Abstract
Recent reports from animal models and from cross-sectional studies have suggested that host responses to anti-Lutzomyia longipalpis saliva antibodies may be related to delayed-type hypersensitivity to Leishmania antigen. In a prospective cohort study, we evaluated 1,080 children from two endemic areas for visceral leishmaniasis (VL) by means of Kaplan-Meier analysis. The incidence rate of delayed-type hypersensitivity to Leishmania antigen, measured at the 24th follow-up month, was higher among those reactive to Lu. longipalpis saliva antibodies at the beginning of the study (0.0217 cases per person-month) than among those previously negative (0.0131 cases per person-month) (P value for the log-rank test = 0.0006). It seems that mounting an anti-saliva immune response helps the development of a cell-mediated anti-Leishmania response.
Visceral leishmaniasis (VL) is a serious public health problem in many regions of the world. Apart from its high incidence, VL is spreading across new places and showing worrying signs of urbanization. Furthermore, severe cases of VL resulting in death have been described.1,2
The main strategies for controlling VL include the early diagnosis and treatment of human cases and measures directed at the vector and the canine reservoir and health education activities. However, these measures are insufficient for proper control of the epidemic. In Brazil, the epidemic is still spreading: there were a total of 24,977 new cases from 1999 to 2005.2 Therefore, the search for new measures to curb the epidemic is extremely important.2,3
Mounting evidence suggests that the sand fly saliva may influence the course of VL infection. Studies performed in murine models have shown that salivary components of sand flies may exacerbate infection when injected together with Leishmania.4–9 Conversely, pre-exposure to sand fly saliva may even protect against Leishmania infection, leading to the development and testing of new vaccines on the basis of inoculation with sand flies' salivary proteins.8,10–12
On exposure to uninfected Lutzomyia longipalpis bites, normal human volunteers develop anti-sand fly saliva antibodies and cell-mediated immune response.13 Host responses to anti-Lu. longipalpis saliva antibodies may be related to the development of delayed-type hypersensitivity to Leishmania antigen. In a cross-sectional study, development of anti-Lu. longipalpis saliva antibodies was associated with increased delayed-type hypersensitivity to Leishmania antigen among children from a VL endemic area.14 This observation is in accordance with experimental reports that have observed that immunity to specific vector salivary protein helps or accelerates the development of cell mediated immunity to a Leishmania antigen.15 Herein, we report on a prospective cohort study aimed at evaluating the presence of anti-Lu. longipalpis saliva antibodies and their relationship with the development of an anti-leishmanial response measured by delayed type hypersensitivity (DTH) positivity. Children residing in two VL endemic areas (Vila Nova and Bom Viver) in Raposa County, São Luis Island (2°32′S and 44″ and 43′W) State of Maranhão, Brazil were enrolled. The villages of Vila Nova and Bom Viver have an approximate population of 2,600 and 4,307, respectively. The study was approved by the Research Ethics Committee of Maranhão Federal University Hospital. Parents or guardians of all participants signed an informed consent.
Children were enrolled according to the following inclusion criteria: age < 10 years; resident of Vila Nova or Bom Viver (in Raposa County, MA); no history of or current infection with VL. Initial population census identified 1,297 children < 10 years of age who were invited for an interview by health care attendants. Of the 1,297 who were invited in the first phase of the study, 49 were excluded because of a previous or current VL infection and 168 did not present for an interview, resulting in 1,080 children (83.3% of total eligible population).
A complete physical examination and determination of Lu. longipalpis immunoglobulin G (IgG) antibodies was performed at 12-month intervals from January 2003 to July 2005. The DTH test was done with 0.1 mL of 25 μg/mL of Leishmania antigen applied intradermally to the anterior side of the right forearm as previously described.16 A response was considered positive when the largest diameter of indurated area was > 5 mm. Enzyme-linked immunosorbent assay for anti-Phlebotomine saliva was performed as previously described.17 A reading above 0.05 (cutoff determined by the mean plus two standard deviations of 20 healthy individuals) was considered positive. Salivary glands from female Lu. longipalpis and the determination of anti-Phlebotomine saliva antibodies were obtained, prepared, and evaluated as previously described.17 On follow-up, children with positive DTH were excluded.
Of the 1,080 children examined, 343 were DTH positive. Thus, for the first follow-up, performed 12 months after the baseline examination, only 737 DTH-negative children were eligible for reexamination. At the 12th month of follow-up there were 103 losses (4 refusals and 99 moved out of the area). A total of 156 out of 634 evaluated children were DTH positive. At the 24th follow-up month 478 DTH-negative children were eligible for inclusion; 61 losses were registered (6 refusals and 55 moved out of the area). Therefore, 417 children were evaluated. Of these, 30 were DTH positive (Figure 1).
Figure 1.
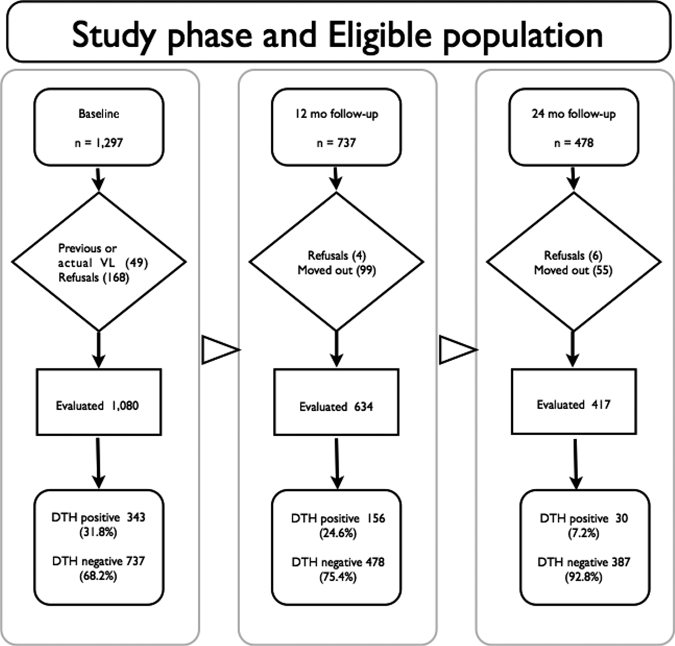
Study flow chart. The study was divided into three phases, baseline, 12-month follow-up, and 24-month follow-up with one cohort from the endemic area of visceral leishmaniasis (VL) in Maranhão State, Brazil to address anti-Leishmania delayed-type hypersensitivity (DTH) and antibody responsiveness to Lutzomyia longipalpis salivary proteins. At the end of the first phase there were 737 DTH-negative children who constituted the eligible population for the phase 2 (12-month follow-up). The 478 children who were DTH at phase 2 included the eligible population of phase 3 (24-month follow-up). The study design details are described in the text.
Data were entered and analyzed using Epi Info version 3.3 (CDC, Atlanta-EUA) and Stata statistic software version 8.0 (2003, Stata Corporation, College Station, TX). To evaluate if the presence of anti-Lu. longipalpis saliva antibodies was associated with subsequent mounting of delayed-type hypersensitivity to Leishmania antigen, Kaplan-Meier and the log-rank test were used. Significance level was set at 0.05.
Prevalence proportion of DTH positivity at time zero was 31.8%. At 12 months, the DTH incidence proportion was 24.6% and at 24 months, it was 7.2% (Figure 1). The prevalence proportion of anti-saliva IgG antibodies was 16.1% at time zero of the study. Incidence proportions of anti-saliva IgG antibodies at the 12th and 24th months of observation were 3.8% and 2.7%, respectively (data not shown).
The total observation time of children with negative anti-saliva antibodies summed to 10,176 months, whereas for those with positive antibodies it totaled 2,448 months. Incidence rate of delayed-type hypersensitivity to Leishmania antigen, measured at to the 24th follow-up month, was 0.0131 cases per person-month for those negative for sandflies' anti-saliva antibodies at the beginning of the study (time zero) and 0.0217 cases per person-month for those with positive anti-saliva antibodies (Table 1).
Table 1.
Association between presence of anti-Lutzomyia longipalpis saliva antibodies at the beginning of the study and the development of delayed-type hypersensitivity to Leishmania antigen up to the 24th follow-up month among children residing in two visceral leishmaniasis (VL) endemic areas, in Raposa County, São Luis, State of Maranhão, Brazil*
| Anti-Lu. longipalpis saliva antibodies at the beginning of the study | Events (DTH-positive to Leishmania) | Sum of time under observation (months) | Incidence rate (cases per person-month) | ||
|---|---|---|---|---|---|
| 12 months | 24 months | Total | |||
| Negative | 116 | 17 | 133 | 10.176 | 0.0131 |
| Positive | 40 | 13 | 53 | 2.448 | 0.0217 |
| Total | 156 | 30 | 186 | 12.624 | 0.0147 |
P value of the log-rank test < 0.0006.
We have previously shown that positive antibody response to sand fly saliva correlates to delayed-type hypersensitivity against Leishmania antigen17 and that the development of both types of immunity is time-coincident.14 Furthermore, DTH responses to sand fly bites in humans, increases blood flow at the site of the bite.18 It is possible that such changes in previously saliva-sensitized individuals help them to mount a cell-mediated immune response against Leishmania after exposure to infected sand flies. In this report, the positive association between anti-sand fly saliva antibodies and anti-Leishmania DTH was confirmed in a longitudinal prospective design. These findings add strong support to this association as they come from a large cohort study.
Considering that measurable anti-sand fly antibodies develop in only a fraction of exposed children and that a positive anti-Leishmania DTH is associated with resistance, it is tempting to speculate that mounting an anti-saliva immune response helps in the development of a protective anti-Leishmania response. However, increased anti-Leishmania DTH response in persons reactive to Lu. longipalpis saliva antibodies at the beginning of the study could quite well reflect increased parasite challenge in individuals who have been bitten more intensively by the sand flay vector. Thus, caution is necessary in extending laboratory results to field situations. Further testing of this hypothesis will probably rely on the use of recombinant salivary proteins to overcome the limitations of using crude sand fly salivary gland sonicates. We have recently showed the feasibility of using recombinant proteins from Lu. longipalpis saliva for large epidemiological studies,19 which opens up the possibility of performing larger studies in endemic areas with a high incidence of VL to address the question of the relationship between anti-saliva response and protection against VL.
Footnotes
Financial support: This work was supported by grants from CNPq – Portuguese acronym for the Brazilian National Research Council (CYTED and Renorbio). MBN, AAMS, and AB are senior investigators from CNPq.
Authors' addresses: Dorlene M. C. Aquino and Arlene J. M. Caldas, Departamento de Enfermagem, Universidade Federal do Maranhão, São Luís, Maranhão, Brazil. José Carlos Miranda, Laboratório de Imunoparasitologia (LIP), Centro de Pesquisas Gonçalo Moniz (CPqGM), Fundação Oswaldo Cruz – FIOCRUZ - Bahia, Salvador, Bahia, Brazil. Antonio A. M. Silva, Departamento de Saúde Pública, Universidade Federal do Maranhão, São Luís, Maranhão, Brazil. Manoel Barral-Netto, Laboratório de Imuno-regulação (LIMI), Centro de Pesquisas Gonçalo Moniz (CPqGM), Fundação Oswaldo Cruz – FIOCRUZ - Bahia, Salvador, Bahia, Brazil; Faculdade de Medicina, Universidade Federal da Bahia, Salvador, Bahia, Brazil; and Instituto Nacional de Ciência e Tecnologia de Investigação em Imunologia - iii - INCT, Salvador, Bahia, Brazil. Aldina Barral, Laboratório de Imunoparasitologia (LIP), Centro de Pesquisas Gonçalo Moniz (CPqGM), Fundação Oswaldo Cruz – FIOCRUZ - Bahia, Salvador, Bahia, Brazil; Faculdade de Medicina, Universidade Federal da Bahia, Salvador, Bahia, Brazil; and Instituto Nacional de Ciência e Tecnologia de Investigação em Imunologia - iii - INCT, Salvador, Bahia, Brazil.
References
- 1.World Health Organization Urbanization: an increasing risk factor for leishmaniasis. Wkly Epidemiol Rec. 2002;77:6. [PubMed] [Google Scholar]
- 2.Brazil Ministério da Saúde. Leishmaniose visceral (calazar). Distribuição de casos confirmados, por Unidade Federada. Brasil: 2006. pp. 1980–2005.http://portal.saúde.gov.br/portal/arquivos/pdf/leish_visceral.pdf Available at. [Google Scholar]
- 3.World Health Organization Research to support the elimination of visceral leishmaniasis. 2010. http://apps.who.int/tdr/svc/research/visceral-leishmaniasis-elimination Available at.
- 4.Theodos CM, Ribeiro JM, Titus RG. Analysis of enhancing effect of sand fly saliva on Leishmania infection in mice. Infect Immun. 1991;59:1592–1598. doi: 10.1128/iai.59.5.1592-1598.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuelson J, Lerner E, Tesh R, Titus R. A mouse model of Leishmania braziliensis braziliensis infection produced by coinjection with sand fly saliva. J Exp Med. 1991;173:49–54. doi: 10.1084/jem.173.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warburg A, Saraiva E, Lanzaro GC, Titus RG, Neva F. Saliva of Lutzomyia longipalpis sibling species differs in its composition and capacity to enhance leishmaniasis. Philos Trans R Soc Lond B Biol Sci. 1994;345:223–230. doi: 10.1098/rstb.1994.0097. [DOI] [PubMed] [Google Scholar]
- 7.Lima HC, Titus RG. Effects of sand fly vector saliva on development of cutaneous lesions and the immune response to Leishmania braziliensis in BALB/c mice. Infect Immun. 1996;64:5442–5445. doi: 10.1128/iai.64.12.5442-5445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, Ribeiro J, Sacks DL. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norsworthy NB, Sun J, Elnaiem D, Lanzaro G, Soong L. Sand fly saliva enhances Leishmania amazonensis infection by modulating interleukin-10 production. Infect Immun. 2004;72:1240–1247. doi: 10.1128/IAI.72.3.1240-1247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valenzuela JG, Belkaid Y, Garfield MK, Mendez S, Kamhawi S, Rowton ED, Sacks DL, Ribeiro JM. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol. 2001;167:5226–5230. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- 12.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290:1351–1354. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 13.Vinhas V, Andrade BB, Paes F, Bomura A, Clarencio J, Miranda JC, Báfica A, Barral A, Barral-Netto M. Human anti-saliva immune response following experimental exposure to the visceral leishmaniasis vector, Lutzomyia longipalpis. Eur J Immunol. 2007;37:3111–3121. doi: 10.1002/eji.200737431. [DOI] [PubMed] [Google Scholar]
- 14.Gomes RB, Brodskyn C, de Oliveira CI, Costa J, Miranda JC, Caldas A, Valenzuela JG, Barral-Netto M, Barral A. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. J Infect Dis. 2002;186:1530–1534. doi: 10.1086/344733. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira F, Lawyer PG, Kamhawi S, Valenzuela JG. Immunity to distinct sand fly salivary proteins primes the anti-Leishmania immune response towards protection or exacerbation of disease. PLoS Negl Trop Dis. 2002;2:e226. doi: 10.1371/journal.pntd.0000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed SG, Badaro R, Masur H, Carvalho EM, Lorenco R, Lisboa A, Teixeira R, Johnson WD, Jr, Jones TC. Selection of a skin test antigen for American visceral leishmaniasis. Am J Trop Med Hyg. 1986;35:79–85. doi: 10.4269/ajtmh.1986.35.79. [DOI] [PubMed] [Google Scholar]
- 17.Barral A, Honda E, Caldas A, Costa J, Vinhas V, Rowton ED, Valenzuela JG, Charlab R, Barral-Netto M, Ribeiro M. Human immune response to sand fly salivary gland antigens: a useful epidemiological marker? Am J Trop Med Hyg. 2000;62:740–745. doi: 10.4269/ajtmh.2000.62.740. [DOI] [PubMed] [Google Scholar]
- 18.Belkaid Y, Valenzuela JG, Kamhawi S, Rowton E, Sacks DL, Ribeiro JM. Delayed-type hypersensitivity to Phlebotomus papatasi sand fly bite: an adaptive response induced by the fly? Proc Natl Acad Sci USA. 2000;97:6704–6709. doi: 10.1073/pnas.97.12.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souza AP, Andrade BB, Aquino D, Entringer P, Miranda JC, Alcantara R, Ruiz D, Soto M, Teixeira CR, Valenzuela JG, de Oliveira CI, Brodskyn CI, Barral-Netto M, Barral A. Using recombinant proteins from Lutzomyia longipalpis saliva to estimate human vector exposure in visceral leishmaniasis endemic areas. PLoS Negl Trop Dis. 2010;4:e649. doi: 10.1371/journal.pntd.0000649. [DOI] [PMC free article] [PubMed] [Google Scholar]


