Abstract
The aim of this study was to define the clinical presentation of brucellosis in northern Australia and to assess the long-term impact of brucellosis on individual lives. A retrospective review was conducted to assess 32 patients with brucellosis caused by Brucella suis in Townsville, Australia during 1996–2009. All patients were Caucasian males with a mean age of 35 years. The greatest risk factor for brucellosis was feral pig hunting (94%, n = 30). There was one laboratory-acquired case. The most frequent clinical features included fever (94%, n = 30), fatigue (78%, n = 25) and arthralgia (78%, n = 25). The complication rate was 25% (n = 8). A delay in diagnosis of more than 14 days significantly increased the risk of the patient developing complications (95% confidence interval = 3.20–198.96, P = 0.002). The long-term impact of brucellosis was significant; 64% (16) of the patients reporting recurrent symptoms. This study highlights the need for further research on brucellosis caused by B. suis.
Brucellosis is one of the world's most widespread zoonoses. It causes significant human morbidity and enormous economic losses in animal husbandry.1 There are approximately 500,000 cases reported worldwide each year. However, this number is considered to be greatly underestimated. The incidence of this disease in Australia is 0.2 cases/100,000 population and 80% of cases occur in the state of Queensland.2 Currently, Brucella suis is the only cause of endemic brucellosis in Queensland.
Brucella suis is a gram-negative coccobacillus that has the feral pig (Sus scrofa) as its reservoir.3 The organism is shed in large numbers in body fluids and is transmitted to humans by direct contact through abrasions in the skin or inhalation of infected aerosols.3 The most common cause of human brucellosis in Australia is from exposure to infected feral pigs by recreational or occupational hunting. The clinical manifestations of human brucellosis are protean and non-specific and pose a diagnostic challenge to clinicians.4 This study is the first to describe human brucellosis in Australia since 1993 and one of only three case series exclusively investigating B. suis infection internationally.5,6 We describe the clinical manifestations and long-term impact for patients diagnosed with brucellosis in northern Australia during 1996–2009.
The study was reviewed and approved by the Townsville Health Services District Ethics Committee (35/07) and the James Cook University Human Ethics Committee (H3039). A retrospective chart review was conducted to assess 32 patients with brucellosis admitted during 1996–2009 to the Townsville Hospital in Queensland. The diagnosis of brucellosis was made by positive culture or positive Brucella serologic analysis that showed one high agglutination titer ≥ 1:160 or a four-fold increase in acute-phase and convalescent-phase serum samples by using the standard agglutination test. Clinical interviews were conducted with 25 patients to determine the long-term sequelae of B. suis infection. The epidemiology of brucellosis in northern Australia was compared with published data from another region in Australia.5
We found a widespread distribution of cases across northern Australia; 81% (n = 26) of patients resided in rural and remote communities (Figure 1). Patient age ranged from 17 to 68 years (mean = 35 years). All patients were men. The overwhelming male predisposition for brucellosis in northern Australia has not been reported in studies outside Australia where disease transmission is caused by consumption of unpasteurized goat's milk.7–10
Figure 1.
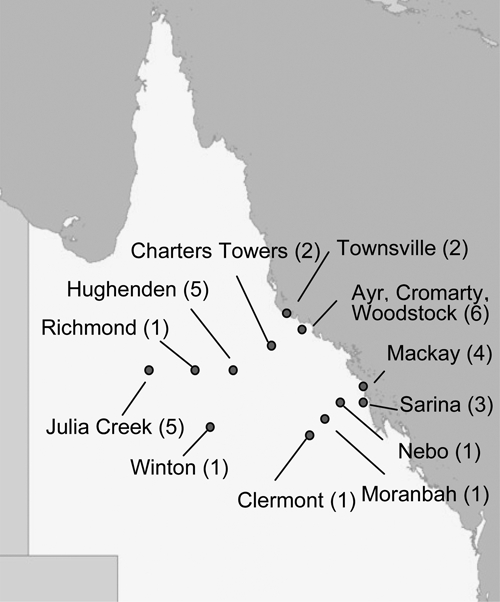
Distribution of cases of brucellosis in northern Queensland, Australia, 1996–2009.
The greatest risk factor for brucellosis in Queensland is feral pig hunting (94%, n = 30). In recent years, there has been a market for export of wild boar meat to Europe. Accredited hunters shoot or trap feral pigs and transport the freshly killed carcasses to ice box depots. Transmission of brucellosis occurs during slaughter by direct contact with feral pig blood or body fluids or by aerosol spread. However, the specific route of infection in individual patients who are hunters is difficult to determine. Of the patients included in this study, 38% (n = 12) had respiratory symptoms. Aerosol spread in these patients may have been the likely route of infection. There are 2,100 licensed professional feral pig hunters in Queensland. However, most hunters in Queensland are recreational and do not have hunting licenses.11 None of the hunters included in this study used protective equipment prior to infection.
Another important mode of transmission is by laboratory exposure to Brucella spp., which is one of the most common laboratory-acquired infections.12,13 There was one case of laboratory-acquired brucellosis in this study. Clinicians need to be aware that laboratory-acquired infections occur. These infections can potentially put laboratory staff at risk by neglecting to write clinical notes on request forms if they suspect brucellosis.
The clinical manifestations of brucellosis in northern Australia were compared with data from another region in Australia and various brucellosis-endemic countries (Table 1). The most common symptoms were fever (94%, n = 30), fatigue (78%, n = 25), arthralgia (72%, n = 23), sweats (63%, n = 20) and myalgia (59%, n = 19). Clinical manifestations of disease were similar to those in a previous study in Australia, except that the frequency of headache and dizziness was significantly lower in the current study. Disease manifestations of brucellosis in Australia are similar to those in other brucellosis-endemic countries.4,8–10,14,15 Clinical features of brucellosis are similar to those of Q fever, and risk factors for both of these diseases often coexist in a similar patient demographic.16 In the current study, 10% (n = 3) of patients were also positive for Q fever by serologic analysis.
Table 1.
Comparison of clinical signs and symptoms of brucellosis in Australia and other brucellosis-endemic countries*
| Characteristic | Australia | India8 (n = 495) | Turkey9 (n = 138) | Greece10 (n = 144) | |
|---|---|---|---|---|---|
| Northern Queensland (n = 32) | Southern Queensland5 (n = 32) | ||||
| Fever | 30 (94) | 31 (97) | 417 (84.2) | 108 (78.3) | 144 (100) |
| Fatigue | 25 (78) | 31 (97) | 6 (1.2) | 98 (71) | 140 (97) |
| Arthralgia | 25 (78) | 25 (78) | 117 (23.6) | 107 (77.5) | 125 (87) |
| Sweats | 23 (72) | 31 (97) | 19 (3.8) | 100 (72.5) | 138 (96) |
| Myalgia | 20 (63) | 25 (78) | NA | 80 (50.8) | NA |
| Headache | 19 (59)† | 27 (84) | 8 (1.6) | 71 (51.4) | 95 (66) |
| Back pain | 15 (47) | 19 (59) | 89 (17.9) | NA | 104 (72) |
| Dizziness | 6 (19)‡ | 15 (47) | NA | NA | NA |
| Splenomegaly | 5 (16) | NA | 95 (19.2) | 50 (36.2) | 74 (51) |
| Hepatomegaly | 3 (9) | NA | 56 (11.3) | 37 (26.8) | 36 (25) |
| Species | B. suis | B. melitensis | |||
Values are no. (%). NA = not available.
95% confidence interval = 0.08–0.89, P = 0.05.
95% confidence interval = 0.09–0.81, P = 0.032.
The complication rate observed in patients was 25% (n = 8). Relapse is a commonly reported complication that occurs in 5–24% of patients.17 In the current study, relapse occurred in 9% (n = 3) of the patients; osteomyelitis also developed in one patient. Osteoarticular complications are common in brucellosis and occur in 28–69% of patients.18,19 In the current study, osteomyelitis or sacroiliitis occurred in 9% (n = 3) of patients. Splenic abscesses occurred in 6% (n = 2) of patients in this study. However, this finding is believed to be extremely rare.20 Epididymoorchitis developed in one patient.
Delay in diagnosis has been shown to increase the rate of complications, a finding that was consistent with our findings. A delay in diagnosis was defined as time taken from first signs and symptoms to definitive diagnosis. A delay in diagnosis of more than 14 days occurred in 12 (38%) patients. This finding was shown to significantly increase the risk of a complication developing in a patient (odds ratio = 26.6, 95% confidence interval = 3.20–198.96, P = 0.002) and highlights the importance of early diagnosis. The delay typically occurred at the level of the primary practitioner where symptoms of brucellosis were not recognized. There was also a delay in requesting the appropriate laboratory investigations. In one case, lateness in obtaining laboratory results contributed to the delay in diagnosis.
The diagnosis of brucellosis was made by positive culture in 59% (n = 19) of the patients. In one patient with sacroiliitis, Brucella organisms were cultured from a bone biopsy. The most common abnormalities found by hematologic analysis were anemia (42%, n = 13), neutropenia (19%, n = 6), monocytosis (17%, n = 5) and lymphopenia (10%, n = 3). Liver function test results were frequently abnormal and showed increased alkaline phosphatase levels in 14 (48%).
Optimal antibiotic therapy for brucellosis is still debated. Therefore, many different regimens are used.21 The most commonly prescribed regimen in northern Australia was a triple therapy combination of doxycycline, gentamicin, and rifampicin (38%, n = 12) for a mean duration of more than six weeks. One patient in the current study received inadequate treatment with a monotherapy regimen, which has been associated with unacceptably high relapse rates.22 Relapse is usually a consequence of inadequate treatment or non-compliance.17 In all patients who showed relapse or in whom complications developed, treatment was deemed appropriate.
Brucellosis has the potential to cause significant long-term morbidity (Table 2). Of the 25 patients interviewed, 64% (n = 16) continued to experience brucellosis-like symptoms that included recurrent fevers, fatigue, residual joint pain, and testicular pain at follow-up (range = 13 days–11 years). Psychiatric manifestations, such as depression and psychosis, have been reported for patients with brucellosis.23 In this study, mental illness was also observed. The diagnosis of depression was made by using a General Practitioner in 20% (n = 5) of patients. A total of 41% (n = 9) had a Kessler Psychological Distress Scale score that indicated moderate-to-high levels of psychological distress at follow-up. Brucellosis caused significant morbidity and financial strain for patients and their family members; 64% (n = 16) had to take more than one month off from work and 12% (n = 3) were unable to work for more than 12 months. Altered recreational and occupational hunting practices, such as wearing personal protective equipment and increased sanitation, were adopted by 52% (n = 13) of patients after initial diagnosis of brucellosis.
Table 2.
Long-term effect of brucellosis, northern Queensland, Australia, 1996–2009*
| Effect | Long-term impact | Patients, no. (%) (n = 25) |
|---|---|---|
| Physical | No effect | 9 (36) |
| Symptoms | Current | 16 (64) |
| Mental | Depression | 5 (20) |
| State | Kessler Psychological Distress Scale score > 20 | 9 (41) |
| Occupational | Time off from work > 1 month | 16 (64) |
| Impact | Time off from work > 12 months | 3 (12) |
In conclusion, this study highlights the need for further research into human brucellosis caused by B. suis, in particular the different complications encountered and how best to optimize the treatment of affected patients. In addition, there is a need for educating medical practitioners and the public that engage in pig shooting about the continuous presence of brucellosis in northern Australia.
Acknowledgments
We acknowledge the support of the Royal College of Pathologist of Australasia in conducting this study.
Footnotes
Authors' addresses: Katie M. Eales and Natkunam Ketheesan, School of Medicine and Dentistry, James Cook University, Townsville, Queensland, Australia, E-mails: katie_eales@health.qld.gov.au and n.ketheesan@jcu.edu.au. Robert E. Norton, Pathology Queensland, Townsville Hospital, Townsville, Queensland, Australia, E-mail: robert_norton@health.qld.gov.au.
References
- 1.Godfroid J, Cloeckaert A, Liautard JP, Kohler S, Fretin D, Walravens K, Garin-Bastuji B, Letesson JJ. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet Res. 2005;36:313–326. doi: 10.1051/vetres:2005003. [DOI] [PubMed] [Google Scholar]
- 2.National Notifiable Diseases Surveillance System Notifications of Brucellosis. 2007. http://www9.health.gov.au/cda/source/cda-index.cfm Available at. Accessed May 2, 2009.
- 3.Young EJ. In: Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. Fifth edition. Mandell GL, Douglas RG, Bennett JE, Dolin R, editors. Philadelphia, PA: Churchill Livingstone; 2000. pp. 1031–1034. (Brucella species). [Google Scholar]
- 4.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 5.Robson J, Harrison M, Wood R, Tilse MH, McKay AB, Brodribb TR. Brucellosis: re-emergence and changing epidemiology in Queensland. Med J Aust. 1993;159:153–158. doi: 10.5694/j.1326-5377.1993.tb137777.x. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Controland Prevention Brucella suis infection associated with feral swine hunting – three states, 2007–2008. MMWR Morb Mortal Wkly Rep. 2009;58:618–621. [PubMed] [Google Scholar]
- 7.Mantur BG, Biradar M, Bidri RC, Mulimani MS, Veerappa K, Kariholu P, Patil SB, Mangalgi SS. Protean clinical manifestations and diagnostic challenges of human brucellosis in adults: 16 years' experience in an endemic area. J Med Microbiol. 2006;55:897–903. doi: 10.1099/jmm.0.46097-0. [DOI] [PubMed] [Google Scholar]
- 8.Memish Z, Mah M, Al Mahmoud S, Al Shaalan M, Khan MY. Brucella bacteraemia: clinical and laboratory observations in 160 patients. J Infect. 2000;40:59–63. doi: 10.1053/jinf.1999.0586. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Mesa JD, Sanchez-Gonzalex J, Reguera JM, Martin L, Lopez-Palmero S, Colmenero JD. Rose Bengal test: diagnostic yield and use for the rapid diagnosis of human brucellosis in emergency departments in endemic areas. Clin Microbiol Infect. 2005;11:221–225. doi: 10.1111/j.1469-0691.2004.01063.x. [DOI] [PubMed] [Google Scholar]
- 10.Hasanjani Roushan MR, Mohrez M, Smailnejad Gangi SM, Soleimani Amiri MJ, Hajiahmadi M. Epidemiological features and clinical manifestations in 469 adult patients with brucellosis in Babol, northern Iran. Epidemiol Infect. 2004;132:1109–1114. doi: 10.1017/s0950268804002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strategy QFPM The State of Queensland: Queensland Government Department of Primary Industries, April 2004. 2010. http://www.dpi.qld.gov.au/documents/Biosecurity_EnvironmentalPests/IPA-Feral-Pig-Strategy.pdf Available at. Accessed May 24.
- 12.Robichaud S, Libman M, Behr M, Rubin E. Prevention of laboratory-acquired brucellosis. Clin Infect Dis. 2004;38:e119–e122. doi: 10.1086/421024. [DOI] [PubMed] [Google Scholar]
- 13.Bouza E, Sanchez-Carrilo C, Hernangómez S, González MJ. Laboratory-acquired brucellosis: a Spanish national survey. J Hosp Infect. 2005;61:80–83. doi: 10.1016/j.jhin.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Kokoglu OF, Hosoglu S, Geyik MF, Ayaz C, Akalin S, Buyukbese MA, Cetinkaya A. Clinical and laboratory features of brucellosis in two university hospitals in southeast Turkey. Trop Doct. 2006;36:49–51. doi: 10.1258/004947506775598752. [DOI] [PubMed] [Google Scholar]
- 15.Andriopoulos P, Tsironi M, Deftereos S, Aessopos A, Assimakopoulos G. Acute brucellosis: presentation, diagnosis, and treatment of 144 cases. Int J Infect Dis. 2007;11:52–57. doi: 10.1016/j.ijid.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Cutler S, Bouzid M, Cutler R. Q fever. J Infect. 2007;54:313–318. doi: 10.1016/j.jinf.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 18.González-Gay MA, Garcia-Porrua C, Ibañez D, García-País MJ. Osteoarticular complications of brucellosis in an Atlantic area of Spain. J Rheumatol. 1999;26:141–145. [PubMed] [Google Scholar]
- 19.Geyik MF, Gur A, Nas K, Cevik R, Sarac J, Dikici B, Ayaz C. Musculoskeletal involvement of brucellosis in different age groups: a study of 195 cases. Swiss Med Wkly. 2002;132:98–105. doi: 10.57187/smw.2002.09900. [DOI] [PubMed] [Google Scholar]
- 20.Spink W. Host-parasite relationship in human brucellosis with prolonged illness due to suppuration of the liver and spleen. Am J Med Sci. 1964;247:129–136. doi: 10.1097/00000441-196402000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Skalsky K, Yahav D, Bishara J, Pitlik S, Leibovici L, Paul M. Treatment of human brucellosis: systematic review and meta-analysis of randomised control trials. BMJ. 2008;336:701–704. doi: 10.1136/bmj.39497.500903.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spink W. Current status of therapy of brucellosis in human beings. JAMA. 1960;172:697–698. doi: 10.1001/jama.1960.63020070004016. [DOI] [PubMed] [Google Scholar]
- 23.Eren S, Bayam G, Ergönül O, Celikbaş A, Pazvantoğlu O, Baykam N, Dokuzoğuz B, Dilbaz N. Cognitive and emotional changes in neurobrucellosis. J Infect. 2006;53:184–189. doi: 10.1016/j.jinf.2005.10.029. [DOI] [PubMed] [Google Scholar]


