Abstract
Most rapid diagnostic tests (RDTs) available use histidine-rich protein 2 (HRP2) as a target. However, it has been reported that sequence variations of this protein affects its sensitivity. Currently, there is insufficient evidence for HRP2 variability in Plasmodium falciparum isolates from Colombia and its relationship with RDT performance. To determine possible geographic differences and their effects on the performance of RDTs, 22 blood samples from patients with P. falciparum malaria from Tumaco and Buenaventura, Colombia were assessed by measurement of HRP2 concentration by an HRP2 enzyme-linked immunosorbent assay, RDTs, and thick blood smear. Statistical analysis showed an association between RDT performance and HRP2 concentrations. No significant difference was found between locations. A large variation of antigen concentration in samples was found at same parasitemia. In contrast to previously reports, there was no correlation between initial parasitemia and HRP2 concentration. Our results indicate that antigen quantity should be studied more carefully because the sensitivity of the RDT is affected more by antigen concentration than by parasitemia.
In Colombia, more than half of the population is at risk for malaria. In 2007, 110,389 malaria cases were reported, 19 of them fatal.1 Most of the fatal cases occurred because of complications caused by delays in diagnosis and treatment. Therefore, one of the principal objectives of malaria control strategies recommended by the World Health Organization (WHO) is to improve access to diagnosis and effective treatment.2,3
Currently, the gold standard diagnosis method for malaria is based on identification of the parasite in Giemsa-stained blood smears by using microscopy. Its use in remote malaria-endemic areas is restricted by a lack of qualified personal and basic infrastructure. Such drawbacks have led to development of simpler diagnostic strategies, including rapid diagnostic tests (RDTs).3 Currently, malaria RDTs are widely used in countries in Africa, and their use is spreading in countries in South America, such as Peru, where much progress has been made in malaria control.2,4
The RDTs are immunocromatographic tests that detect specific parasite antigens through antibody capture.3 The main antigens detected by RDTs are lactate dehydrogenase, aldolase (panspecific, which is present in all Plasmodium species), and histidine-rich protein 2 (HRP2), which is specific for Plasmodium falciparum.3 Most RDTs available use HRP2 as a target. It has been reported that sequence variations of this antigen, particularly the types and numbers of specific amino acid repeats, can affect the sensitivity of HRP2-based RDTs at low parasitaemias.5,6 Currently, there is insufficient evidence about HRP2 variability in isolates from Colombia and its relationship with RDT performance.
The purpose of this study was to evaluate the amount of HRP2 in P. falciparum isolates from two malaria-endemic cities on the Pacific coast of Colombia to determine changes between locations and their effects on RDT performance.
Symptomatic patients with a P. falciparum-positive thick smear were enrolled in the study if they had a parasitemia ≥ 3,000 parasites/µL, had not taken antimalarial drugs in the last two months (negative by the Saker-Solomon test), and provided a signed informed consent that was approved by the Centro Internacional de Entrenamiento e Investigaciones Médicas (Cali, Colombia) institutional review board.
We evaluated P. falciparum blood samples from Buenaventura (Valle Department) and Tumaco (Nariño Department), which are cities located on the Pacific coast of Colombia. The 12 samples collected in Buenaventura were independently analyzed and reported as part of a larger multi-center collaboration between the Foundation for Innovative New Diagnostics and WHO.
Differences in protein expression levels were determined by measurement of HRP2 concentration using an enzyme-linked immunosorbent assay (ELISA) for HRP2.7 Statistical analyses were conducted to determine the association between geographic origin and parasitemia with HRP2 concentrations. Additionally, malaria RDTs were performed with a dilution of the patient blood to determine sensitivity.
Five milliliters of venous blood was collected into tubes containing EDTA, and two thick blood smears were prepared. Parasitemia was determined by two expert microscopists at Centro Internacional de Entrenamiento e Investigaciones Médicas, and the mean value was calculated. A third microscopist was consulted if the variation coefficient between counting was > 20%. Blood was mixed for thirty minutes, and each sample was diluted to a parasitemia of 200 parasites/µL using blood from healthy donors, according to the WHO–West Pacific Regional Office protocol for preparing quality-control samples of malaria RDTs.8 The HRP2 ELISA and RDT were used for testing the dilutions containing 200 parasites/μL. This parasite density enables interpretable and comparable antigen quantification from all samples, and is close to the RDT detection limit of 100 parasites/µL recommended by past WHO consultations in 1999 and 2003.8 Parasitemias of the dilutions were corroborated by using the Earle-Pérez thick blood film method and thin blood films.9
The RDT (Parahit® Span Diagnostics Ltd., Surat, India) was performed immediately after dilutions were made according to the manufacturer's specifications.10 The RDT results were classified into three groups based on intensities of the test lines and using the control line intensity as reference: one cross, two crosses, and three crosses if the test line intensity was weaker, the same, or stronger than the control line, respectively. The RDT results were considered negative if only the control line was seen, and they were invalidated and repeated when no line was shown. After RDT reading, the dilutions were stored at –20°C until ELISA was conducted.
The HRP2 concentrations were measured by using the Malaria Ag CELISA® Kit (Cellabs, Sydney, New South Wales, Australia.7 A standard curve was established with a recombinant protein (P. falciparum HRP2-HB3) kindly provided by Dr. David Sullivan (Johns Hopkins School of Public Health, Baltimore, MD) after confirming its stock concentration by using the technique of Bradford.11
Ten serial dilutions were prepared from the protein stock by using phosphate-buffered saline–0.05% Tween 20 as diluent. Each dilution was tested in triplicate, and the experiment repeated 11 times. The negative control value was established by measuring the optical density (OD) of five non-infected venous blood samples, testing in duplicate, and calculating the mean ± OD (0.093 ± 0.004, maximum = 0.098, minimum = 0.090). For each sample, the dilution of 200 parasites/µL was tested in triplicate, and the concentrations were determined by comparison with a recombinant HRP2 standard curve run in parallel. Data were analyzed by using Stata Statistical Sofware version 9.2 (StataCorp, College Station, TX). A P value > 0.05 was considered statistically significant.
During January–November 2008, we obtained 22 samples, 10 from Tumaco and 12 from Buenaventura. There was no statistically significant difference between parasitemias observed in patients from these two cities (P = 0.990; geometric means = 8,915 parasites/μL and 8,569 parasites/µL, respectively).
The HRP2 standard curve had a large linearity range between 0.49 ng/mL and 31.5 ng/mL of HRP2 protein, with a positive correlation of R = 0.948 (P < 0.001), which showed a strong linear relationship between HRP2 concentration and OD. Large HRP2 concentration variations were found in the 22 samples assessed at a concentration of 200 parasites/µL; values ranged from 1.6 ng/mL to 52.7 ng/mL of HRP2 (Figure 1). Two samples were additionally diluted to 1:2 with phosphate-buffered saline to estimate the HRP2 concentration because their ODs at a concentration of 200 parasites/μL was above the detection limit.
Figure 1.
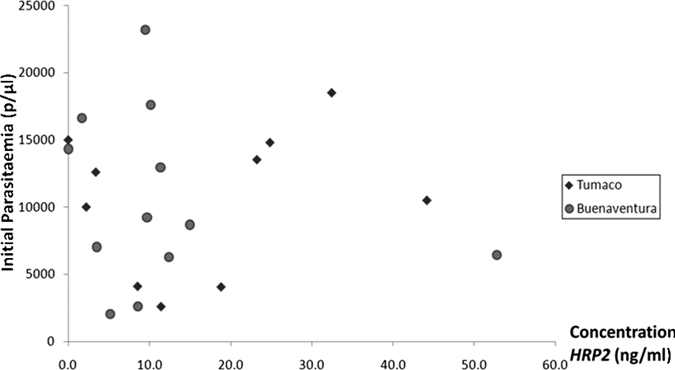
Histidine-rich protein 2 of Plasmodium falciparum isolates from Tumaco and Buenaventura, Colombia, at a concentration of 200 parasites/μL with respect to the initial parasitemia of the patient infections. A poor association was found between the two variables (correlation coefficient = 0.503, 95% confidence interval = 0.09–0.77, P = 0.703). Two samples could not be analyzed by an enzyme-linked immunosorbent assay.
Three samples showed negative results or could not be detected by one or both detection methods when diluted to a concentration of 200 parasites/µL (Figure 2). One of these three samples (RDT negative and ELISA result below the detection limit of 0.49 ng/mL) had a relatively high initial parasitemia of 14,327 parasites/µL (Figure 2B).
Figure 2.

A, Association between Plasmodium falciparum histidine-rich protein 2 (HRP2) concentration at a parasite concentration of 200 parasites/µL and rapid diagnostic test (RDT) results for samples from Tumaco and Buenaventura, Colombia. B, Comparison between initial parasitemia and RDT results at a concentration of 200 parasites/µL in samples from both cities. The RDT intensities of samples from both cities showed a correlation with initial HRP2 concentration (P = 0.005), but not with parasite density of the samples. 0 = negative RDT result; 1 = test band intensity weaker than that of the control band; 2 = test band intensity equal to that of the control band.
The HRP2 concentrations observed in dilutions of 200 parasites/µL in samples from Tumaco were higher than in samples from Buenaventura (geometric mean = 13.12 ng/mL and 8.86 ng/mL, respectively), but this difference was not statistically significant (P = 0.331). All 10 samples from Tumaco had positive RDT results at a dilution of 200 parasites/µL; 50% of these samples had an RDT intensity of two crosses. In contrast, 2 of the 12 samples from Buenaventura at the same dilution showed negative results (Figure 2).
The RDT results of dilutions of 200 parasites/µL showed a correlation with the concentration of HRP2 at the same dilution (Figure 2A). The highest intensities in the RDT were obtained for samples with higher HRP2 concentrations. Nevertheless, the initial parasitemia was not correlated with RDT results of the dilutions of 200 parasites/µL (Figure 2B). This finding indicates that the intensity of RDT results reflects the amount of HRP2, but not necessarily the initial parasitemia.
The most striking result of this study is that there is a large variation of antigen concentration in the samples, even though all dilutions were to the same parasite density of 200 parasites/μL of blood. There are various potential explanations for this finding. One possibility is that polymorphism in the HRP2 sequences lead to differences in the HRP2 detection signal by the ELISA, and consequently in antigen concentration. Lee and others showed that genetic variability of HRP2 affects sensitivity of techniques based on the detection of this antigen by monoclonal antibodies (malaria RDTs, ELISA kits).6 Baker and others reported differences between geographically widespread parasites originating from various countries.12 However, the possible HRP2 sequence variations between wild-type parasites from the same country remain unknown. It would be interesting to compare P. falciparum hrp2 gene sequences and copy number variation of collected parasite samples and include samples from other areas of the same country.
Furthermore, antigen concentration can depend on several characteristics of the infection because antigen accumulates during infection and varies in expression levels during the parasite life cycle.13,14 We also observed no clear correlation between initial parasite density of infection and P. falciparum HRP2 content in the dilutions containing 200 parasites/µL. Similarly, we found no correlation between the initial parasite density of infection and pfHRP2 content in the whole sample. These results differ from previous findings in parasites isolated from Thailand, where a high correlation was observed between parasitemias and protein concentrations.13 Our study also indicated a slight variation in HRP2 levels between study sites, although without statistical significance. A larger study would be necessary to assess this hypothesis.
Our results indicate that pfHRP2 sequences and their expression levels should be studied more carefully in various malaria-endemic areas of Colombia. Variations in both findings could have significant consequences on the performance of malaria RDTs.
Acknowledgments
We thank Madeline Montenegro and the patients for help and cooperation during this study, Dr. Sandra Incardona for comments on this report, Dr. David Sullivan (Johns Hopkins School of Public Health) for kindly providing recombinant protein P. falciparum HRP2-HB3, and the Foundation for Innovative New Diagnosis and the World Health Organization–Tropical Disease Research Program for supporting collection of samples in Buenaventura.
Footnotes
Financial support: This study was supported by the Young Researcher Program (ID 098-2007) of Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnología.
Authors' address: Zuleima Pava, Diego F. Echeverry, Gustavo Díaz, and Claribel Murillo, Centro Internacional de Entrenamiento e Investigaciones Médicas, Carrera 125 No. 19-225, Cali, Colombia.
References
- 1.SIVIGILA . Informe Final 2007 Enfermedades Transmitidas por Vectores. Cali, Colombia: SIVIGILA; 2007. [Google Scholar]
- 2.World Health Organization World Malaria Report 2008. 2009. www.who.com Available at. Accessed July 16, 2010.
- 3.WHO/USAID/DFID/AusAID . Malaria Rapid Diagnosis—Making It Work. Geneva: World Health Organization; 2003. [Google Scholar]
- 4.Organización Panamericana de la Salud, Red Amazónica de Vigilancia de la Resistencia a las Antimaláricos . Investigación Operacional sobre la Implementación del Uso de Pruebas Rápidas de Diagnóstico de Malaria. Guayaquil, Ecuador: Organización Panamericana de La Salud; 2005. [Google Scholar]
- 5.World Health Organization . List of Known Commercially-Available Antigen-Detecting Malaria RDTs. Geneva: World Health Organization; 2005. [Google Scholar]
- 6.Lee N, Baker J, Andrews K, Gatton M, Bell D, Cheng Q, McCarthy J. Effect of sequence variation in Plasmodium falciparum histidine-rich protein 2 on binding of specific monoclonal antibodies: implications for rapid diagnostic tests for malaria. J Clin Microbiol. 2006;44:2773–2778. doi: 10.1128/JCM.02557-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cellabs Malaria Ag ELISA. 2004. http://www.tcsbiosciences.co.uk/downloads/CellabsMalariaELISA.pdf Available at. Accessed July 16, 2010.
- 8.World Health Organization . Training in Tropical Diseases Methods Manual for Laboratory Quality Control Testing of Malaria RDTs. SOP. 3.08. Geneva: World Health Organization; 2006. [Google Scholar]
- 9.Earle WC, Pérez M. Enumeration of parasites in the blood of malaria patients. J Lab Clin Med. 1932;11:1124–1130. [Google Scholar]
- 10.Span Diagnostics Ltd. Parahit Rapid Diagnostic Test. Surat, India: Span Diagnostics Ltd; 2007. [Google Scholar]
- 11.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Baker J, McCarthy J, Gatton M, Kyle D, Belizario V, Luchavez J, Bell D, Cheng Q. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J Infect Dis. 2005;192:870–877. doi: 10.1086/432010. [DOI] [PubMed] [Google Scholar]
- 13.Desakorn V, Dondorp A, Silamut K, Pongtavornpinyo W, Sahassananda D, Chotivanich K, Pitisuttithum P, Smithyman A, Day N, White N. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Trans R Soc Trop Med Hyg. 2005;99:517–524. doi: 10.1016/j.trstmh.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Kifude C, Rajasekariah H, Sullivan DJ, Stewart V, Angov E, Martin S, Diggs C, Waitumbi J. Enzyme-linked immunosorbent assay for detection of Plasmodium falciparum histidine-rich protein 2 in blood, plasma, and serum. Clin Vaccine Immunol. 2008;15:1012–1018. doi: 10.1128/CVI.00385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


