Abstract
Critically ill patients with disseminated strongyloidiasis may not be candidates for oral treatment. We report four patients with disseminated strongyloidiasis, believed to be unable to absorb oral therapy, who were treated with ivermectin by rectal and/or subcutaneous administration. Obtaining subcutaneous ivermectin and dosing it appropriately is a challenge. These cases underscore the need for improved access to subcutaneous ivermectin and more pharmacological data to guide use of this treatment approach.
Introduction
The preferred treatment for intestinal strongyloidiasis is oral ivermectin.1,2 However, many patients with disseminated infection with Strongyloides stercoralis experience paralytic ileus, profuse vomiting, and diarrhea, which limits delivery and absorption of oral medications, including ivermectin.3,4 Such cases present a therapeutic challenge. There is no U.S. Food and Drug Administration (FDA)–approved treatment for disseminated disease, and oral therapy is unlikely to be effective in the setting of the gastrointestinal dysfunction often seen in patients with disseminated strongyloidiasis. Whereas no parenteral antihelminthic medications are licensed for human use, parenteral ivermectin (Stromectol®; Merck, Whitehouse Station, NJ) is approved for veterinary use and has been used as subcutaneous treatment in a limited number of patients with severe strongyloidiasis refractory to oral agents.5–14
This report describes four patients with disseminated strongyloidiasis treated at New York Presbyterian Hospital/Weill Cornell Medical Center in 2007–2009. None of the four patients were candidates for oral ivermectin because of ileus, diarrhea, or vomiting, which led to concern for poor drug absorption. Three major themes emerge from these patients: 1) the mortality rate of disseminated strongyloidiasis remains high, 2) parenteral ivermectin is difficult to obtain, and 3) there is little information to guide appropriate dosing. These latter two hurdles impede optimal therapy of a life-threatening disease.
Patient 1
A 43-year-old women with diabetes and infected with human T lymphotropic virus (HTLV-1) had emigrated from Ghana six years ago and had abdominal pain, weight loss, and an eosinophil count of 300 cells/μL. She had been previously given a diagnosis of intestinal strongyloidiasis by routine stool examination for ova and parasites at another center and had been treated with multiple courses of oral ivermectin, most recently three months before admission. At that time, she had abdominal pain, weight loss, and diarrhea, and was treated with a full course (200 μg/kg/day, twice a day) of oral ivermectin, which she completed without vomiting or complication. Symptoms improved initially with treatment. However, three weeks before admission, abdominal pain followed by nausea, vomiting, diarrhea, and weight loss developed again in the patient.
On admission, an abdominal computed tomography scan showed ileal wall thickening, and stool and duodenal aspirates contained many S. stercoralis larvae. Respiratory distress developed despite five days of oral ivermectin (12 mg/day) which required mechanical ventilation. Bronchoalveolar lavage (BAL) and an intradermal biopsy specimen of a serpiginous rash both showed many larvae.
Bowel ileus developed and repeat BAL still showed numerous larvae. Because of presumed poor absorption of oral ivermectin, rectal administration was initiated (6 mg, twice a day15), and oral albendazole (400 mg/day) was added because of concern for ivermectin resistance. The patient improved slowly, and larvae were not present on repeat duodenal aspirate or stool examination by concentrated wet mount technique (hereafter referred to as routine stool examination). She was discharged on day 46 after completing a total of 37 days of treatment with oral ivermectin and seven days of rectal therapy. There was no evidence of relapse by symptoms, and the results of seven subsequent stool examinations over the following month were negative.
Patient 2
A previously reported14 61-year-old woman from South Korea underwent renal allograft transplantation and was admitted for high-dose corticosteroid treatment (methylprednisolone, 500 mg/day) for acute rejection. In this setting, severe disseminated strongyloidiasis with pulmonary involvement developed and she was treated with oral and rectal ivermectin (12 mg/day orally and 12 mg/day rectally) and oral albendazole (400 mg, twice a day). Treatment with steroids were tapered and discontinued during the seven days after diagnosis of strongyloidiasis. One week after diagnosis, BAL remained positive for larvae and the eosinophil count peaked at 700 cells/μL. Poor absorption of anti-helminthic therapy was suspected.
Emergency FDA and institutional review board (IRB) approvals were obtained for subcutaneous ivermectin on a compassionate use basis, under Investigational New Drug (IND) # 078849. The patient's family provided informed consent and the nearby Animal Medical Center provided veterinary parenteral ivermectin at no cost. The approval/procurement process took more than 24 hours. Ivermectin was administered at a dose of 200 μg/kg divided into two aliquots (one in each arm). This treatment was given three times with 48 hours between treatments. Repeat BAL three days after treatment initiation showed no larvae, she responded clinically, and there was no recurrence of infection. The latter finding was deduced by the absence of larvae in five bronchial washings through one-month post-admission, and no larvae were seen in routine stool examinations one, five, and seven months after admission.
Patient 3
A 76-year-old woman had emigrated from Jamaica at the age of 18 years. She had severe non-bloody diarrhea. She had a history of relapsed Waldenstrom macroglobulinemia complicated by autoimmune thrombocytopenia and was treated with dexamethasone (8 mg/day), with no recent increase in dose, and intermittent rituximab. On admission, she was febrile, hypotensive, and obtunded with decreased breath sounds and a distended tender abdomen. Chest and abdomen computed tomography scans showed bilateral pulmonary infiltrates, pulmonary edema, and colon edema consistent with colitis. She was intubated endotracheally and resuscitated, and broad-spectrum antibacterial therapy and hydrocortisone (50 mg intravenously every six hours) were initiated. Stool examination and a BAL specimen demonstrated many S. stercoralis larvae. Oral ivermectin (12 mg) was given daily by nasogastric tube and steroids were discontinued. Blood cultures grew Escherichia coli and treatment with piperacillin-tazobactam was continued. Large volume diarrhea persisted, as did the requirement for mechanical ventilation. Repeat stool and BAL samples showed persistent larvae. The peak eosinophil count was 5,200 cells/μL.
On day 11, veterinary parenteral ivermectin was obtained by the same compassionate use process described for patient 2, which again took over 24 hours (IND # 104510). Subcutaneous ivermectin (200 μg/kg) was administered in two aliquots, one in each arm, every 72 hours, for a total of three doses. The patient showed defervescence after the first treatment. Tracheal aspirates and routine stool examinations were negative for larvae on day 16, as were four additional respiratory samples over the next month and 11 stool samples over the next 2.5 months. Hydrocortisone (50 mg three times/day) was reinstituted to treat thrombocytopenia, and was given in three pulses during 10 of the next 82 days. Her course was complicated by bacterial infections, multiple organ dysfunction syndrome, and malnutrition. The decision was made on day 102 to pursue comfort care only. The patient died one week later; no post-mortem examination was obtained.
Patient 4
A 59-year-old man who had emigrated from Puerto Rico 33 years previously had pleuritic chest pain, dyspnea, dysphagia, and diarrhea. Nine months before admission, chemotherapy for metastatic thymoma was started along with prednisone, 60 mg/day, for treatment of autoimmune hemolytic anemia. He was afebrile, his lungs were clear, and results of an abdominal examination were normal. Eosinophil count was zero, and remained so throughout admission. Findings of a chest radiograph were normal, but electrocardiogram showed evidence of pericarditis that responded to treatment with colchicine, ibuprofen, and continued prednisone. However, weakness, severe abdominal pain, and distension, followed by fever and hypotension, developed. Computed tomography of the abdomen and pelvis showed ileus and bowel wall edema. Treatment with prednisone was tapered starting on day seven to 16 mg/day by day 16 and continued throughout admission thereafter.
Stool examination on day nine demonstrated many S. stercoralis larvae, and one dose of oral ivermectin (12 mg/day) was given by nasogastric tube (Figure 1 and Table 1). However, in view of a requirement for continuous nasogastric suction, the FDA-and IRB-approval processes for parenteral ivermectin were initiated simultaneously, and drug was available 24 hours later. On day 10, in the setting of respiratory distress requiring mechanical ventilation, E. coli bacteremia, and septic shock, BAL demonstrated many live larvae and subcutaneous ivermectin was administered once at a total dose of 200 μg/kg (100 μg/kg in each arm), under IND #104740. No clinical response was observed over the next two days, repeat respiratory samples showed persistent larvae in high numbers, and a serpiginous, erythematous rash developed on the abdomen, consistent with larva currens.
Figure 1.
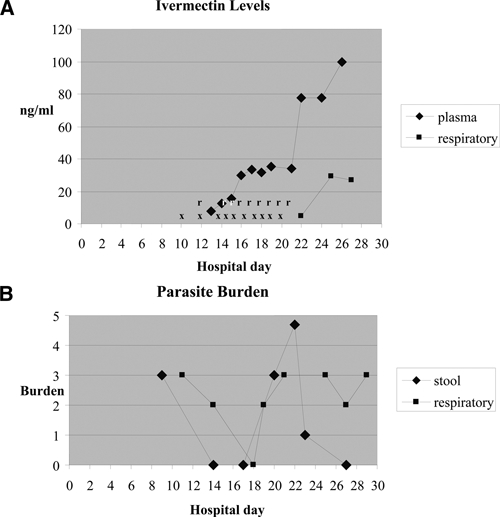
Data for patient 4. A, Plasma and respiratory ivermectin trough levels (ng/mL). X = doses of subcutaneous ivermectin; r = doses of rectal ivermectin. Respiratory levels were obtained from tracheal aspirates. B, Stool and respiratory parasite burdens. 0 = none; 1 = sparse; 2 = moderate; 3 = many. Respiratory samples included bronchoalveolar fluid on days 11 and 20; tracheal aspirate on days 14, 18, 19, 25, and 27; and sputum on day 23.
Table 1.
Antihelminthic therapy and plasma and respiratory tract ivermectin concentrations for patient 4
| Hospital day | Ivermectin dose | Albendazole dose (by nasogastric tube) | Plasma concentration (ng/mL) | Respiratory tract concentration (ng/mL) |
|---|---|---|---|---|
| 9 | ||||
| 10 | 200 μg/kg2 by nasogastric tube, 200 μg/kg2 | |||
| 11 | ||||
| 12 | 200 μg/kg2 (×2), 200 μg/kg rectal | |||
| 13 | 7.9 | |||
| 14 | 200 μg/kg2, 200 μg/kg rectal | 12.7 | ||
| 15 | 200 μg/kg2, 200 μg/kg rectal | 15.7 | ||
| 16 | 200 μg/kg2, 200 μg/kg rectal | 29.7 | ||
| 17 | 200 μg/kg2, 200 μg/kg rectal | 33.6 | ||
| 18 | 200 μg/kg2, 200 μg/kg rectal | 31.6 | ||
| 19 | 200 μg/kg2, 200 μg/kg rectal | 35.4 | ||
| 20 | 200 μg/kg2, 200 μg/kg rectal | |||
| 21 | 200 μg/kg2, 200 μg/kg rectal | 400 mg, twice a day | 33.9 | |
| 22 | 400 mg, twice a day | 77.4 | 4.7 | |
| 23 | 400 mg, twice a day | |||
| 24 | 400 mg, twice a day | |||
| 25 | 400 mg, twice a day | 77.9 | 29.3 | |
| 26 | 400 mg, twice a day | |||
| 27 | 400 mg, twice a day | 99.8 | 26.9 | |
| 28 | 400 mg, twice a day | |||
| 29 | 400 mg, twice a day | |||
On day 12, diarrhea had resolved and rectal ivermectin (12 mg/day) by retention enema was begun. Additional doses of subcutaneous ivermectin were also administered (Table 1). On hospital day 14, results of a stool examination were negative, but tracheal aspirate showed larvae, although fewer in number. Persistent respiratory distress and fevers suggested inadequate treatment. Thus, on day 14, the interval for subcutaneous ivermectin dosing was increased to daily. Plasma and/or respiratory secretions were obtained starting on day 13 and tested for ivermectin concentrations.7 On day 13, after treatments on day 5, 10 and 12, ivermectin was readily detected at a concentration of 7.9 ng/mL (Table 1).
Results for ivermectin concentrations in plasma and respiratory specimens and parasitologic response during the next 14 days are shown in Figure 1. On day 22, ivermectin was detected at a concentration of 4.7 ng/mL in respiratory secretions. Oral albendazole (400 mg, twice a day) was then added by nasogastric tube given the lack of parasite clearance. Plasma ivermectin concentrations on days 16–21, which suggested an apparent steady state value of 30–35 ng/mL, levels in the range (13.9–101.1 ng/mL) seen in healthy volunteers given single 12-mg doses of oral ivermectin without adverse effect.16,17 Anuria and ultimately fatal multiple organ dysfunction syndrome subsequently developed in the patient. The maximum plasma ivermectin concentration was 99.8 ng/mL, and the concentration in tracheal aspirates increased to 26.9–29.3 ng/mL. Levels of liver enzymes remained normal except for alanine aminotransferase (84 U/L). Treatment with subcutaneous and rectal ivermectin were discontinued on day 22, and the patient died eight days later. Three days before death, stool but not tracheal aspirate was free of larvae. Post-mortem examination was not performed.
Discussion
Although Strongyloides hyperinfection syndrome is recognized as an emerging global infectious disease with high mortality rates,16 timely diagnosis remains a problem17 and systematic preventive therapy is not used routinely.18 For example, in the US, treatment errors occurred more often among providers unfamiliar with immigrant health. When presented with a hypothetical case scenario, physicians-in-training in the United States had poor recognition (9%) of the need for parasite screening and frequently advocated empiric corticosteroids (23%).19 Delayed treatment leads to increased likelihood of dissemination and severe disease. However, treatment options for severe disease are limited, and are adapted from recommendations for chronic, uncomplicated strongyloidiasis, for which ivermectin has been identified as optimal treatment.20
The experience in our ivermectin-treated patients illustrates four main points. First, the mortality rate of disseminated strongyloidiasis remains high. Second, in critically ill patients, absorption of oral ivermectin is likely to be suboptimal. Third, access to parenteral ivermectin remains restricted such that timely drug procurement is difficult under the best of circumstances. Fourth, in disseminated strongyloidiasis, the pharmacokinetics, pharmacodynamics, and overall efficacy of ivermectin are poorly understood.
For our patients, conventional oral ivermectin therapy failed, as judged by persistent parasite burden. Treatment failure has been associated previously with HTLV-1 infection and steroid use.18,21 In our cases, failure was associated with HTLV-1 infection of only one patient. Ivermectin failure in the other three cases was likely related to corticosteroid use, which may impair host immune responses or alter parasite population kinetics.22 In all four of these cases, failure of oral ivermectin therapy appeared to be related to poor drug absorption caused by some combination of vomiting, diarrhea, and/or ileus. Each patient was critically ill with pulmonary dissemination, all required intensive care and mechanical ventilation, and two patients died. These cases underscore the severity of disseminated strongyloidiasis and the difficulty of providing treatment in the likely presence of poor gastrointestinal absorption.
In our cases, ivermectin was ultimately delivered by one or more non-approved methods, either by the rectal and/or subcutaneous route. This treatment appeared to eradicate infection in three of four patients, although our results may be limited by the use of routine stool examination. Fresh stool examinations by the Baermann sedimentation technique or stool culture on agar media would have had greater sensitivity and are now recommended.23 Rectal dosing of ivermectin required no special procedure, but provision of subcutaneous ivermectin required labor-intensive coordination among the primary caregivers, the IRB, the FDA officer assigned to compassionate IND reviews, a pharmacist, the Animal Medical Center and the infectious diseases service. Physical proximity of our medical center, five blocks from the Animal Medical Center, facilitated access to veterinary ivermectin. In addition, we have now developed a clinical team to facilitate ivermectin access and have created form documents for approval and consent for compassionate use of ivermectin, all of which expedited the access process for our later cases. The maximum number of emergency, compassionate use IRB approvals allowed for a single drug at our institution is three, which necessitated formal submission of a research protocol to continue to offer parenteral ivermectin therapy, anticipating that we will continue to encounter disseminated strongyloidiasis.
This case series and similar case reports6–14 raise the question of whether and when subcutaneous ivermectin will become more accessible. The number of cases for which this therapy is indicated but not sought or obtained is unknown. More centralized data collection related to disseminated strongyloidiasis may provide incentive for industry to market this drug for humans.
The pharmacokinetics, pharmacodynamics, and efficacy of ivermectin in disseminated strongyloidiasis remain poorly understood. Subcutaneous ivermectin is absorbed slowly and the kinetics of rectal absorption are unknown. Because of high mortality and potential delays in diagnosis and gaining access to subcutaneous ivermectin, there is a desire to achieve systemic levels of ivermectin without delay. The optimal mode of ivermectin administration, therapeutic level, and dose and duration of treatment have yet to be established. Use of rectal and subcutaneous routes and splitting the site of subcutaneous injections are strategies to enhance the absorption of ivermectin, but there is little or no pharmacokinetic data to support this practice. It is also worth noting that some patients have failed to respond to subcutaneous ivermectin, including patient 4 in this report.11,13
Thus, the delivery and efficacy of parenteral ivermectin in extraintestinal Strongyloides infection merits further evaluation. Early studies of ivermectin for intestinal versus non-intestinal infection were performed in canines by using oral formulation ivermectin only.24,25 One study showed efficacy against extraintestinal Strongyloides,24 whereas a second study found that oral ivermectin was ineffective against tissue dwelling, third-stage larvae.25 Published human data on the use of subcutaneous ivermectin for treatment of disseminated strongyloidiasis are limited to the case reports mentioned above, and include both successes and failures. In our experience, gastrointestinal clearance can occur despite ongoing pulmonary infection (cases 2–4). This observation raises the question of whether there is a failure of drug delivery or failure of efficacy at a major site of involvement, the respiratory tract.
This report is the first to document that subcutaneous ivermectin can reach measurable concentrations in respiratory secretions (case 4, Figure 1 and Table 1). In this patient, plasma ivermectin concentrations also reached 99.8 ng/mL, which is in the range of well-tolerated levels in healthy subjects (50–100 ng/mL).26,27 However, because this patient was sedated and paralyzed for mechanical ventilation, physical examination was of limited use in assessing for possible ivermectin-associated neurotoxicity.6
In case 4, the sharp increase in concentrations after the final ivermectin dose likely indicates a depot effect from subcutaneous administration, as described.6 The concentrations in plasma and respiratory secretions in case 4 are well above the reported value sufficient to paralyze 50% of S. ratti and S. venezuelensis in vitro (2.4 ng/mL), although the therapeutic concentration required in humans against S. stercoralis is unknown.28 Despite seemingly adequate concentrations, pulmonary infection persisted in case 4. Possible explanations include ivermectin-resistant Strongyloides, limited ivermectin efficacy against non-intestinal stages of Strongyloides, or inadequate concentration of free ivermectin (despite detectable total ivermectin concentrations). In each of our cases, ivermectin was used off-label because there are no approved non-oral medications available for disseminated disease. Of note, the package insert for oral ivermectin states that “ivermectin activity against Strongyloides stercoralis is limited to the intestinal stages.” As judged by previous reports of successful parasite eradication by using parenteral ivermectin6–10,12,14 and our cases 2 and 3, parenteral therapy may become the standard approach for disseminated strongyloidiasis by default during periods of likely poor absorption, but with sparse data to guide its use.
Acknowledgment
We thank Animal Medical Center for providing parenteral ivermectin repeatedly at no cost and on short notice.
Footnotes
Financial support: This study was supported by grants T32 AI 007613 (Dahlene N. Fusco, Michael J. Satlin, and Meera Pahuja) and T32 HS000066 (Jennifer A. Downs).
Authors' addresses: Dahlene N. Fusco, Massachusetts General Hospital, Boston MA, E-mail: dnfusco@partners.org. Jennifer A. Downs, Michael J. Satlin, Meera Pahuja, and Henry W. Muray, Division of Infectious Diseases, Weill Cornell Medical College, New York-Presbyterian Hospital, New York, NY, E-mails: jna2002@med.cornell.edu, mjs9012@med.cornell.edu, mep2002@med.cornell.edu, and hwmurray@med.cornell.edu. Liz Ramos, Pharmacy Department, New York-Presbyterian Hospital, New York, NY, E-mail: lir9012@nyp.org. Philip S. Barie, Departments of Surgery and Public Health, Weill Cornell Medical College, New York-Presbyterian Hospital, New York, NY, E-mail: pbarie@med.cornell.edu. Lawrence Fleckenstein, Division of Pharmaceutics and Experimental Therapeutics, University of Iowa College of Pharmacy, Iowa City, IA, E-mail: l-fleckenstein@uiowa.edu.
References
- 1.Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, Sánchez-Sánchez P, Matogo-Oyana J, Rodríguez-Calabuig D. Efficacy and safety of ivermectin and thiabendazole in the treatment of Strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615–2619. doi: 10.1517/14656566.5.12.2615. [DOI] [PubMed] [Google Scholar]
- 2.Marti H, Haji JH, Savioli L, Chwaya HM, Mgeni AF, Ameir JS, Hatz C. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyoides stercoralis and other soil-transmitted helminth infections in children. Am J Trop Med Hyg. 1996;55:477–481. doi: 10.4269/ajtmh.1996.55.477. [DOI] [PubMed] [Google Scholar]
- 3.Igra-Siegman Y, Kapila R, Sen P, Kaminski ZC, Louria DB. Syndrome of hyperinfection with Strongyloides stercoralis. Rev Infect Dis. 1981;3:397–407. doi: 10.1093/clinids/3.3.397. [DOI] [PubMed] [Google Scholar]
- 4.Cruz T, Reboucas G, Rocha H. Fatal Strongyloidiasis in patients receiving corticosteroids. N Engl J Med. 1966;275:1093–1096. doi: 10.1056/NEJM196611172752003. [DOI] [PubMed] [Google Scholar]
- 5.Chiodini PL, Reid AJ, Wiselka MJ, Firmin R, Fowerake J. Parenteral ivermectin in Strongyloides hyperinfection. Lancet. 2000;355:43–44. doi: 10.1016/s0140-6736(99)02744-0. [DOI] [PubMed] [Google Scholar]
- 6.Turner SA, Maclean D, Fleckenstein L, Greenaway C. Parenteral administration of ivermectin in a patient with disseminated Strongyloidiasis. Am J Trop Med Hyg. 2005;73:911–914. [PubMed] [Google Scholar]
- 7.Marty FM, Lowry CM, Rodriguez M, Milner D, Pieciak W, Sinha A, Fleckenstein L, Baden LR. Treatment of human disseminated Strongyloidiasis with a parenteral veterinary formulation of ivermectin. Clin Infect Dis. 2005;41:e5–e8. doi: 10.1086/430827. [DOI] [PubMed] [Google Scholar]
- 8.Hauber HP, Galle J, Chiodini PL, Rupp J, Birke R, Vollmer E, Zabel P, Lange C. Fatal Outcome of a hyperinfection syndrome despite successful eradication of Strongyloides with subcutaneous ivermectin. Infection. 2005;33:383–386. doi: 10.1007/s15010-005-5060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenberger P, Rosa-Cunha I, Morris M, Nishida S, Akpinar E, Gaitan J, Tzakis A, Doblecki-Lewis S. Hyperinfection Strongyloidiasis in a liver transplant recipient treated with parenteral ivermectin. Transpl Infect Dis. 2009;11:137–142. doi: 10.1111/j.1399-3062.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- 10.Pacanowski J, Santos MD, Roux A, Maignan C, Guillot J, Lavarde V, Cornet M. Subcutaneous ivermectin as safe salvage treatment in Strongyloides stercoralis hyperinfection syndrome: a case report. Am J Trop Med Hyg. 2005;73:122–124. [PubMed] [Google Scholar]
- 11.Leung V, Al-Rawahi GN, Frant J, Fleckenstein L, Bowie W. Case report: failure of subcutaneous ivermectin in treating Stronygloides hyperinfection. Am J Trop Med Hyg. 2008;79:853–855. [PubMed] [Google Scholar]
- 12.Miller MA, Church LW, Salgado CD. Strongyloides hyperinfection: a treatment dilemma. Am J Med Sci. 2008;336:358–361. doi: 10.1097/MAJ.0b013e31815cff89. [DOI] [PubMed] [Google Scholar]
- 13.Grein J, Mathisen G, Donovan S, Fleckenstein L. Serum ivermectin levels after enteral and subcutaneous administration for Strongyloides hyperinfection: a case report. Scandanavian Journal Infect Dis. 2010;42:234–236. doi: 10.3109/00365540903443165. [DOI] [PubMed] [Google Scholar]
- 14.Huston JM, Eachempati SR, Rodney JR, Cayci C, Fusco D, Mathew M, Shou J, Goldstein MJ, Kapur S, Barie PS. Treatment of Strongyloides stercoralis hyperinfection-associated septic shock and acute respiratory distress syndrome with drotecogin alfa (activated) in a renal transplant recipient. Transpl Infect Dis. 2009;11:227–280. doi: 10.1111/j.1399-3062.2009.00386.x. [DOI] [PubMed] [Google Scholar]
- 15.Tarr PE, Miele PS, Peregoy K, Smith M, Neva F, Lucey D. Case report: rectal administration of ivermectin to a patient with Strongyloides hyperinfection syndrome. Am J Trop Med Hyg. 2003;68:453–455. [PubMed] [Google Scholar]
- 16.Marcos LA, Terashima A, Dupont HL, Gotuzzo E. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg. 2008;102:314–318. doi: 10.1016/j.trstmh.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Qu Z, Kundu UR, Abadeer RA, Wanger A. Strongyloides colitis is a lethal mimic of ulcerative colitis: the key morphologic differential diagnosis. Hum Pathol. 2009;40:572–577. doi: 10.1016/j.humpath.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Fardet L, Genereau T, Cabane J, Kettaneh A. Severe strongyloidiasis in corticosteroid-treated patients. Clin Microbiol Infect. 2006;12:945–947. doi: 10.1111/j.1469-0691.2006.01443.x. [DOI] [PubMed] [Google Scholar]
- 19.Boulware DR, Stauffer WM, Hendel-Paterson BR, Rocha JL, Seet RC, Summer AP, Nield LS, Supparatpinyo K, Chaiwarith R, Walker PF. Maltreatment of Strongyloides infection: case series and worldwide physicians-in-training survey. Am J Med. 2007;120:e1–e8. doi: 10.1016/j.amjmed.2006.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suputtamongkol Y, Kungpanichkul N, Silpasakorn S, Beeching NJ. Efficacy and safety of a single-dose veterinary preparation of ivermectin versus 7 day high-dose albendazole for chronic strongyloidiasis. Int J Antimicrob Agents. 2008;31:46–49. doi: 10.1016/j.ijantimicag.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Terashima A, Alvarez H, Tello R, Infante R, Freedman DO, Gotuzzo E. Treatment failure in intestinal strongyloidiasis: an indicator of HTLV-I infection. Int J Infect Dis. 2002;6:28–30. doi: 10.1016/s1201-9712(02)90132-3. [DOI] [PubMed] [Google Scholar]
- 22.Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5:345–355. doi: 10.1128/cmr.5.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.March Blatt J, Cantos G. Evaluation of techniques for the diagnosis of Strongyloides stercoralis in human immunodeficiency virus (HIV) positive and HIV negative individuals in the city of Itajai, Brazil. Braz J Infect Dis. 2003;7:402–408. doi: 10.1590/s1413-86702003000600008. [DOI] [PubMed] [Google Scholar]
- 24.Campbell WC. In: Ivermectin and Abamectin. Campbell WC, editor. New York: Springer-Verlag; 1989. p. 157. (Safety of ivermectin in target animals). [Google Scholar]
- 25.Mansfield LS, Schad GA. Ivermectin treatment of naturally acquired and experimentally induced Strongyloides stercoralis infections in dogs. J Am Vet Med Assoc. 1992;201:726–730. [PubMed] [Google Scholar]
- 26.Stromectol. 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050742s022lbl.pdf Available at. Accessed March 8.
- 27.Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, Sciberras DG, Hseih JY, Lasseter KC. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002;42:1122–1133. doi: 10.1177/009127002401382731. [DOI] [PubMed] [Google Scholar]
- 28.Satou T, Koga M, Koike K, Taka I, Nikaido T. Nematocidal activities of thiabendazole and ivermectin against the larvae of Strongyloides ratti and S. venezuelensis. Vet Parasitol. 2001;99:311–322. doi: 10.1016/s0304-4017(01)00472-1. [DOI] [PubMed] [Google Scholar]


