sciurus niger) associations with West Nile virus. Am J Trop Med Hyg. 2007;76:782–784. [PubMed] [Google Scholar]
- 7.Gómez A, Kilpatrick AM, Kramer LD, Dupuis AP, Maffei JG, Goetz SJ, Marra PP, Daszak P, Aguirre AA. Land use and West Nile virus seroprevalence in wild mammals. Emerg Infect Dis. 2008;14:962–965. doi: 10.3201/eid1406.070352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blitvich BJ, Juarez LI, Tucker BJ, Rowley WA, Platt KB. Antibodies to West Nile virus in raccoons and other wild peridomestic mammals in Iowa. J Wildl Dis. 2009;45:1163–1168. doi: 10.7589/0090-3558-45.4.1163. [DOI] [PubMed] [Google Scholar]
- 9.Docherty DE, Samuel MD, Egstad KF, Griffin KM, Nolden CA, Karwal L, Ip HS. Short report: changes in West Nile virus seroprevalence and antibody titers among Wisconsin mesopredators 2003–2006. Am J Trop Med Hyg. 2009;81:177–179. [PubMed] [Google Scholar]
- 10.Blitvich BJ, Bowen RA, Marlenee NL, Hall RA, Bunning ML, Beaty BJ. Epitope-blocking enzyme-linked immunosorbent assays for detection of West Nile virus antibodies in domestic mammals. J Clin Microbiol. 2003;41:2676–2679. doi: 10.1128/JCM.41.6.2676-2679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesh RB, Siirin M, Guzman H, Travassos da Rosa AP, Wu X, Duan T, Lei H, Nunes MR, Xiao SY. Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis. 2005;192:287–295. doi: 10.1086/431153. [DOI] [PubMed] [Google Scholar]
- 12.Root JJ, Oesterle PT, Nemeth N, Klenk K, Gould D, McLean RG, Clark L, Hall JS. Experimental infection of fox squirrels (Sciurus niger) with West Nile virus. Am J Trop Med Hyg. 2006;75:697–701. [PubMed] [Google Scholar]
- 13.Platt KB, Tucker BJ, Halbur PG, Blitvich BJ, Fabiosa FG, Mullin K, Parikh GR, Kitikoon P, Barthalomay LC, Rowley WA. Fox squirrels (Sciurus niger) develop West Nile virus viremias sufficient for infecting select mosquito species. Vector Borne Zoonotic Dis. 2008;8:225–233. doi: 10.1089/vbz.2007.0182. [DOI] [PubMed] [Google Scholar]
- 14.Tiawsirisup S, Blitvich BJ, Tucker BJ, Halbur PJ, Bartholomay LC, Rowley WA, Platt KB. Susceptibility of fox squirrels (Sciurus niger) to West Nile virus by oral exposure. Vector Borne Zoonotic Dis. 2010;10:207–209. doi: 10.1089/vbz.2008.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gómez A, Kramer LD, Dupuis AP, Kilpatrick AM, Davis LJ, Jones MJ, Daszak P, Aguirre AA. Experimental infection of eastern gray squirrels (Sciurus carolinensis) with West Nile virus. Am J Trop Med Hyg. 2008;79:447–451. [PMC free article] [PubMed] [Google Scholar]
- 16.Platt KB, Tucker BJ, Halbur PG, Tiawsirisup S, Blitvich BJ, Fabiosa FG, Bartholomay LC, Rowley WA. West Nile virus viremia in eastern chipmunks (Tamias striatus) sufficient for infecting different mosquitoes. Emerg Infect Dis. 2007;13:831–837. doi: 10.3201/eid1306.061008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiawsirisup S, Platt KB, Tucker BJ, Rowley WA. Eastern cottontail rabbits (Sylvilagus floridanus) develop West Nile virus viremias sufficient for infecting select mosquito species. Vector Borne Zoonotic Dis. 2005;5:342–350. doi: 10.1089/vbz.2005.5.342. [DOI] [PubMed] [Google Scholar]
- 18.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald JP, Meaney CA, Armstrong DM. Mammals of Colorado. Niwot, CO: University Press of Colorado; 1994. [Google Scholar]
- 20.Schmidly DJ. The Mammals of Texas. Revised Edition. Austin, TX: University of Texas Press; 2004. [Google Scholar]
- 21.Rupprecht CE, Smith JS. Raccoon rabies: the re-emergence of an epizootic in a densely populated area. Semin Virol. 1994;5:155–164. [Google Scholar]
- 22.Sorvillo F, Ash LR, Berlin OG, Yatabe J, Degiorgio C, Morse SA. Baylisascaris procyonis: an emerging helminthes zoonoses. Emerg Infect Dis. 2002;8:355–359. doi: 10.3201/eid0804.010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall JS, Bentler KT, Landolt G, Elmore SA, Minnis RB, Campbell TA, Barras SC, Root JJ, Pilon J, Pabilonia K, Driscoll C, Slate D, Sullivan H, McLean RG. Influenza infection in wild raccoons. Emerg Infect Dis. 2008;14:1842–1848. doi: 10.3201/eid1412.071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLean RG, Ubico SR, Bourne D, Komar N. West Nile virus in livestock and wildlife. Curr Top Microbiol Immunol. 2002;67:271–308. doi: 10.1007/978-3-642-59403-8_14. [DOI] [PubMed] [Google Scholar]
- 25.Bunning ML, Bowen RA, Cropp CB, Sullivan KG, Davis BS, Komar N, Godsey MS, Baker D, Hettler DL, Holmes DA, Biggerstaff BJ, Mitchell CJ. Experimental infection of horses with West Nile virus. Emerg Infect Dis. 2002;8:380–386. doi: 10.3201/eid0804.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaty BJ, Calisher CH, Shope RE. In: Arboviruses. Lennette EH, Lennette DA, Lennette ET, editors. Washington, DC: American Public Health Association; 1995. pp. 189–212. (Diagnostic procedures for viral, rickettsial, and chlamydial infections). [Google Scholar]
- 27.Nemeth NM, Beckett S, Edwards E, Klenk K, Komar N. Avian mortality surveillance for West Nile virus in Colorado. Am J Trop Med Hyg. 2007;76:431–437. [PubMed] [Google Scholar]
- 28.McLean RG, Francy DB, Campos EG. Experimental studies of St. Louis encephalitis virus in vertebrates. J Wildl Dis. 1985;21:85–93. doi: 10.7589/0090-3558-21.2.85. [DOI] [PubMed] [Google Scholar]
- 29.Styer LM, Bernard KA, Kramer LD. Enhanced early West Nile virus infection in young chickens infected by mosquito bite: effect of viral dose. Am J Trop Med Hyg. 2006;75:337–345. [PubMed] [Google Scholar]
- 30.Austgen LE, Bowen RA, Bunning ML, Davis BS, Mitchell CJ, Chang GJ. Experimental infection of cats and dogs with West Nile virus. Emerg Infect Dis. 2004;10:82–86. doi: 10.3201/eid1001.020616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page LK, Swihart LK, Kazacos KR. Implications of raccoon latrines in the epizootiology of baylisascariasis. J Wildl Dis. 1999;35:474–480. doi: 10.7589/0090-3558-35.3.474. [DOI] [PubMed] [Google Scholar]
- 32.Tonry JH, Brown CB, Cropp CB, Co JK, Bennett SN, Nerurkar VR, Kuberski T, Gubler DJ. West Nile virus detection in urine. Emerg Infect Dis. 2005;11:1294–1296. doi: 10.3201/eid1108.050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray K, Walker C, Herrington E, Lewis JA, McCormick J, Beasley DW, Tesh RB, Fischer-Hoch S. Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201:2–4. doi: 10.1086/648731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiawsirisup S, Platt KB, Evan RB, Rowley WA. Susceptibility of Ochlerotatus trivittatus (Coq.), Aedes albopictus (Skuse), and Culex pipiens (L.) to West Nile virus infection. Vector Borne Zoonotic Dis. 2004;4:190–197. doi: 10.1089/vbz.2004.4.190. [DOI] [PubMed] [Google Scholar]
- 35.McGee CE, Schneider BS, Girard YA, Vanlandingham DL, Higgs S. Nonviremic transmission of West Nile virus: evaluation of the effects of space, time, and mosquito species. Am J Trop Med Hyg. 2007;76:424–430. [PubMed] [Google Scholar]


