Abstract
Human landing catches (HLCs) are currently the preferred method to determine vector human biting rates (HBRs), which are key determinants of entomologic inoculation rates and important measures for assessing the impact of vector control efforts. Although HLCs are the most direct means of establishing HBRs, they are labor-intensive, and their use is facing increasing ethical concerns. The relationship between Centers for Disease Control (CDC) light traps and HLC collections was evaluated in Macha, Zambia during the 2007–2008 and 2008–2009 rainy seasons. A CDC light trap captured on average 1.91 (95% confidence interval = 1.16–2.28) times as many An. arabiensis per night as an indoor HLC. Additionally, nets treated with deltamethrin did not affect the numbers of An. arabiensis collected. Our results suggest that in regions where use of vector control interventions is high and vector densities are low, CDC light traps can be used to monitor An. arabiensis HBRs.
Introduction
In the fight against malaria and the push toward eradication, interventions must be effectively used and accurately evaluated. Current strategies to reduce malaria transmission rely heavily on vector control, specifically the use of insecticide-treated bed nets (ITNs), indoor residual spraying, and source reduction. The most direct method for assessing these vector management measures is the entomologic inoculation rate (EIR) because it quantifies the tendency of a mosquito population to transmit infectious sporozoites to humans.1 The EIR, defined as the number of infectious bites received by an individual per unit time, is calculated by multiplying the proportion of mosquitoes in a vector population harboring sporozoite-stage parasites in their salivary glands by the nightly biting pressure of the vector on a human population (the human biting rate [HBR]).2 Human biting rates for particular vectors can be highly variable, even at a fine geographic scale.3
The gold standard method for determining the HBR is the human landing catch (HLC) because mosquitoes are captured by aspiration as they land and attempt to feed on collectors.2 However, in many regions where vector control efforts are underway, extensive use of HLCs may not be practical. In addition to being non-standardized because of variability in the attractiveness and skill of collectors,4 HLCs are extremely labor-intensive, which limits the number of data points that may be simultaneously collected. Additionally, they require vigilance throughout the night by collectors and intense supervision to ensure that the information gathered is reliable. Furthermore, as has been noted by others, there are increasing ethical and worker safety concerns that this collection method increases the risk of exposure of catchers to infectious mosquitoes.4–8 The ethical dilemma is compounded in areas of drug-resistant malaria and when collectors would otherwise have the opportunity to be protected from infectious bites by sleeping under an ITN. Institutional Review Boards have begun to voice concerns about use of HLCs, going so far as to deem this method an occupational hazard.9
Consequently, work has been conducted to evaluate alternative methods of determining HBRs that would be as sensitive as the HLC, and be cost-effective, exposure-free, and widely deployable. Indoor resting collections are largely unsuitable for this purpose because insecticides on walls or bed nets inherently reduce estimates of indoor biting by pyrethrum spray catches. Likewise, the recently developed Mbita traps have proven not to be sensitive enough for collections in areas of low mosquito densities.7,10,11 One promising alternative to the HLC is the Ifakara tent trap being developed in Tanzania.7 This collection method has been shown to correlate well with HLC collections independent of vector density, but it is still being modified and is not yet available as a commercial product.
Consequently, Centers for Disease Control (CDC) light traps hung beside occupied beds protected by bed nets remain a preferred alternative to an indoor HLC for collecting host-seeking vectors over a wide range of mosquito densities. The CDC light traps are affordable, easy to use, and have relatively high sampling efficiency,12 although the relationship between CDC trap and indoor HLC collections needs to be verified locally for each study area2 (Table 1) because vector species composition and intraspecific variation in feeding and resting behavior can have a significant impact on the quantitative association between the two methods.
Table 1.
Published relationships between CDC light trap and HLC collections of Anopheles gambiae s.l. in Africa*
| Study | Relative sensitivity of CDC trap versus that of HLC (95% CI) | Density dependent | Species composition | Location | Reference |
|---|---|---|---|---|---|
| 1 | 0.33 (0.24–0.46) | Yes | Not reported | Ulanga District, Tanzania | Okumu and others, 200812 |
| 2 | 0.56 (0.49–0.66) | No | An. arabiensis | Ahero, Kenya | Mathenge and others, 200511 |
| 3 | 1.06 (0.88–1.26) | No | An. gambaie s.s. | Bo District, Sierra Leone | Magbity and others, 20028 |
| 4 | 1.07 (0.89–1.29)† | No | > 90% An. gambaie s.s. | Muheza District, Tanzania | Lines and others, 19915 |
| 5 | 1.08 | No | ~70% An. gambaie s.s. | Noungou, Burkino Faso | Costantini and others, 19986 |
| 6 | 1.18‡ | No | < 40–100% An. gambaie s.s. | Bagamoyo District, Tanzania | Davis and others, 1995,21 Shiff and others, 199532 |
| 7 | 1.3 | Yes | 12% An. gambaie s.s. | Ulanga District, Tanzania | Govella and others, 20097 |
| 8 | 1.86 (1.73–2.00) | No | 55% An. arabiensis | Suba District, Kenya | Mathenge and others, 200410 |
CDC = Centers for Disease Control; HLC = human landing catch; CI = confidence interval.
Comparison between 3 CDC trap collections and 2 HLC collectors.
Comparison between 1 CDC trap collection and 2 HLC collectors.
As part of the National Malaria Strategic Plan of Zambia, the Macha area of Southern Province received 4,800 ITNs in 2007 (Thuma PE, unpublished data), which largely eliminated use of insecticide spray catches to monitor Anopheles arabiensis, the principal vector of Plasmodium falciparum in the region.13,14 With both practical and ethical restrictions on the widespread use of HLCs to assess differences in EIRs across the Macha region, studies to evaluate the relationship between CDC light trap and indoor HLC collections were performed in Macha, an area with overall low vector densities,13 during the 2007–2008 and 2008–2009 rainy seasons. Investigations of the impact of insecticide treatment of bed nets on the numbers of An. arabiensis collected by CDC light trap were conducted in conjunction with these studies because of the high rate of ITN use in the research area.
Materials and Methods
Mosquito collecting and handling.
The Johns Hopkins Malaria Research Institute field station is located in Macha, Zambia at 16.39292S, 26.79061E. Mosquitoes were collected for this study in two village areas, Chidakwa and Namwalinda, both located < 10 km from the field station. Collections were performed during the peak of the rainy season, January through April in 2008 and 2009.
For the comparison between indoor HLC and CDC light trap collections, three houses were chosen in Namwalinda and three houses were chosen in Chidakwa. The HLC and CDC light trap collections were performed in the same houses on alternating nights, with CDC light traps hung next to occupants sleeping under untreated bed nets. If the trapping room had more than one bed, the other occupants were instructed to use their existing bed nets, or if they had none, additional nets were provided. Six teams of two HLC collectors were randomly rotated among the houses on successive collection nights so that each HLC collection team collected at each house before the rotation began again. The HLC and CDC trap collections were performed from 7:00 pm to 7:00 am up to 3 times per week for a given house for a total of 176 trap nights for each collection method. Because HLC collections were only performed for the first half hour of every hour, the CDC light trap catch was compared with the total An. arabiensis captured by both HLC collectors.
For the comparison of catch results by CDC trap suspended near treated and untreated bed nets, six households in Namwalinda with more than one sleeping house were randomly chosen for the study. Pairs of deltamethrin-treated and untreated bed nets were allocated to neighboring sleeping houses. The CDC traps were hung nightly next to an occupied bed in each house. Every other night the nets were exchanged between the houses to remove any sampling bias.
DNA isolation and polymerase chain reaction.
Collected mosquitoes were identified morphologically15 at the field station and individually placed in tubes containing silica gel desiccant (Fisher Scientific, Fair Lawn, NJ) and cotton for stable storage until they were processed for molecular analysis. Heads/thoraces were separated from abdomens before homogenization and rehydrated at room temperature in 20 μL of double-distilled water for 10 minutes. The DNA was extracted from mosquito heads/thoraces and abdomens by a modified salt procedure as previously described.16 DNA pellets were resuspended in 50 μL of double-distilled water. Host source of blood fed mosquitoes was determined by polymerase chain reaction (PCR) on abdominal DNA.14,16 The DNA from head/thorax extractions was used to confirm species by the PCR assay of Scott and others.17 Additionally, head/thorax DNA was screened for P. falciparum by nested PCR.18
Data analysis.
As an exploratory step, the An. arabiensis totals for all trap nights for both the CDC light trap and HLC methods were graphed and Pearson's correlation coefficient for linear association was calculated. Initially, we used the Bland-Altman analysis19 used in previous studies5,8,20,21 to assess whether the differences observed between the nightly means calculated for each collection method were density dependent and to estimate the bias associated with the collection methods. However, handling non-normally distributed data by using a logarithmic transformation with the addition of one to count totals of zero may bias the results.22 Therefore, to avoid artificially converting zeros to non-zero values when many sampling occasions in this study yielded no mosquitoes, a negative binomial regression analysis was performed using STATA version 10 (StataCorp., College Station, TX). The negative binomial distribution has been used previously to model overdispersed mosquito count collections.10,11,23
The difference in the sampling efficiency of CDC light traps relative to the HLC reference method was evaluated after controlling for month and year of collection and clustering on household. Using the negative binomial model, we assumed that total mosquito counts followed a Poisson distribution in which there is an overdispersion parameter to account for variance that is greater than that expected under a true Poisson distribution. The log of the expected counts is modeled as the function log(E(Yijk)) = α + βX+ ɛijk, where Yijk is the monthly mosquito count for household i in month j and year k and X are the covariates of sampling method, collection year, and collection month. This method enabled standard errors to be adjusted for correlated observations within collection households. Density dependence was evaluated by generating a new variable to present the tertiles of the total combined numbers of An. arabiensis collected by CDC trap and HLC. Each tertile represented one of three parts of the ordered dataset distribution, and each contained one-third of the population. The above analysis was then repeated with the inclusion of interaction terms for sampling method and tertile density.
The hypothesis that CDC light traps collect the same number of mosquitoes whether suspended next to people sleeping under deltamethrin-treated or untreated bed nets was examined by using paired t-tests. For the 2009 data, the significance of the difference in the proportion of unfed mosquitoes collected in light traps hung next to treated or untreated nets was tested by using a two-proportion z-test.
Results
For the CDC light trap/HLC comparison collections, 497 An. gambiae complex mosquitoes were captured: 19 An. quadriannulatus and 478 An. arabiensis. However, 113 of the An. arabiensis were caught during a single trap night (108 from the CDC trap and 5 from the corresponding HLC). This trap night was an extreme outlier because the next largest number of An. arabiensis caught in a trap night was 47. Because inclusion of this data point significantly skewed the results obtained it was dropped from the analysis, leaving a total of 365 An. arabiensis (225 from CDC traps and 140 from HLCs) collected during 175 trap nights. All mosquitoes caught by light trap or landing catch were negative for P. falciparum sporozoites.
Preliminary analysis showed that CDC light traps tended to catch more mosquitoes overall (Figure 1). As expected, there was a statistically significant correlation between the numbers of An. arabiensis caught by CDC trap and indoor HLC collection (r = 0.51, P < 0.001). The negative binomial regression analysis performed on the nightly collection data revealed that a CDC light trap captured on average 1.91 (95% confidence interval = 1.61–2.28, P < 0.001) times as many An. arabiensis per night as an indoor HLC pair, after controlling for month and year. All coefficients for month and year of collection were statistically significant in the model, which suggested that there were more captures in February than in January, but decreased captures in March and April as compared to January. Similarly, there was a statistically significant decrease in collection counts in 2009 as compared to 2008. None of the interaction terms for sampling method and tertile were statistically significant, which suggested that CDC light trap efficiency was not density dependent.
Figure 1.
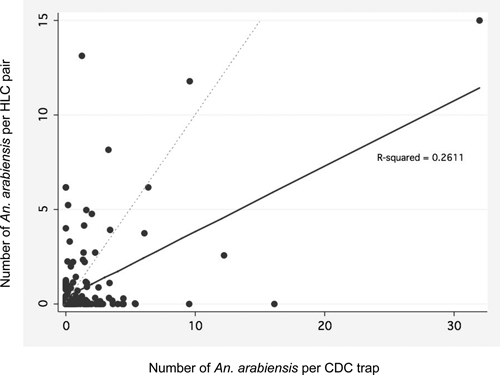
Nightly scatter plot of paired Anopheles arabiensis collections for 175 trap nights. The solid line indicates the regression line of the dataset and is shown in comparison with the dashed identity line. Each point represents the collections from one household per night.
For the comparison of CDC light traps hung next to treated or untreated bed nets, a total of 518 An. arabiensis and 19 An. quadriannulatus were captured. Paired t-tests for each year showed no statistically significant mean differences between catches from paired traps. One hundred twenty pairs were analyzed in 2008 (P = 0.36), and 96 pairs were examined in 2009 (P = 0.26). No statistically significant difference was observed in the proportion of unfed mosquitoes caught by traps suspended next to treated or untreated nets during the 2009 season (P = 0.355) (Table 2). Of those mosquitoes that were blood fed, 98% of blood meals were taken from human hosts.
Table 2.
Number of Anopheles gambiae s.l. complex mosquitoes collected by CDC light traps near ITNs*
| Year (no. mosquitoes) | Trap or net | No. An. quadriannulatus | No. An. arabiensis | (% unfed) |
|---|---|---|---|---|
| 2008 (120) | Bayer K-O Tab (deltamethrin) | 11 | 60 | – |
| Untreated bed net | 6 | 78 | – | |
| 2009 (96) | Permanet (deltamethrin) | 2 | 210 | 80.8 |
| Untreated bed net | 0 | 171 | 76.8 |
CDC = Centers for Disease Control; ITNs = insecticide-treated bed nets.
Discussion
In this study, CDC light traps in Macha, Zambia caught on average 1.91 times as many mosquitoes as a pair of indoor HLC collectors. Although this relative sampling efficiency is higher than those previously published for the An. gambiae complex (Table 1), the difference might be largely explained by the sibling species composition at each locality and the considerable variation in An. arabiensis foraging behavior throughout Africa. There has been only one other CDC/HLC comparison study conducted in an area where An. arabiensis was the sole sibling species present. In Ahero, Kenya, a CDC light trap caught approximately 60% of the number of An arabiensis as an HLC.11 However, unlike in Macha where An. arabiensis is highly anthropophilic,13,14 in the rice irrigation region of Kenya where the previous work was undertaken, An. arabiensis is known to be predominately zoophilic.24 The CDC traps in this study might also have caught more mosquitoes because the population of An. arabiensis in Macha displays higher post-prandial endophily. The light traps may be attracting not only host-seeking An. arabiensis, but also some proportion of indoor resting mosquitoes.20 In our comparison of CDC light trap efficiency by bed net type, on average only 78.8% (Table 2) of An. arabiensis captured by light trap were unfed. It has been shown that the added stimulus of light from incandescent bulbs increases the numbers of An. gambiae s.l. caught by CDC light trap by approximately 2.5 times.6 Perhaps this added stimulus was enough to attract a portion of non–host-seeking An. arabiensis. Furthermore, the positioning of the CDC light trap in relation to the host acting as bait has been shown to have a significant impact on catch sizes.25 It is therefore possible that some of the observed difference in the sampling efficiency of CDC traps between this study and others might be caused by trap placement.
Although it has been reported that the efficiency of CDC light traps as a substitute for HLCs may vary as a function of vector abundance,7,20 most studies in Africa have shown the correlation between the two methods to be independent of density.5,6,8,10,11,21 Importantly, the relative sampling efficiency of CDC light traps in this study was not density dependent. Therefore, even with the relatively low numbers of An. arabiensis collected in Macha, we should be able to predict what the results of an HLC collection would have been from the catch total of a CDC light trap. Because neither trapping method yielded sporozoite-positive mosquitoes, we were unable to assess the influence of trapping method on sporozoite prevalence. Similar studies have found conflicting results regarding the influence of trapping method on infection rates. Some studies have shown higher sporozoite rates in An. gambiae s.l.20,21 collected by light trap than in those collected by HLCs, whereas other investigations have found the methods to yield similar rates.5,10 Further investigation will need to be undertaken in areas with higher P. falciparum transmission rates to determine if trapping method has an effect on sporozoite prevalence in southern Zambia.
It has been suggested that the excito-repellent properties of ITNs can reduce the numbers of mosquitoes that enter sleeping huts or cause those that do to exit more quickly.26,27 However, in our study, the presence of ITNs did not affect the utility of CDC traps. This is a critical finding because surveys completed during the 2008 and 2009 rainy seasons in Macha found that 72–86% of the population, depending on village area, reported sleeping under a bed net treated with deltamethrin the previous night (unpublished data). If CDC light traps are to be used as a replacement for indoor HLCs, it would be impractical and unethical in an area with high ITN use to replace all existing nets with untreated bed nets for monitoring purposes. Previous studies have shown a slight decrease in8 or no effect on28 the sampling efficiency of CDC traps in the presence of ITNs. However, although surrounding occupied beds were covered with ITNs, the CDC traps in both of these investigations were hung next to untreated bed nets. Our data are the first to directly compare the catch totals of An. arabiensis by CDC trap hung beside each bed net type. These results will have to be confirmed elsewhere, but the finding is encouraging in that control programs throughout southern Africa might benefit from the ability to use existing ITNs in conjunction with CDC traps as part of their surveillance efforts.
A major drawback to using CDC light traps as a substitute for HLCs is that although HLCs can be performed inside and outside, CDC light traps are known to be ineffective for collecting An. gambiae complex mosquitoes when hung outdoors.6,29 Consequently, light traps can only be used to sample the indoor-biting fraction of the vector population. Although An. arabiensis in Macha has been shown to be predominately exophagic,30 CDC light traps were efficient at collecting the indoor host-seeking mosquitoes that are responsible for most bites after people have gone to bed. We acknowledge that HLCs remain the best sampling technique to glean information on the degree of exophagy in a population and vector biting times. However, when HLCs become prohibitive because of logistical issues and ethical concerns, the results presented here indicate that CDC light traps may be used as a stopgap measure to sample indoor, host-seeking An. arabiensis until better tools are developed, proven effective and cost-efficient, and become widely available.
This is the first study to report that the relationship between a CDC light trap and indoor HLC collection can be established for An. arabiensis in an area of low vector density where ongoing vector control interventions are being used. The situation in Macha is one that will be seen more and more as the malaria map begins to shrink. Although the Southern Province of Zambia has historically had hyperendemic transmission of P. falciparum, there has been a significant decrease in the number of malaria cases in the Macha region since 2003 when Zambia adopted artemisinin combination therapy as the national standard for the treatment of uncomplicated malaria.31 As malaria rates in Macha and similar sites throughout Africa are reduced further through vector control efforts, it will become even more important to monitor EIRs over a wider region to identify remaining foci of malaria transmission. To calculate HBRs for this purpose, we show that as long as known caveats are kept in mind and the relationship between catch methods is established locally, CDC light traps can be used next to treated bed nets as a substitute for indoor HLCs.
Acknowledgments
We thank Dr. Marie Diener-West for her statistical insights; Shadrack Habbanti for his time and effort spent coordinating field team operations in Zambia; Musapa Mulenga for managing our collections in Macha; and our field mosquito collectors (Corrence Munsanje, Nathan Phiri, Clement Mwaanga, Fines Mwaanga, Twaambo Moono, Gift Shamapani, Miyanda Moono, Guide Hansumo, Mathias Muleka, Chaltone Munsanje, Pathias Chibambo, Malony Mulota, Haggard Musyutila, Cliff Singanga, Paul Haakaloba, and Ojukwu Himumwe) for their assistance.
Footnotes
Financial support: This study was supported by the Johns Hopkins Malaria Research Institute (Douglas E. Norris), National Institutes of Health training grant T32AI007417 (Laura C. Norris), and a Simpson Student Award from the Tropical Medicine Dinner Club of Baltimore and a Johns Hopkins Bloomberg School of Public Health Sommer Scholarship (Christen M. Fornadel).
Authors' addresses: Christen M. Fornadel, Laura C. Norris, and Douglas E. Norris, The W. Harry Feinstone Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, 615 North Wolfe Street, Baltimore, MD 21205, E-mails: cfornade@jhsph.edu, lnorris@jhsph.edu, and dnorris@jhsph.edu.
References
- 1.Shaukat A, Breman J, McKenzie FE. Using the entomological inoculation rate to assess the impact of vector control on malaria parasite transmission and elimination. Malar J. 2010;9:122. doi: 10.1186/1475-2875-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beier JC. Vector incrimination and entomological inoculation rates. Methods Mol Med. 2002;72:3–10. doi: 10.1385/1-59259-271-6:01. [DOI] [PubMed] [Google Scholar]
- 3.Cano J, Descalzo MA, Moreno M, Chen Z, Nzambo S, Bobuakasi L, Buatiche JN, Ondo M, Micha F, Benito A. Spatial variability in the density, distribution and vectorial capacity of anopheline species in a high transmission village (Equatorial Guinea) Malar J. 2006;5:21. doi: 10.1186/1475-2875-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mboera LE. Sampling techniques for adult Afrotropical malaria vectors and their reliability in the estimation of entomological inoculation rate. Tanzania Journal of Health Research. 2005;7:117. doi: 10.4314/thrb.v7i3.14248. [DOI] [PubMed] [Google Scholar]
- 5.Lines JD, Curtis CF, Wilkes TJ, Njunwak KJ. Monitoring human-biting mosquitoes (Diptera, Culicidae) in Tanzania with light-traps hung beside mosquito nets. Bull Entomol Res. 1991;81:77–84. [Google Scholar]
- 6.Costantini C, Sagnon N, Sanogo E, Merzagora L, Coluzzi M. Relationship to human biting collections and influence of light and bednet in CDC light-trap catches of West African malaria vectors. Bull Entomol Res. 1998;88:511. [Google Scholar]
- 7.Govella NJ, Chaki PP, Geissbuhler Y, Kannady K, Okumu F, Charlwood JD, Anderson RA, Killeen GF. A new tent trap for sampling exophagic and endophagic members of the Anopheles gambiae complex. Malar J. 2009;8:157. doi: 10.1186/1475-2875-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magbity EB, Lines JD, Marbiah MT, David K, Peterson E. How reliable are light traps in estimating biting rates of adult Anopheles gambiae s.l. (Diptera: Culicidae) in the presence of treated bed nets? Bull Entomol Res. 2002;92:71–76. doi: 10.1079/BER2001131. [DOI] [PubMed] [Google Scholar]
- 9.Mirabello L, Vineis JH, Yanoviak SP, Scarpassa VM, Povoa MM, Padilla N, Achee NL, Conn JE. Microsatellite data suggest significant population structure and differentiation within the malaria vector Anopheles darlingi in Central and South America. BMC Ecol. 2008;8:3. doi: 10.1186/1472-6785-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathenge EM, Omweri GO, Irungu LW, Ndegwa PN, Walczak E, Smith TA, Killeen GF, Knols BG. Comparative field evaluation of the Mbita trap, the Centers for Disease Control light trap, and the human landing catch for sampling of malaria vectors in western Kenya. Am J Trop Med Hyg. 2004;70:33–37. [PubMed] [Google Scholar]
- 11.Mathenge EM, Misiani GO, Oulo DO, Irungu LW, Ndegwa PN, Smith TA, Killeen GF, Knols BG. Comparative performance of the Mbita trap, CDC light trap and the human landing catch in the sampling of Anopheles arabiensis, An. funestus and culicine species in a rice irrigation in western Kenya. Malar J. 2005;4:7. doi: 10.1186/1475-2875-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okumu FO, Kotas ME, Kihonda J, Mathenge E, Killeen GF, Moore SJ. Comparative evaluation of methods used for sampling malaria vectors in the Kilombero Valley, South Eastern Tanzania. The Open Tropical Medicine Journal. 2008;1:51–55. [Google Scholar]
- 13.Kent RJ, Thuma PE, Mharakurwa S, Norris DE. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am J Trop Med Hyg. 2007;76:267–274. [PMC free article] [PubMed] [Google Scholar]
- 14.Fornadel CM, Norris DE. Increased endophily by the malaria vector Anopheles arabiensis in southern Aambia and identification of digested blood meals. Am J Trop Med Hyg. 2008;79:876–880. [PMC free article] [PubMed] [Google Scholar]
- 15.Gillies MT, Coetzee M. A Supplement to the Anophelinae of Africa South of the Sahara. Johannesburg: South African Institute for Medical Research; 1987. [Google Scholar]
- 16.Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005;73:336–342. [PMC free article] [PubMed] [Google Scholar]
- 17.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 18.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 19.Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Statistician. 1983;32:307–317. [Google Scholar]
- 20.Mbogo CN, Glass GE, Forster D, Kabiru EW, Githure JI, Ouma JH, Beier JC. Evaluation of light traps for sampling anopheline mosquitoes in Kilifi, Kenya. J Am Mosq Control Assoc. 1993;9:260–263. [PubMed] [Google Scholar]
- 21.Davis JR, Hall T, Chee EM, Majala A, Minjas J, Shiff CJ. Comparison of sampling anopheline mosquitoes by light-trap and human-bait collections indoors at Bagamoyo, Tanzania. Med Vet Entomol. 1995;9:249–255. doi: 10.1111/j.1365-2915.1995.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith T. Proportionality between light trap catches and biting densities of malaria vectors. J Am Mosq Control Assoc. 1995;11:377–378. [PubMed] [Google Scholar]
- 23.Hii JL, Smith T, Mai A, Ibam E, Alpers MP. Comparison between anopheline mosquitoes (Diptera: Culicidae) caught using different methods in a malaria endemic area of Papua New Guinea. Bull Entomol Res. 2000;90:211–219. doi: 10.1017/s000748530000033x. [DOI] [PubMed] [Google Scholar]
- 24.Githeko AK, Adungo NI, Karanja DM, Hawley WA, Vulule JM, Seroney IK, Ofulla AV, Atieli FK, Ondijo SO, Genga IO, Odada PK, Situbi PA, Oloo JA. Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol. 1996;82:306–315. doi: 10.1006/expr.1996.0038. [DOI] [PubMed] [Google Scholar]
- 25.Mboera LE, Kihonda J, Braks MA, Knols BG. Short report: influence of Centers for Disease Control light trap position, relative to a human-baited bed net, on catches of Anopheles gambiae and Culex quinquefasciatus in Tanzania. Am J Trop Med Hyg. 1998;59:595–596. doi: 10.4269/ajtmh.1998.59.595. [DOI] [PubMed] [Google Scholar]
- 26.Lines JD, Myamba J, Curtis CF. Experimental hut trials of permethrin-impregnated mosquito nets and eave curtains against malaria vectors in Tanzania. Med Vet Entomol. 1987;1:37–51. doi: 10.1111/j.1365-2915.1987.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller JE, Lindsay SW, Armstrong JR. Experimental hut trials of bednets impregnated with synthetic pyrethroid or organophosphate insecticide for mosquito control in The Gambia. Med Vet Entomol. 1991;5:465–476. doi: 10.1111/j.1365-2915.1991.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 28.Kirby MJ, Green C, Milligan PM, Sismanidis C, Jasseh M, Conway DJ, Lindsay SW. Risk factors for house-entry by malaria vectors in a rural town and satellite villages in The Gambia. Malar J. 2008;7:2. doi: 10.1186/1475-2875-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Githeko AK, Service MW, Mbogo CM, Atieli FA, Juma FO. Sampling Anopheles arabiensis, A. gambiae sensu lato and A. funestus (Diptera: Culicidae) with CDC light-traps near a rice irrigation area and a sugarcane belt in western Kenya. Bull Entomol Res. 1994;84:319–324. [Google Scholar]
- 30.Fornadel CM, Norris LC, Glass GE, Norris DE. Analysis of Anopheles arabiensis blood feeding behavior in southern Zambia during the two years after introduction of insecticide-treated bed nets. Am J Trop Med Hyg. 2010;83:848–853. doi: 10.4269/ajtmh.2010.10-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thuma P. Changes in inpatient pediatric malaria case load at Macha hospital after the introduction of artemether/lumefantrine in a rural Zambian community. Symposium 54. 56th Annual Meeting of the American Society for Tropical Medicine and Hygiene; Philadelphia, PA: 2007. November 2007. [Google Scholar]
- 32.Shiff CJ, Minjas JN, Hall T, Hunt RH, Lyimo S, Davis JR. Malaria infection potential of anopheline mosquitoes sampled by light trapping indoors in coastal Tanzanian villages. Med Vet Entomol. 1995;9:256–262. doi: 10.1111/j.1365-2915.1995.tb00131.x. [DOI] [PubMed] [Google Scholar]


