Abstract
Anopheles arabiensis mosquitoes are the primary vector responsible for Plasmodium falciparum transmission in Macha, Zambia. Because insecticide-treated bed nets (ITNs) have the potential to alter host feeding behavior, the extent of the zoophilic and exophagic tendencies of the vector was evaluated during the two rainy seasons after ITN introduction. Centers for Disease Control light traps, paired indoor/outdoor human landing catches, and outdoor cattle-baited collections were used to assess potential changes in host preference. Results support the hypothesis that An. arabiensis mosquitoes in Macha remain highly anthropophilic despite high ITN use. Anopheles arabiensis mosquitoes in Macha appear to be relatively exophagic and have been caught biting outdoors immediately after sunset and before sunrise, potentially circumventing some of the protective effects of ITNs.
Introduction
Since 2003, the National Malaria Control Program in Zambia has redoubled its efforts to reduce the country's malaria burden. In 2003, the Ministry of Health introduced intermittent presumptive treatment of pregnant women and adopted artemesinin combination therapy as the standard treatment of uncomplicated malaria.1 In 2004, the National Malaria Control Program added the implementation of large-scale integrated vector control efforts, including indoor residual house spraying, larviciding, environmental management, and the distribution of insecticide-treated bed nets (ITNs).2 By 2007, more than 5.3 million ITNs were distributed throughout the country free of charge.2 The Macha area of Southern Province received 4,800 long-lasting ITNs impregnated with deltamethrin (Thuma PE, unpublished data).
The major vector of Plasmodium falciparum parasites in the Macha area are Anopheles arabiensis Patton mosquites.3 In contrast to the other major malaria vectors in sub-Saharan Africa, An. gambiae s.s Giles and An. funestus s.s Giles, which are primarily anthropophilic, endophagic, and endophilic,4,5 An. arabiensis is more variable its foraging behavior. Although the vector can be found feeding and resting indoors in some localities,6–11 in other areas it is mainly exophagic12–15 and exophilic.12,16,17 Additionally, An. arabiensis has been reported to be highly anthropophilic in some regions,9,18–21 including southern Zambia,3,22 whereas elsewhere it predominantly displays zoophagic foraging tendencies.16,17,23,24 Furthermore, human blood indices (HBIs) for the vector have been shown to vary even between households in the same village based on cattle ownership.17,25
The pyrethroid insecticides that are used to impregnate ITNs affect anopheline mosquitoes in a number of ways. Implementation of ITNs may reduce vector survival and suppress vector populations.26–28 These nets can also alter foraging behavior. It has been reported that ITNs can shift anopheline biting outdoors,26,29 earlier in the evening,26,29,30 or to alternate hosts.28,30,31 Additionally, ITNs may have contact irritancy and/or non-contact excito-repellency effects, decreasing the numbers of An. gambiae s.l. that enter sleeping houses and causing those that do enter to exit more quickly.32,33 The goal of this study was to assess the foraging behavior of An. arabiensis in Macha during the two years after large-scale ITN introduction.
Materials and Methods
Mosquito collecting and handling.
Anopheles arabiensis were captured from December through May during the 2007–2008 and 2008–2009 rainy seasons after ITN introduction near the Johns Hopkins Malaria Research Institute field station. This field site is located at 16.39292°S, 26.79061°E in the Southern Province of Zambia. Collections were performed in two village-areas, Chidakwa and Namwalinda, situated 5 km and 10 km from the field station, respectively. After capture, all mosquitoes were killed by freezing, identified morphologically,34 and packaged on silica gel desiccant (Fisher Scientific, Fair Lawn, NJ) and cotton in individual tubes for stable storage and transport.
The degree of exophagy of An. arabiensis and the activity level of the vector throughout the night was assessed by paired indoor/outdoor human landing catches (HLCs). Teams of two HLC collectors stationed inside and outside seven selected sleeping houses captured mosquitoes by manual aspiration, keeping hourly collections in separately labeled paper cups. Collections were performed from 8:00 pm to 6:00 am during the 2007–2008 season and were extended to 7:00 pm to 7:00 am during the 2008–2009 season. Collections were performed for 246 trap nights. Collectors were rotated between collection locations each night.
Anthropophily of An. arabiensis was examined by paired outdoor HLC and cattle-baited trap (CBT) collections performed simultaneously at three different households over 72 nights for 216 trap nights. Outdoor HLC and CBT collections were conducted by teams of two trained field personnel from dusk until dawn. The CBTs were conducted in Chidakwa and Namwalinda at seven households. Each CBT was a kraal, made of felled trees and branches, just large enough to hold one calf. After being led and tied into the kraal, the calf was covered with a double bed–sized untreated bed net. The net was hung above the kraal so that it lay on the outside of the pen, resting approximately 30 cm off the ground. The upper portion of the net was fashioned into a funnel that collected most of the resting mosquitoes and was cinched closed in the morning. The calf was then released and the remaining mosquitoes were collected by aspirator.
Additionally, anthropophily was assessed by determining the human blood indices of engorged An. arabiensis collected by Centers for Disease Control and Prevention (CDC) light traps during each season. The CDC traps were hung next to occupied beds protected by bed nets. If there was more than one bed in a trapping room, the other occupants were instructed to also use bed nets. Household members were shown how to turn on the trap before they went to sleep and how to cinch the bag closed and disconnect the battery in the morning. Trapping was performed in approximately 10 households in both village-areas multiple times per month.
Bed net use survey.
At the peak of each rainy season, household censuses were performed in Chidakwa and Namwalinda. The total numbers of people who slept in each structure and the number of people who slept under an ITN the previous night were enumerated during the 2007–2008 and 2008–2009 rainy seasons. Additionally, during the second year, the ages and sexes of those not sleeping under a bed net were noted. Results were averaged across three months each year to account for movements between sleeping structures. An odds ratio was calculated to determine if there was a gender bias in bed net use.
DNA isolation and polymerase chain reaction.
Heads/thoraces were separated from mosquito abdomens so that DNA could be extracted from each separately. Prior to homogenization, specimens were rehydrated at room temperature for 10 minutes in 20 μL of double-distilled water. DNA was extracted by using a modified salt procedure35 and resuspended in 50 μL of double-distilled water. DNA from engorged abdomens was used as template to determine host source by polymerase chain reaction.22,35 DNA from head/thoraces was used to confirm species by using the diagnostic procedure of Scott et al.36 and also to screen for P. falciparum. Plasmodium infection status was assessed by using a nested PCR.37
Data analysis.
Mosquito collections were modeled as overdispersed Poisson populations.38–40 Comparisons of CBT and outdoor HLC collections and comparisons of indoor and outdoor HLCs were performed using separate negative binomial regression analyses in STATA version 10 (Stata Corp., College Station, TX). Mosquito counts were assumed to follow a Poisson distribution with an overdispersion parameter to account for increased variance as compared with the mean. The log of the expected counts was modeled as the function log(E(Yijk)) = α + βX + ɛijk, where Yijk is the monthly mosquito count for household i in month j and year k, and X are the covariates of sampling method, collection year, and collection month. This method enabled standard errors to be adjusted for correlated observations within collection households.
Results
Across the three months during the 2007–2008 rainy season when household censuses were conducted, an average of 343 persons in 43 households were interviewed about their bed net use. During the 2008–2009 rainy season, an average of 305 persons in 39 households were surveyed. Every household owned at least one ITN. During the first year 82% of people self-reported sleeping under a bed net the previous night. Bed net coverage was 87% in Chidakwa (n = 159) and 78% in Namwalinda (n = 184). Coverage was still high the following year, but the proportion of people self-reporting bed net use decreased to 75% overall, 85% in Chidakwa (n = 149) and 65% in Namwalinda (n = 156). More detailed surveys were conducted during the 2008–2009 season. The odds of a female reporting to have slept under a bed net were 1.87 (95% confidence interval [CI] = 1.11–3.16) times that of a male. The age group reporting the largest number of individuals not sleeping under a bed net was persons 5–14 years of age. Only one child less than 1 year of age was reported to not have slept under a net (Figure 1).
Figure 1.

Individuals not protected by a bed net during the 2008–2009 season, partitioned by age class, Macha, Zambia.
Prior to the distribution of ITNs, An. arabiensis in Macha was highly anthropohilic; published HBIs were 0.872 and 0.923 for indoor-resting mosquitoes.3,22 This study found that the vector remained highly anthropophilic in the Macha region during the two years after the introduction of ITNs. Outdoor HLC teams caught nearly double the numbers of An. arabiensis as paired CBTs, although CBTs caught more mosquitoes overall. In total, CBTs caught 10,002 mosquitoes, and outdoor HLCs caught 1,878. Anopheles arabiensis catch totals were 285 and 147 for outdoor HLCs and CBTs, respectively. A negative binomial regression analysis demonstrated that the average number of An. arabiensis caught by a HLC pair was approximately 2.2 (95% CI = 0.99–4.9) times the number captured by a CBT collection.
Additionally, high HBIs were calculated for blood fed mosquitoes captured by CDC light trap (Table 1). Although everyone who slept in a room where CDC light trapping occurred was instructed to use a bed net, a sizeable percentage of An. arabiensis in the traps were blood fed. During the 2007–2008 season, 28% of the mosquitoes captured were engorged. Likewise, during the 2008–2009 season, 25% were blood fed. Human blood indices of 0.94 and 0.96 were determined for the two consecutive years, with the remaining blood meals found to be from cattle, dogs, and goats (Table 1). Two An. funestus s.s., one unfed and one engorged on human blood, were also captured by CDC light trap during the 2008–2009 season. None of the An. arabiensis collected by HLC, CBT, or CDC light trap were positive for P. falciparum sporozoites.
Table 1.
Abdominal status and host source of engorged Anopheles arabiensis mosquitoes collected by Centers for Disease Control light trap, Macha, Zambia
| Season | Unfed | Gravid | Blood fed | Host source, no (%) | |||
|---|---|---|---|---|---|---|---|
| Human | Cow | Dog | Goat | ||||
| 2007–2008 | 588 | 8 | 235 | 204 (94) | 14 (6) | 0 | 0 |
| 2008–2009 | 681 | 38 | 233 | 204 (96) | 4 (1.9) | 3 (1.4) | 2 (0.9) |
Over the course of this study, An. arabiensis consistently displayed exophagic behavior (Figure 2). During the first year, outdoor HLC pairs captured 279 An. arabiensis, and indoor teams caught 130 specimens. The following year 96 An. arabiensis were caught outside, and 61 were captured inside. A negative binomial regression analysis showed that outdoor HLCs caught an average of 1.7 (95% CI = 1.2–2.5) times as many An. arabiensis as indoor HLCs. Furthermore, throughout the night there was consistently more biting activity outdoors (Figure 3). Anopheles arabiensis began biting indoors and outdoors by 7:00 pm and continued to be active until 6:00 am inside and 7:00 am outside. Peak biting for the vector occurred between 10:00 pm and 2:00 am, decreasing thereafter. A total of 14% of An. arabiensis biting occurred before 10:00 pm.
Figure 2.
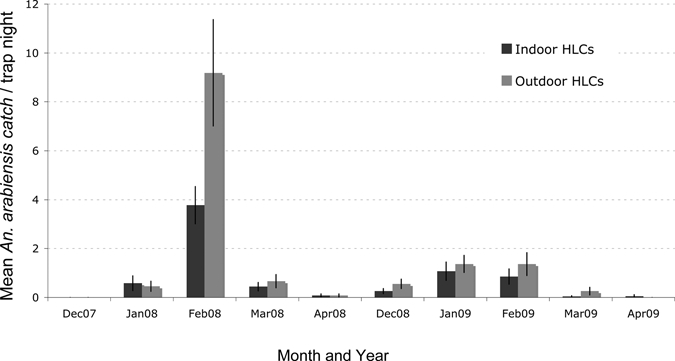
Seasonality of Anopheles arabiensis mosquitoes caught by human landing catch pairs during the 2007–2008 and 2008–2009 rainy seasons, Macha, Zambia. Bars indicate monthly means plus one standard error.
Figure 3.

Total Anopheles arabiensis mosquitoes caught each hour by human landing catch pairs December–April, Macha, Zambia.
Discussion
The foraging behavior of An. arabiensis in Macha, Zambia during the two years after introduction of ITNs was assessed in this study. Over the two-year period, the vector remained highly anthropophilic, as shown by high HBIs from CDC light trap collections and paired outdoor HLC and CBT comparisons. This study is the second report of a direct comparison between HLCs and CBTs for An. arabiensis. As underscored previously,12 the relative efficiency of the two techniques cannot be known. It may be that more mosquitoes were attracted to the cattle bait but that the trap was less efficient at catching them than the human collectors. However, when taken in conjunction with the HBI data, there is strong support for continued anthropophily in An. arabiensis in the Macha region. There have been reports of shifts in foraging by anophelines to alternate hosts after introduction of ITNs in India,28 Papua New Guinea30 and Kenya.31 However, similar to the data from Macha, other investigations of An. gambiae complex mosquitoes in Africa have not seen this effect.26,29,41
Other potential behavioral changes that have been observed in anophelines with the introduction of ITNs are shifts toward outdoor and/or earlier biting. Like other aspects of its behavior, the nightly biting activity of An. arabiensis varies dramatically across Africa. Peak biting after midnight has been observed in Senegal,9,10 Chad,8 and Kenya.24 However, in Mozambique13 and Tanzania,14 high activity levels were seen as early as 9:00 pm, and in Ethiopia peak biting was seen from 7:00 pm until 9:00 pm.42 In Macha, An. arabiensis biting occurs throughout the night, with peak activity starting before midnight at approximately 10:00 pm. Although most persons have gone to bed by this hour, it is important to note that 14% of An. arabiensis biting occurs prior to this time, when residents are finishing dinner and preparing for bed and are not protected by ITNs.
It is difficult to interpret whether ITNs are having an effect on An. arabiensis biting times in Macha because similar collections were not performed in the pre-intervention year. Likewise, it is unknown whether the high degree of exophagy observed in the population is a result of ITN introduction. Anopheles arabiensis in Macha might be inherently exophagic as it is elsewhere,12–15 or the vector might have adapted rapidly to feeding outdoors because indoor hosts are now mostly inaccessible. However, it is likely that the exophagy is not caused by a decrease in An. arabiensis entering behavior. Centers for Disease Control and Prevention light traps have been shown to catch similar numbers of mosquitoes when placed next to either an untreated bed net or ITN in Macha,43 which suggests that nets treated with deltamethrin produce little non-contact repellency.
The numbers of An. arabiensis captured by CBTs and HLCs were significantly reduced during the second year of trapping. However, this was probably not caused by community-wide suppression of the vector as reported for An. gambaie s.l. populations in Kenya27 and Tanzania26 because the total numbers of mosquitoes captured, including zoophilic culicines collected by CBT, were also reduced. Instead, differences in mosquito densities are likely a result of interannual variation in rainfall because An. arabiensis numbers have been shown to fluctuate greatly with the amount and frequency of precipitation received in the Macha area.3 Furthermore, it is evident that rainfall greatly influences An. funestus s.s. in Macha. Prior to an extended drought during the 2004–2005 rainy season, An. funestus s.s. was a recognized vector in the region. However, since that drought year it had not been observed until this report with the collection of two specimens by CDC light trapping during the 2008–2009 season.
Overall, CDC light traps in Macha captured a high percentage (26.5%) of blood fed An. arabiensis, with greater than 93% of the blood meals coming from human hosts, even though all occupants of trapping houses were instructed to use a bed net. Other studies have found CDC light traps to catch between 1.8% and 9% blood fed An. gambiae s.l. when used in conjunction with bed nets.44–47 Even when used without nets, the numbers of engorged An. gambiae s.l. captured by CDC trap have been lower than the proportions reported here.43,46 The high percentage of blood fed mosquitoes collected from CDC traps in Macha might be a consequence of capturing some fraction of exophagic but endophilic An. arabiensis. Additionally, it is possible that the host sources of the blood meals are occupants of the trapping rooms. Perhaps bed nets are being used improperly or the vector might be feeding through the bed net. Although none of the mosquitoes collected for this study were sporozoite positive, during the two rainy seasons of trapping after introduction of ITNs, malaria cases continued to be seen at the Macha Mission Hospital. To further decrease malaria transmission, there needs to be a better understanding of who is being bitten, and therefore, who is at greatest risk for contracting malaria or being a gametocyte donor.
It is evident that regardless of whether shifts in feeding location or biting times by An. arabiensis in Macha have occurred, the vector remains anthropophilic. This preference, in combination with the exophagic behavior of the vector and potential for early evening biting, might abrogate ITN protection as intervention use continues. Therefore, the foraging behavior of An. arabiensis in the Macha region will need to be monitored during future rainy seasons.
Acknowledgments
We thank Dr. Marie Diener-West for statistical insights; Shadrack Habbanti for time and effort spent coordinating field team operations in Zambia; Musapa Mulenga for managing collections in Macha; and the field mosquito collectors (Corrence Munsanje, Nathan Phiri, Clement Mwaanga, Fines Mwaanga, Twaambo Moono, Gift Shamapani, Miyanda Moono, Guide Hansumo, Mathias Muleka, Chaltone Munsanje, Pathias Chibambo, Malony Mulota, Haggard Musyutila, Cliff Singanga, Paul Haakaloba, and Ojukwu Himumwe) for assistance.
Footnotes
Financial support: Research support was provided to Douglas E. Norris and Gregory E. Glass from the Johns Hopkins Malaria Research Institute, National Institutes of Health grant T32AI007417 support to Laura C. Norris, a Johns Hopkins Bloomberg School of Public Health Sommer Scholarship award to Christen M. Fornadel, and additional training support from the Fogarty Foundation (5D43TW001587).
Authors' addresses: Christen M. Fornadel, Laura C. Norris, Gregory E. Glass, and Douglas E. Norris, The W. Harry Feinstone Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mails: cfornade@jhsph.edu, lnorris@jhsph.edu, gglass@jhsph.edu, and dnorris@jhsph.edu.
References
- 1.Ministry of Health of Zambia A Six-Year Strategic Plan: A Roadmap for the Impact on Malaria in Zambia, 2006–2011. 2006. http://www.nmcc.org.zm/files/6NMCPStrategicPlanZMOH.doc Available at.
- 2.Chanda E, Masaninga F, Coleman M, Sikaala C, Katebe C, Macdonald M, Baboo KS, Govere J, Manga L. Integrated vector management: the Zambian experience. Malar J. 2008;7:164. doi: 10.1186/1475-2875-7-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kent RJ, Thuma PE, Mharakurwa S, Norris DE. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am J Trop Med Hyg. 2007;76:267–274. [PMC free article] [PubMed] [Google Scholar]
- 4.Gillies MT, DeMeillon B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region) Second edition. Johannesburg: South African Institute for Medical Research; 1968. [Google Scholar]
- 5.White GB, Magayuka SA, Brocham PF. Comparative studies on sibling species of the Anopheles gambiae Giles complex (Diptera: Culicidae): bionomics and vectorial activity at Segera, Tanzania. Bull Entomol Res. 1972;62:295–317. [Google Scholar]
- 6.Antonio-Nkondjio C, Kerah CH, Simard F, Awono-Ambene P, Chouaibou M, Tchuinkam T, Fontenille D. Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J Med Entomol. 2006;43:1215–1221. doi: 10.1603/0022-2585(2006)43[1215:cotmvs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Taye A, Hadis M, Adugna N, Tilahun D, Wirtz RA. Biting behavior and Plasmodium infection rates of anopheles arabiensis from Sille, Ethiopia. Acta Trop. 2006;97:50–54. doi: 10.1016/j.actatropica.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Kerah-Hinzoumbe C, Peka M, Antonio-Nkondjio C, Donan-Gouni I, Awono-Ambene P, Same-Ekobo A, Simard F. Malaria vectors and transmission dynamics in Goulmoun, a rural city in south-western Chad. BMC Infect Dis. 2009;9:71. doi: 10.1186/1471-2334-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontenille D, Lochouarn L, Diatta M, Sokhna C, Dia I, Diagne N, Lemasson JJ, Ba K, Tall A, Rogier C, Trape JF. Four years' entomological study of the transmission of seasonal malaria in Senegal and the bionomics of Anopheles gambiae and A. arabiensis. Trans R Soc Trop Med Hyg. 1997;91:647–652. doi: 10.1016/s0035-9203(97)90506-x. [DOI] [PubMed] [Google Scholar]
- 10.Lemasson JJ, Fontenille D, Lochouarn L, Dia I, Simard F, Ba K, Diop A, Diatta M, Molez J. Comparison of behavior and vector efficiency of Anopheles gambiae and An. arabiensis (Diptera: Culicidae) in Barkedji, a Sahelian area of Senegal. J Med Entomol. 1997;34:396–403. doi: 10.1093/jmedent/34.4.396. [DOI] [PubMed] [Google Scholar]
- 11.Ameneshewa B, Service MW. Resting habits of Anopheles arabiensis in the Awash River Valley of Ethiopia. Ann Trop Med Parasitol. 1996;90:515–521. doi: 10.1080/00034983.1996.11813077. [DOI] [PubMed] [Google Scholar]
- 12.Tirados I, Costantini C, Gibson G, Torr SJ. Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol. 2006;20:425–437. doi: 10.1111/j.1365-2915.2006.652.x. [DOI] [PubMed] [Google Scholar]
- 13.Mendis C, Jacobsen JL, Gamage-Mendis A, Bule E, Dgedge M, Thompson R, Cuamba N, Barreto J, Begtrup K, Sinden RE, Hogh B. Anopheles arabiensis and An. funestus are equally important vectors of malaria in Matola coastal suburb of Maputo, southern Mozambique. Med Vet Entomol. 2000;14:171–180. doi: 10.1046/j.1365-2915.2000.00228.x. [DOI] [PubMed] [Google Scholar]
- 14.Geissbuhler Y, Chaki P, Emidi B, Govella NJ, Shirima R, Mayagaya V, Mtasiwa D, Mshinda H, Fillinger U, Lindsay SW, Kannady K, de Castro MC, Tanner M, Killeen GF. Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania. Malar J. 2007;6:126. doi: 10.1186/1475-2875-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oyewole I, Awolola T, Ibidapo C, Oduola A, Okwa O, Obansa J. Behaviour and population dynamics of the major anopheline vectors in a malaria endemic area in southern Nigeria. J Vector Borne Dis. 2007;44:56. [PubMed] [Google Scholar]
- 16.Ralisoa Randrianasolo BO, Coluzzi M. Genetical investigations on zoophilic and exophilic Anopheles arabiensis from Antananarivo area (Madagascar) Parassitologia. 1987;29:93–97. [PubMed] [Google Scholar]
- 17.Mahande A, Mosha F, Mahande J, Kweka E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar J. 2007;6:100. doi: 10.1186/1475-2875-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adugna N, Petros B. Determination of the human blood index of some anopheline mosquitos by using ELISA. Ethiop Med J. 1996;34:1–10. [PubMed] [Google Scholar]
- 19.Hadis M, Lulu M, Makonnen Y, Asfaw T. Host choice by indoor-resting Anopheles arabiensis in Ethiopia. Trans R Soc Trop Med Hyg. 1997;91:376–378. doi: 10.1016/s0035-9203(97)90245-5. [DOI] [PubMed] [Google Scholar]
- 20.Garrett-Jones C, Boreham PF, Pant CP. Feeding habits of anophelines (Diptera: Culicidae) in 1971–78, with reference to the human blood index: a review. Bull Entomol Res. 1980;70:165–185. [Google Scholar]
- 21.Joshi GP, Service MW, Pradhan GD. A survey of species A and B of the Anopheles gambiae Giles complex in the Kisumu area of Kenya prior to insecticidal spraying with OMS-43 (fenitrothion) Ann Trop Med Parasitol. 1975;69:91–104. doi: 10.1080/00034983.1975.11686988. [DOI] [PubMed] [Google Scholar]
- 22.Fornadel CM, Norris DE. Increased endophily by the malaria vector Anopheles arabiensis in southern Zambia and identification of digested blood meals. Am J Trop Med Hyg. 2008;79:876–880. [PMC free article] [PubMed] [Google Scholar]
- 23.Muriu SM, Muturi EJ, Shililu JI, Mbogo CM, Mwangangi JM, Jacob BG, Irungu LW, Mukabana RW, Githure JI, Novak RJ. Host choice and multiple blood feeding behaviour of malaria vectors and other anophelines in Mwea rice scheme, Kenya. Malar J. 2008;7:43. doi: 10.1186/1475-2875-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Githeko AK, Adungo NI, Karanja DM, Hawley WA, Vulule JM, Seroney IK, Ofulla AV, Atieli FK, Ondijo SO, Genga IO, Odada PK, Situbi PA, Oloo JA. Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol. 1996;82:306–315. doi: 10.1006/expr.1996.0038. [DOI] [PubMed] [Google Scholar]
- 25.Bøgh C, Clarke SE, Pinder M, Sanyang F, Lindsay SW. Effect of passive zooprophylaxis on malaria transmission in The Gambia. J Med Entomol. 2001;38:822–828. doi: 10.1603/0022-2585-38.6.822. [DOI] [PubMed] [Google Scholar]
- 26.Magesa SM, Wilkes TJ, Mnzava AE, Njunwa KJ, Myamba J, Kivuyo MD, Hill N, Lines JD, Curtis CF. Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. Part 2. Effects on the malaria vector population. Acta Trop. 1991;49:97–108. doi: 10.1016/0001-706x(91)90057-q. [DOI] [PubMed] [Google Scholar]
- 27.Gimnig JE, Kolczak MS, Hightower AW, Vulule JM, Schoute E, Kamau L, Phillips-Howard PA, ter Kuile FO, Nahlen BL, Hawley WA. Effect of permethrin-treated bed nets on the spatial distribution of malaria vectors in western Kenya. Am J Trop Med Hyg. 2003;68:115. [PubMed] [Google Scholar]
- 28.Sampath TR, Yadav RS, Sharma VP, Adak T. Evaluation of lambdacyhalothrin-impregnated bednets in a malaria endemic area of India. Part 2. Impact on malaria vectors. J Am Mosq Control Assoc. 1998;14:437–443. [PubMed] [Google Scholar]
- 29.Mbogo CN, Baya NM, Ofulla AV, Githure JI, Snow RW. The impact of permethrin-impregnated bednets on malaria vectors of the Kenyan coast. Med Vet Entomol. 1996;10:251–259. doi: 10.1111/j.1365-2915.1996.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 30.Charlwood JD, Graves PM. The effect of permethrin-impregnated bednets on a population of Anopheles farauti in coastal Papua New Guinea. Med Vet Entomol. 1987;1:319–327. doi: 10.1111/j.1365-2915.1987.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 31.Bøgh C, Pedersen EM, Mukoko DA, Ouma JH. Permethrin-impregnated bednet effects on resting and feeding behaviour of lymphatic filariasis vector mosquitoes in Kenya. Med Vet Entomol. 1998;12:52–59. doi: 10.1046/j.1365-2915.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- 32.Lines JD, Myamba J, Curtis CF. Experimental hut trials of permethrin-impregnated mosquito nets and eave curtains against malaria vectors in Tanzania. Med Vet Entomol. 1987;1:37–51. doi: 10.1111/j.1365-2915.1987.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 33.Miller JE, Lindsay SW, Armstrong JR. Experimental hut trials of bednets impregnated with synthetic pyrethroid or organophosphate insecticide for mosquito control in The Gambia. Med Vet Entomol. 1991;5:465–476. doi: 10.1111/j.1365-2915.1991.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 34.Gillies MT, Coetzee M. A Supplement to the Anophelinae of Africa South of the Sahara. Johannesburg: South African Institute for Medical Research; 1987. [Google Scholar]
- 35.Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005;73:336–342. [PMC free article] [PubMed] [Google Scholar]
- 36.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 37.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 38.Mathenge EM, Misiani GO, Oulo DO, Irungu LW, Ndegwa PN, Smith TA, Killeen GF, Knols BG. Comparative performance of the Mbita trap, CDC light trap and the human landing catch in the sampling of Anopheles arabiensis, An. funestus and culicine species in a rice irrigation in western Kenya. Malar J. 2005;4:7. doi: 10.1186/1475-2875-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathenge EM, Omweri GO, Irungu LW, Ndegwa PN, Walczak E, Smith TA, Killeen GF, Knols BG. Comparative field evaluation of the Mbita trap, the Centers for Disease Control light trap, and the human landing catch for sampling of malaria vectors in western Kenya. Am J Trop Med Hyg. 2004;70:33–37. [PubMed] [Google Scholar]
- 40.Hii JL, Smith T, Mai A, Ibam E, Alpers MP. Comparison between anopheline mosquitoes (Diptera: Culicidae) caught using different methods in a malaria endemic area of Papua New Guinea. Bull Entomol Res. 2000;90:211–219. doi: 10.1017/s000748530000033x. [DOI] [PubMed] [Google Scholar]
- 41.Lindsay SW, Alonso PL, Schellenberg JR, Hemingway J, Adiamah JH, Shenton FC, Jawara M, Greenwood BM. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, West Africa: 7. Impact of permethrin-impregnated bed nets on malaria vectors. Trans R Soc Trop Med Hyg. 1993;87:45–51. doi: 10.1016/0035-9203(93)90175-p. [DOI] [PubMed] [Google Scholar]
- 42.Yohannes M, Haile M, Ghebreyesus TA, Witten KH, Getachew A, Byass P, Lindsay SW. Can source reduction of mosquito larval habitat reduce malaria transmission in Tigray, Ethiopia? Trop Med Int Health. 2005;10:1274–1285. doi: 10.1111/j.1365-3156.2005.01512.x. [DOI] [PubMed] [Google Scholar]
- 43.Fornadel CM, Norris LC, Norris DE. Centers for Disease Control light traps for monitoring Anopheles arabiensis human biting rates in an area with low vector density and high insecticide-treated bed net use. Am J Trop Med Hyg. 2010;83:838–842. doi: 10.4269/ajtmh.2010.10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Githeko AK, Service MW, Mbogo CM, Atieli FA, Juma FO. Sampling Anopheles arabiensis, A. gambiae sensu lato and A. funestus (Diptera: Culicidae) with CDC light-traps near a rice irrigation area and a sugarcane belt in western Kenya. Bull Entomol Res. 1994;84:319–324. [Google Scholar]
- 45.Davis JR, Hall T, Chee EM, Majala A, Minjas J, Shiff CJ. Comparison of sampling anopheline mosquitoes by light-trap and human-bait collections indoors at Bagamoyo, Tanzania. Med Vet Entomol. 1995;9:249–255. doi: 10.1111/j.1365-2915.1995.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 46.Govella NJ, Chaki PP, Geissbuhler Y, Kannady K, Okumu F, Charlwood JD, Anderson RA, Killeen GF. A new tent trap for sampling exophagic and endophagic members of the Anopheles gambiae complex. Malar J. 2009;8:157. doi: 10.1186/1475-2875-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mbogo CN, Glass GE, Forster D, Kabiru EW, Githure JI, Ouma JH, Beier JC. Evaluation of light traps for sampling anopheline mosquitoes in Kilifi, Kenya. J Am Mosq Control Assoc. 1993;9:260–263. [PubMed] [Google Scholar]


