Abstract
Leptospira borgpetersenii serovar Arborea is an emerging cause of leptospirosis in Australia. It was not previously recognized as an endemic serovar before the 1990s, but at that point, human infections with the serovar increased significantly. Using fluorescent-amplified fragment-length polymorphism (FAFLP) molecular typing, human and rodent isolates were compared genetically. Typing revealed 11 unique profiles among the 23 isolates examined; however, there was no clonality revealed between the human and rodent isolates. There was clonality among rodent isolates from geographically related areas. This study highlights the utility of Leptospira culture combined with FAFLP for the examination of the epidemiology of this disease.
Leptospirosis is the disease caused by the gram-negative spirocheate Leptospira. Leptospira are divided into 20 species that are determined by DNA–DNA hybridization and 16S rRNA analysis; serologically, there are approximately 200 serovars.1,2 The majority of leptospirosis infections in Australia occurs in the state of Queensland with an incidence rate of approximately 3.2 cases per 100,000 people, and the majority of infections occur in the far northeastern coastal areas.3 Of particular interest is the emergence of novel serovars of Leptospira, particularly those that affect areas where leptospirosis is not endemic; one such serovar is L. borgpetersenii serovar Arborea. L. borgpetersenii sv. Arborea was first isolated in 1955 from a wood mouse (Apodermus sylvaticus) in Arborea-Sardegna, Italy.4 Subsequently, it has been reported throughout the world in Barbados, the Terceira Islands (Azores), Argentina, and most recently, Australia.3,5–7
The aim of this study was to define the molecular epidemiology of this serovar in Australia utilizing strains isolated from humans and rodents. This was performed using the fluorescent-amplified fragment-length polymorphism (FAFLP) methodology that has been previously used successfully to type Leptospira spp.8–10
Leptospira were isolated by culture; briefly, 1 mL of whole blood (human) or kidney tissue (rodent) was inoculated into 3 mL of Ellinghausen–McCullough–Johnson–Harris (EMJH) medium, incubated at 30°C for 6 weeks, and examined weekly by dark-field microscopy. Positive cultures were identified to species level through amplification and sequencing of a 504-bp fragment of the DNA gyrase subunit B (gyrB) gene; this was performed as previously described by Slack and others.11 Isolates were then serologically identified using a cross-agglutinin absorption test (CAAT) against the reference strain L. borgpetersenii sv. Arborea strain Arborea. FAFLP was performed as previously described using the Microbial FAFLP kit (Applied Biosystems).8,9 The fluorescently labeled DNA fragments were separated using the ABI-310 genetic analyzer (Applied Biosystems), and the fragment-sizing data were converted into binaries and analyzed using the Bionumerics software package (Applied Maths). Clustering analysis was performed using the dice coefficient and the unweighted pair-group method with an arithmetic mean (UPGMA) algorithm (Figure 1).
Figure 1.
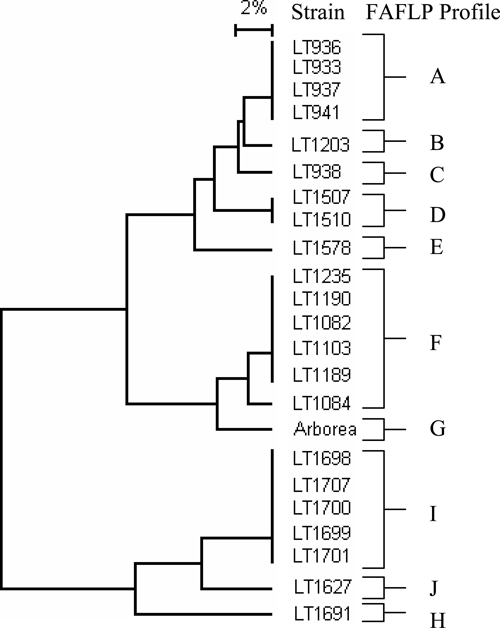
Dendrogram of the L. borgpetersenii serovar Arborea strains constructed from the FAFLP results using the dice coefficient and the UPGMA algorithm. Bar = percent difference.
Molecular typing using FAFLP was performed on 22 L. borgpetersenii isolates of serovar Arborea strains and the reference strain for this serovar (strain Arborea). The 22 isolates were composed of 15 rodent isolates and 7 human isolates. These isolates were found over an 8-year period from 1998 to 2005 and were geographically distributed across the state of Queensland (Table 1).
Table 1.
Details of L. borgpetersenii serovar Arborea isolates examined using FAFLP
| Strain | Location | Source* | Year | FAFLP profile |
|---|---|---|---|---|
| LT 936 | Brisbane | M | 1998 | A |
| LT 933 | Brisbane | M | 1998 | A |
| LT 937 | Brisbane | M | 1998 | A |
| LT 941 | Brisbane | M | 1998 | A |
| LT 1203 | Sunshine Coast | H | 2001 | B |
| LT 938 | Brisbane | R | 1998 | C |
| LT 1507 | Tully | M | 2003 | D |
| LT 1510 | Tully | M | 2003 | D |
| LT 1578 | Innisfail | H | 2004 | E |
| LT 1235 | Innisfail | H | 2002 | F |
| LT 1190 | Sunshine Coast | H | 2001 | F |
| LT 1082 | Brisbane | R | 2000 | F |
| LT 1103 | Brisbane | R | 2000 | F |
| LT 1189 | Sunshine Coast | H | 2001 | F |
| LT 1084 | Brisbane | M | 2000 | G |
| Arborea | Arborea-Sardegna | A | 1955 | H |
| LT 1698 | Sunshine Coast | M | 2005 | I |
| LT 1707 | Sunshine Coast | M | 2005 | I |
| LT 1700 | Sunshine Coast | M | 2005 | I |
| LT 1699 | Sunshine Coast | M | 2005 | I |
| LT 1701 | Sunshine Coast | M | 2005 | I |
| LT 1627 | Innisfail | H | 2004 | J |
| LT 1697 | Innisfail | H | 2004 | K |
M = Mus sp.; H = human; R = Rattus sp.; A = Apodermus sylvaticus.
When clustering analysis was performed, there were 11 unique FAFLP profiles among the 23 isolates (Figure 1). Within the 11 unique profiles, there were seven individual profiles and four profiles that contained two or more strains. Two profiles were of particular interest. Profile A contained isolates obtained from trapped rodents at an urban area in Brisbane, and Profile I contained isolates taken from trapped rodents at a semirural area on the sunshine coast. Both of these areas were not previously known for endemic leptospirosis and had sustained an increase in the number of Leptospira infections since 1999.3 FAFLP Profiles D and F were the only other profiles to contain more than one isolate; Profile D contained two rodent isolates, and Profile F was the only profile containing a mixture of human and rodent isolates from three geographically separate areas ranging from Innisfail in the north of the state to Brisbane in the south of the state, a distance of approximately 1,600 km. The type strain for this serovar, Arborea, was isolated in 1955; it showed a high level of similarity with FAFLP profiles F and G, suggesting some form of relatedness between European and Australian isolates. Given that L. borgpetersenii serovar Arborea was not isolated in Australia before 1998,3 the FAFLP studies could suggest that Arborea was possibly imported through rodents aboard cargo ships entering Australian ports.
Using a powerful molecular epidemiological technique such as FAFLP, we have been able to characterize the L. borgpetersenii serovar Arborea strains isolated from both humans and rodents. Although there was little evidence to suggest that there was direct infection transmission between rodents and humans, this could be influenced by the fact that the majority of human infections were diagnosed serologically by microscopic agglutination test (MAT) testing rather than by culture.3 This study shows that there has been clonal transmission of the serovar among rodents at a local and state level. Additionally, it shows the potential of using culture and FAFLP for the epidemiological typing of Leptospira isolates from both human and rodent populations.
Acknowledgments
The authors would like to thank the staff of the Queensland Health Population Health Units and other submitting laboratories for their assistance with isolates. The authors also wish to thank Queensland Health for their past and present support of Leptospirosis research in Australia.
Footnotes
Authors' addresses: Andrew T. Slack, Meegan L. Symonds, Michael F. Dohnt, Scott B. Craig, and Lee D. Smythe, WHO/FAO/OIE Collaborating Centre for Reference and Research on Leptospirosis, Western Pacific Region, Communicable Disease Unit, Queensland Health Forensic and Scientific Services, Coopers Plains, Queensland, Australia, E-mail: Lee_smythe@health.qld.gov.au.
References
- 1.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slack AT, Khairani-Bejo S, Symonds ML, Dohnt MF, Galloway RL, Steigerwalt AG, Bahaman AR, Craig S, Harrower BJ, Smythe LD. Leptospira kmetyi sp. nov., isolated from an environmental source in Malaysia. Int J Syst Evol Microbiol. 2009;59:705–708. doi: 10.1099/ijs.0.002766-0. [DOI] [PubMed] [Google Scholar]
- 3.Slack AT, Symonds ML, Dohnt MF, Smythe LD. The epidemiology of leptospirosis and the emergence of Leptospira borgpetersenii serovar Arborea in Queensland, Australia, 1998–2004. Epidemiol Infect. 2006;134:1217–1225. doi: 10.1017/S0950268806006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kmety E, Dikken H. Classification of the Species Leptospira interrogans and History of Its Serovars. Groningen, The Netherlands: University Press Groningen; 1993. [Google Scholar]
- 5.Collares-Pereira M, Mathias ML, Santos-Reis M, Ramalhinho MG, Duarte-Rodrigues P. Rodents and Leptospira transmission risk in Terceira island (Azores) Eur J Epidemiol. 2000;16:1151–1157. doi: 10.1023/a:1010916132497. [DOI] [PubMed] [Google Scholar]
- 6.Matthias MA, Levett PN. Leptospiral carriage by mice and mongooses on the island of Barbados. West Indian Med J. 2002;51:10–13. [PubMed] [Google Scholar]
- 7.Vanasco NB, Rossetti C, Sequeira G, Sequeira MD, Calderon G, Tarabla HD. First isolations of leptospires serogroup Ballum serovar arborea in Argentina. Vet Rec. 2000;147:246–247. doi: 10.1136/vr.147.9.246. [DOI] [PubMed] [Google Scholar]
- 8.Slack A, Symonds M, Dohnt M, Smythe L. An improved multiple-locus variable number of tandem repeats analysis for Leptospira interrogans serovar Australis: a comparison with fluorescent amplified fragment length polymorphism analysis and its use to redefine the molecular epidemiology of this serovar in Queensland, Australia. J Med Microbiol. 2006;55:1549–1557. doi: 10.1099/jmm.0.46779-0. [DOI] [PubMed] [Google Scholar]
- 9.Slack AT, Symonds ML, Dohnt MF, Corney BG, Smythe LD. Epidemiology of Leptospira weilii serovar Topaz infections in Australia. Commun Dis Intell. 2007;31:216–222. [PubMed] [Google Scholar]
- 10.Vijayachari P, Ahmed N, Sugunan AP, Ghousunnissa S, Rao KR, Hasnain SE, Sehgal SC. Use of fluorescent amplified fragment length polymorphism for molecular epidemiology of leptospirosis in India. J Clin Microbiol. 2004;42:3575–3580. doi: 10.1128/JCM.42.8.3575-3580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slack AT, Symonds ML, Dohnt MF, Smythe LD. Identification of pathogenic Leptospira species by conventional or real-time PCR and sequencing of the DNA gyrase subunit B encoding gene. BMC Microbiol. 2006;6:95. doi: 10.1186/1471-2180-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]


