Abstract
Lymphatic dilatation, dysfunction, and lymphangiogenesis are hallmarks of patent lymphatic filariasis, observed even in those with subclinical microfilaremia, through processes associated, in part, by vascular endothelial growth factors (VEGFs). A panel of pro-angiogenic factors was measured in the plasma of subjects from filaria-endemic regions using multiplexed immunological assays. Compared with endemic normal control subjects, those with both subclinical microfilaremia, and those with longstanding lymphedema had significantly elevated levels of VEGF-A, VEGF-C, VEGF-D, and angiopoeitins (Ang-1/Ang-2), with only levels of basic fibroblast growth factor (bFGF) and placental growth factor (PlGF) being elevated only if lymphedema was evident. Furthermore, levels of these factors 1-year post-treatment with doxycycline were similar to pretreatment levels suggesting a minimal role, if any, for Wolbachia. Our data support the concept that filarial infection per se is associated with elevated levels of most of the known pro-angiogenic factors, with only a few being associated with the serious pathologic consequences associated with Wuchereria bancrofti infection.
Introduction
The lymphatic vascular system is an important part of immune surveillance, tissue fluid homeostasis, and fat absorption. Any acquired or congenital defect in lymphatic architecture or function can lead to lymphatic dysfunction and lymphedema. Lymphangiogenesis seen in both normal processes (e.g., wound healing or inflammation) and in pathologic conditions such as lymphedema or cancer metastasis is regulated, in part, by the interactions between the vascular endothelial growth factors (VEGF)-C, VEGF-D, and their receptor, VEGFR-3 (reviewed in Reference 1).
Disease following infections with Wuchereria bancrofti (Wb) and Brugia malayi (Bm), the two major causative agents of lymphatic filariasis (LF), is characterized by lymphangitis, hydrocoele, lymphedema, and elephantiasis. The events that lead to these conditions are poorly characterized, but immune and/or inflammatory responses to the parasites are believed to play a significant role in mediating some of these serious clinical manifestations.2–5
Because both Wb and Bm harbor the intracellular Wolbachia endosymbiont, previous studies have implicated Wolbachia as an inducer of VEGF-C that, in turn, could affect the lymphatic vessels.6 In addition, treatment with doxycycline, an antibiotic that targets Wolbachia, in patients with LF has been shown to reduce plasma VEGF-C/VEGFR-3 levels and improve pathology.7
Recently, we have shown that filarial antigens and plasma from infected individuals were able to stimulate human lymphatic endothelial cells to undergo proliferation and remodeling.8 To assess whether lymphatic endothelial cell (LEC) activation and proliferation induced by patient plasma was a consequence of the presence of pro-angiogenic factors in plasma of filaria-infected individuals driving lymphatic remodeling, we used very sensitive methods for measuring VEGF-C and a variety of other VEGFs, soluble VEGFRs, and other angiogenic factors in the plasma of patients with filarial infections. We show that not only the VEGFs but also their receptors and other pro-angiogenic factor levels are elevated in filarial infections regardless of clinical manifestations. Moreover, doxycycline therapy did not result in reduced levels of these factors.
Methods
Patient samples.
Platelet poor plasma samples were used for the entire study. All samples were from archived specimens collected as part of multiple studies on filarial infections, each performed with approval from the Institutional Review Board (IRB) of the National Institute of Allergy and Infectious Diseases along with the IRBs of the Tuberculosis Research Center (for studies in India) and the University of Bamako (studies in Mali). Informed consent was obtained from all subjects and all protocols used contained stored sample language. With the exception of several ongoing clinical trials (NCT00342576, NCT00340691), all other specimens were collected before the existence of internationally recognized clinical trials registries. Thus, all samples were collected from well-characterized patients with Wb infections,9 or Wb/Mansonella perstans coinfections from Mali.10 All samples were obtained after centrifugation of heparinized whole blood, that were stored at −80°C until used. Almost all of the samples had never been thawed previously, with the exception of the pre- and post-treatment samples that had been thawed only one time before use. The demographic and other details of these patients are given in Table 1. All samples were tested for microfilaremia (by night blood collection and Nucleopore filtration of blood) and circulating antigen levels in plasma by Og4C3 kit (TropBio Pty Ltd., Townsville, Queensland, Australia).
Table 1.
Patient population for the study
| Group and populations | Number of patients | Age range (yrs) | MF count | CFA (Og4C3) | ||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||
| INDIA–Wuchereia bancrofti | ||||||
| Screening* | 172 | |||||
| Endemic normals | 59 | 21–51 | 0 | – | 7 (2–35) | – |
| Asymptomatic microfilaraemics | 58 | 17–58 | 180 (1–1,350) | – | 224,614 (4–4,198,147) | – |
| Chronic lymphedema | 55 | 18–65 | 0 | – | 22 (1.3–54) | – |
| MALI–Wuchereria bancrofti and Mansonella perstans | ||||||
| Pre- and post-treatment (12 mo) | 60 | |||||
| Asymptomatic microfilaraemic (doxycycline) | 31 | 19–64 | 476 (17–3,196) | 0 | 1,323 (612–2862) | 572 (207–1,077) |
| Asymptomatic microfilaraemic (no treatment) | 29 | 19–65 | 328 (0–6,222) | 198 (0–3,026) | 401 (162–993) | 192 (73–506) |
Subset of the population (36 from each group) has been assayed for lymphangiogenic panels.
A total of 172 samples from Indian subjects from a W. bancrofti-endemic region of the world, were assayed for VEGF-C by dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA). A subset (N = 108) of these were used for measurement of additional lymphangiogenic factors by multiplex and were chosen based solely on having equivalent numbers of samples within each group and having sufficient plasma volume to test multiple analytes. Paired samples before and after treatment were also assessed (see Table 1 for details); for all studies involving treatment we only used samples that were collected before and 12 months after therapy. By definition, endemic normal (EN) individuals are those residing in filarial-endemic regions of the world and shown to be free of infection (both by microfilarial filtration and by having negative circulating filarial antigen levels). Clinically asymptomatic (subclinical) microfilaraemic (MF) subjects had circulating microfilariae in the bloodstream and were antigen positive. Those termed “CP” were individuals who exhibited various degrees of lymphedema (from stage II to frank elephantiasis); these were typically negative for circulating filarial antigen.
DELFIA, enzyme-linked immunosorbent assay (ELISA), and multiplex analysis.
For the initial measurements of VEGF-C, Immulon-4 plates were coated with mouse monoclonal antibody to VEGF-C (R&D Systems, Minneapolis, MN) overnight at 4°C. Plates were blocked with 5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 2 h at 37°C. Samples and standards were added to plates in duplicate and incubated overnight at 4°C. After washing, the plates were incubated for 1 h at 37°C with a detection antibody (biotinylated goat polyclonal anti-human VEGF-C; R&D Systems), After washing away unbound detection antibody, plates were incubated with europium-labeled streptavidin (Perkin Elmer, Foster City, CA) for 1 h at 37°C. Plates were developed with DELFIA enhancement solution (Perkin Elmer) for 15 min at room temperature and then read with a VICTOR V luminometer (Perkin Elmer). The lower limit of the assay was 32 pg/mL.
The growth factors VEGF-A, sVEGFR-1, basic fibroblast growth factor (bFGF), and placental growth factor (PlGF) were assayed using Meso Scale Discovery's 4-plex growth factors panel-I (Meso Scale Discovery, Gaithersburg, MD). A customized 4-plex panel for VEGF-C, VEGF-D, Ang-1, and Ang-2 was also manufactured by Meso Scale Discovery for performing the assays. The sVEGFR-2 and sVEGFR-3 were measured using kits from R&D Systems. All the assays were carried out per manufacturer's instructions. The upper detection limits by multiplex assays were 40 ng/mL for Ang-1 and Ang-2, 20 ng/mL for VEGF-C and VEGF-D, and 9 ng/mL for bFGF, sVEGFR-1, VEGF-A, and PlGF. The lower detection limits for Ang-1, Ang-2, VEGF-C, VEGF-D, bFGF, sVEGFR-1, VEGF-A, and PlGF were 176.1, 227.6, 37.3, 48.6, 0.9, 3.6, 1.9, and 4.7 pg/mL, respectively.
Statistics.
Data analyses were performed using GraphPad PRISM (GraphPad Software, Inc., San Diego, CA). Unless otherwise noted, geometric means (GM) were used for measurements of central tendency. Statistically significant differences between two groups were analyzed using the non-parametric Mann-Whitney U test. Correlations were calculated by Spearman's correlation test. Wilcoxon signed-rank test was used for all pairwise comparisons. The Dunn's post test was used to correct for multiple comparisons.
Results
Filarial infections have increased levels of VEGF-C.
Using a highly sensitive europium-based assay to detect the plasma concentrations of VEGF-C in 172 individuals from areas endemic for Wb, we found significantly increased levels of VEGF-C in patients with both CP (N = 55, P = 0.04) or MF (N = 58, P = 0.002) compared with the EN (N = 59) (Figure 1). Levels of VEGF-C were comparable between CP and MF individuals. A subset of samples (N = 36) was assayed in parallel with a commercial VEGF-C assay in a multiplex format (Meso Scale Discovery). The results indicated that the europium-based assay was highly correlated with the multiplex assay (P = 0.008).
Figure 1.
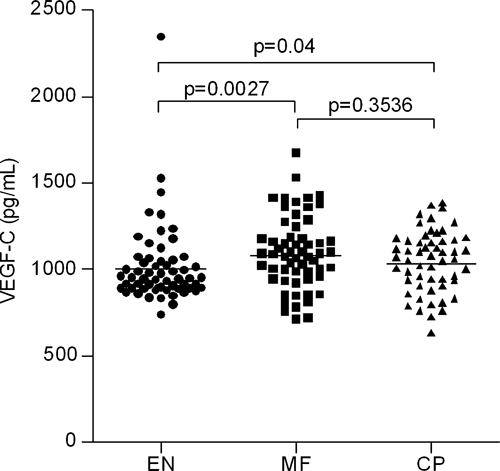
Plasma levels of vascular endothelial growth factors (VEGF)-C in individuals with human lymphatic filarial infections. Endemic normals (EN; N = 59), asymptomatic microfilaremic (MF; N = 58), chronic lymphedema (CP; N = 55).
Plasma lymphangiogenic factors are elevated in filarial patients.
To analyze other soluble growth factor levels in the filarial patients, we assayed levels of VEGF-A, VEGF-D, sVEGFR-1, sVEGFR-2, sVEGFR3, Ang-1, Ang-2, PlGF, and bFGF in 108 individuals from areas endemic for bancroftian filariasis drawn from southern India. Significantly elevated levels of VEGF-A, C, and D could be detected in individuals with both MF and CP compared with EN controls (Figure 2A–C). Interestingly, VEGF-D levels were elevated to an even greater extent in the subclinical MF individuals above that seen in those seen with CP (P < 0.007).
Figure 2.
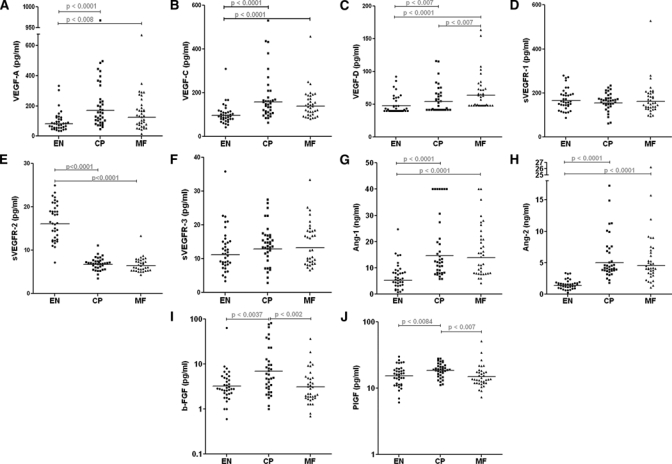
Plasma levels of lymphangiogenic factors in individuals with human lymphatic filarial infections. Endemic normals (EN; N = 36), asymptomatic microfilaremic (MF; N = 36), chronic lymphedema (CP; N = 36).
Analysis of soluble receptor levels indicated that the sVEGFR-1 and sVEGFR-3 (Figure 2D–F) levels did not differ among the three groups of subjects. Notably, the levels of sVEGFR-2 (Figure 2E) were significantly reduced in those with MF (P < 0.0001) and CP (P < 0.0001) compared with EN. There was a significant positive correlation between the levels of VEGF-A (but not VEGF-C/VEGF-D) and VEGF-C in all the groups (EN, r = 0.530, P < 0.0009; MF, r = 0.550, P < 0.0005; CP, r = 0.548, P < 0.0005) and significantly inverse correlations between sVEGFR2 and both VEGF-A (r = −0.227, P = 0.019) and VEGF-C (r = −0.412, P < 0.0001).
Interestingly, the MF and CP individuals also had elevated levels of the pro-angiogenic factors Ang-1 (P < 0.0001) and Ang-2 (P < 0.0001) (Figure 2G–H) compared with the uninfected EN. Levels of PlGF (Figure 2I) and bFGF (Figure 2J) were observed to be higher in subjects with lymphedema (CP) compared with EN (PlGF, P = 0.008; bFGF, P = 0.007) or MF (PlGF, P = 0.003; bFGF, P = 0.002). Together, these results suggest that Wb infections are associated with elevated levels of lymphangiogenic factors; among these, PlGF and bFGF appear to be increased in those with lymphatic disease associated with filarial infection.
There was a significant positive correlation between the levels of VEGF-A (but not VEGF-C/VEGF-D) and VEGF-C in all the groups (EN, r = 0.530, P < 0.0009; MF, r = 0.550, P < 0.0005; CP, r = 0.548, P < 0.0005) and significantly inverse correlations between sVEGFR2 and both VEGF-A (r = −0.227, P = 0.019) and VEGF-C (r = −0.412, P < 0.0001).
Targeting Wolbachia with doxycycline treatment does not alter the levels of lymphangiogenic factors.
With specific attention to the role played by Wolbachia, we evaluated the effects of doxycycline treatment on levels of lymphangiogenic factors pre- and post-treatment in plasma collected from Wb/Mansonella perstans co-infected MF individuals (N = 60) treated with either 200 mg daily doxycycline for 6 weeks (N = 31) or no drug (N = 29) (Figure 3). As shown in Figure 3A–C, there were no significant changes in the levels of VEGF-A, C, or D, or their soluble receptors (Figure 3D–F) at 1 year after therapy, regardless of treatment arm. Similarly, with the exception of Ang-2 (Figure 3H), there were no differences in the levels of other pro-angiogenic factors Ang-1 (Figure 3G), PlGF (Figure 3I), or bFGF (Figure 3J) 1 year after therapy. Interestingly, the levels of Ang-2 were increased in the untreated group compared with the doxycycline-treated group.
Figure 3.
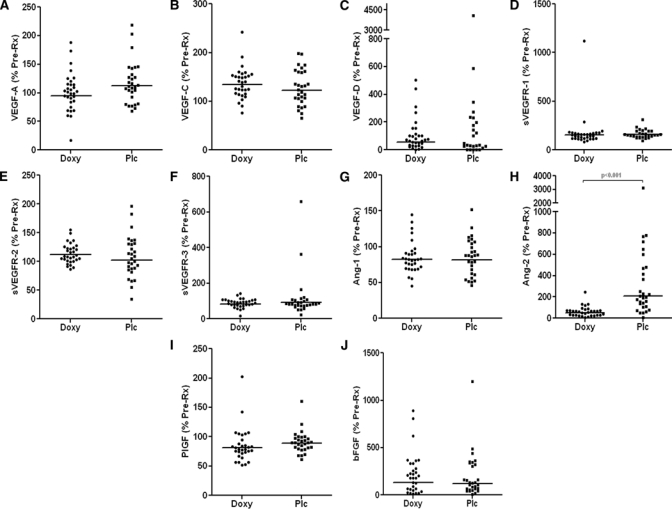
Comparison of plasma levels of lymphangiogenic factors in Wuchereria bancrofti- and Mansonella perstans-infected asymptomatic microfilaremics pre and post doxycycline treatment (1 year). Data are plotted as the percentage pretreatment levels (% Pre-Rx) of the lymphangiogenic factors in the doxycycline-treated group (Doxy; N = 32) and the placebo group (Plc; N = 29).
Interrelationships among the angiogenic factors.
Recently, it has been suggested that the ratios of Ang-1:Ang-2 ratios may reflect changes in stabilization of lymphatic endothelium.11–13 The significantly diminished Ang-1:Ang-2 ratio seen in both the MF (P = 0.002) and CP groups (P = 0.04) (Figure 4A) compared with the EN population may point to alterations in endothelial stability as a mechanism underlying the lymphatic dysfunction seen in LF. Interestingly, doxycycline treatment in patients with LF increased the Ang-1:Ang-2 ratios (P = 0.0014) at 1 year after treatment compared with doxycycline-untreated individuals (P = 0.0004) (Figure 4B–C).
Figure 4.
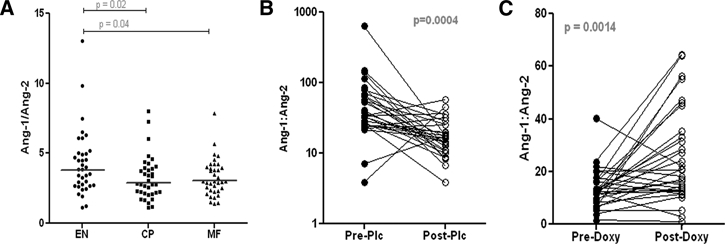
Ang-1:Ang-2 ratios in filarial patients pre-treatment or post-treatment (1year). (A) Wuchereria bancrofti infected endemic normals (EN), microfilaraemics (MF) or chronic lymphedema (CP). (B and C) Ang-1:Ang-2 ratios of pre- and post-treatment in W. bancrofti- and Mansonella perstans-infected microfilaraemic individuals administered (B) no treatment or (C) doxycycline.
Discussion
The role of lymphangiogenesis-promoting factors is an area gaining importance in the study of LF. The results from the current study strongly indicate an active involvement of lymphatic endothelial-acting growth factors in LF. Both VEGF-A14,15 and VEGF-C/sVEGFR-37 levels have been observed to be associated with filarial disease. The improvement in the levels of circulating VEGF-C/sVEGFR3 and amelioration of disease with doxycycline treatment7 suggests that some of the effects may be related to the presence of the endosymbiont Wolbachia. In addition, we have shown previously that filarial antigens and plasma from filaria-infected individuals have the capacity to induce human lymphatic endothelial cells to undergo proliferation and differentiation, a process felt to be mediated by excreted or secreted parasite proteins or lymphangiogenesis promoting factors.8
The VEGFs and angiopoietins are important regulatory growth factors for endothelial function. Elevated levels of plasma VEGF-C have been shown in patients with vascular diseases associated with tumors, lymphedema, lymphangioleiomyomatosis, and inflammatory diseases where the levels of VEGFs have been correlated to measures of endothelial damage/dysfunction (reviewed in Reference 16). Although it is likely that the differences seen in plasma levels of VEGF-A, VEGF-C, VEGF-D, sVEGFR-1, sVEGFR-2, sVEGFR-3, Ang-1, Ang-2, b-FGF, and PlGF, between healthy Europeans (basis for normal ranges in these assays) and the normal population (EN) in this study reflects differences in ethnicity and/or exposure to other (non-filarial) environmental stimuli,7 the fact that the well-defined filarial-infected patients have alterations in many of these factors compared with appropriate controls implicate some of these factors in mediating the lymphatic dysfunction seen in LF. In particular, VEGF-C levels in filaria-infected individuals were significantly elevated compared with uninfected normal individuals from the same endemic region of the world. These elevated levels of VEGF-C correlated closely with levels of VEGF-A, VEGF-D, Ang-1, Ang-2, and bFGF suggesting that VEGF-C is among many factors associated with changes in the lymphatics in filarial infections. Although the elevated levels of VEGFs have been associated with disease7,14 and lymphatic remodeling in LF by adult parasites (alive or dead), it is not clear why some of the angiogenic factors remain elevated in individuals with chronic pathology many of whom are antigen negative.
In terms of pathologic responses, this study seemed to indicate that bFGF was significantly elevated in those with pathology from Wb infection (lymphedema, elephantiasis). The FGF is a multifunctional growth factor that can modulate hematopoiesis,17,18 regulate angiogenesis,19 and stimulate fibroproliferative processes.20,21 The bFGF data from this study—in concert with other angiogenic and mitogenic factors such as VEGF family of proteins, Ang-1, and Ang-2—may provide a link between the processes of lymphangiogenesis and fibrosis, both of which are seen in filaria-associated pathological manifestations.
Expression of VEGF receptors on cells and, more recently, in serum/plasma as soluble receptors have been used as potential biomarkers in tumor biology.22–24 Although both full-length receptors compose an extracellular domain containing seven-immunoglobulin-like loops, a transmembrane domain, and an intracellular catalytic tyrosine kinase domain, sVEGFR-1 is the product of an alternatively spliced messenger RNA (mRNA) composed of only the extracellular domains. In contrast to sVEGFR-1, very little is known about sVEGFR-2. Recent clinical studies have investigated the potential of sVEGFR2 as a surrogate for tumor progression in melanoma,25 myelodysplastic syndromes, or in various leukemias.26 Although there is no information related to the utility of monitoring sVEGFR-2 levels in filarial infections, the inverse relationship between sVEGFR-2 and VEGF-A or VEGF-C is similar to that seen in other conditions.27–29 Similarly, increased levels of VEGF and reduced levels of sVEGFR-2 have been shown with the use of inhibitors of cellular VEGF receptor tyrosine kinases VEGF RTKs, including sunitinib,30 axitinib,31 and telatinib,32 suggesting a receptor downregulation-mediated increase in sVEGFR-2. Together with in vitro studies in which VEGF-induced endocytosis of VEGFR-2, resulting in lower levels of sVEGFR-2,22 it seems possible that sVEGFR-2 levels in the plasma of filaria-infected individuals might influence levels of circulating VEGFs in filarial infections. Alternatively, it is possible that sVEGFR-2, like other soluble receptors such as sVEGFR-1,33 can bind and sequester VEGF in vivo, thereby influencing the binding and activation of the VEGFRs.
Angiopoietins are ligands for Tie-2 receptors on the endothelial cell that are important in maturation and stabilization of the vasculature. Although VEGF initiates endothelial cell growth, proliferation, and differentiation, Ang-1—interacting with its receptor Tie2—results in vessel maturation, maintaining vessel integrity by recruitment of periendothelial cells, reestablishment of basement membrane, and limiting the permeability-inducing effects of VEGF.34–36 Although Ang-2 is known to be an antagonist to Ang-1 activity, it has been shown to behave as an agonist on prolonged exposures and high concentrations; in the presence of elevated levels of VEGF-A, Ang-2 induces vessel formation,37 as well. Thus, the local balance of growth factors can be critical in influencing the microvascular environment toward either vessel stability or angiogenesis. The elevated ratios of Ang-1:Ang-2 in EN compared with MF or CP suggest a possible destabilized nature of the lymphatics in LF. Furthermore, treatment of Wb-infected individuals with doxycycline exhibited a significant increase in Ang-1:Ang-2 ratios, suggesting a change toward more stabilized lymphatics. Whether these changes reflect parasite clearance or a stabilizing effect of doxycycline on lymphatics themselves (as has been recently suggested38) remain an open question.
It is clear from the doxycycline treatment studies that the majority of plasma angiogenic factors did not change significantly 1 year after treatment. Although MF loads (and presumably the Wolbachia load) are reduced post-treatment compared with pre-treatment levels, the levels of circulating antigens (when able to be measured) remained no different (Table 1). The DEC treatment studies (with or without albendazole) have shown antigenemia to be 23–57% of pretreatment levels with wide variations39–43 likely because of the only moderate macrofilaricidal activity of DEC.44–47 Similarly, doxycycline treatment resulted in lower levels of antigenemia at 12 months and 24 months post-treatment compared with pretreatment levels.7,48 Nevertheless, a longitudinal study monitoring levels of circulating filarial antigens in parallel with the pro-angiogenic factors in both the microfilaraemic individuals and those with lymphedema and/or elephantiasis could provide a more definitive answer to this issue.
Although it is appealing to suggest that most of the lymphatic pathology is associated with inflammatory responses against the adult parasites (containing Wolbachia) living in the afferent lymphatics, we show that elevated levels of most lymphangiogenic factors are associated with filarial infection per se with only a few (e.g., bFGF and PIGF) being associated with filarial disease. These data suggest that understanding fully the nature of the excretory/secretory proteins49–51 of the parasite may provide additional insights into those parasite- and Wolbachia-derived molecules that mediate the lymphatic dysfunction seen in LF.
Acknowledgment
We thank NIAID intramural editor Brenda Rae Marshall for assistance.
Footnotes
Authors' addresses: Sasisekhar Bennuru, Grace Maldarelli, Amy D. Klion, and Thomas Nutman, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, E-mails: bennurus@niaid.nih.gov, grace.maldarelli@gmail.com, aklion@niaid.nih.gov, and tnutman@niaid.nih.gov. V. Kumaraswami, Tuberculosis Research Center, ICMR, India, E-mail: kumaraswami@gmail.com.
References
- 1.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 2.Klei TR, Enright FM, Blanchard DP, Uhl SA. Specific hypo-responsive granulomatous tissue reactions in Brugia pahangi-infected birds. Acta Trop. 1981;38:267–276. [PubMed] [Google Scholar]
- 3.Nutman TB, Kumaraswami V, Pao L, Narayanan PR, Ottesen EA. An analysis of in vitro B cell immune responsiveness in human lymphatic filariasis. J Immunol. 1987;138:3954–3959. [PubMed] [Google Scholar]
- 4.Ottesen EA. Immunopathology of lymphatic filariasis in man. Springer Semin Immunopathol. 1980;2:373–385. [Google Scholar]
- 5.Piessens WF, McGreevy PB, Piessens PW, McGreevy M, Koiman I, Saroso JS, Dennis DT. Immune responses in human infections with Brugia malayi: specific cellular unresponsiveness to filarial antigens. J Clin Invest. 1980;65:172–179. doi: 10.1172/JCI109648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ristimaki A, Narko K, Enholm B, Joukov V, Alitalo K. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem. 1998;273:8413–8418. doi: 10.1074/jbc.273.14.8413. [DOI] [PubMed] [Google Scholar]
- 7.Debrah AY, Mand S, Specht S, Marfo-Debrekyei Y, Batsa L, Pfarr K, Larbi J, Lawson B, Taylor M, Adjei O, Hoerauf A. Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PLoS Pathog. 2006;2:e92. doi: 10.1371/journal.ppat.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennuru S, Nutman TB. Lymphangiogenesis and lymphatic remodeling induced by filarial parasites: implications for pathogenesis. PLoS Pathog. 2009;5:e1000688. doi: 10.1371/journal.ppat.1000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopinath R, Hanna LE, Kumaraswami V, Perumal V, Kavitha V, Vijayasekaran V, Nutman TB. Perturbations in eosinophil homeostasis following treatment of lymphatic filariasis. Infect Immun. 2000;68:93–99. doi: 10.1128/iai.68.1.93-99.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulibaly YI, Dembele B, Diallo AA, Lipner EM, Doumbia SS, Coulibaly SY, Konate S, Diallo DA, Yalcouye D, Kubofcik J, Doumbo OK, Traore AK, Keita AD, Fay MP, Traore SF, Nutman TB, Klion AD. A randomized trial of doxycycline for Mansonella perstans infection. N Engl J Med. 2009;361:1448–1458. doi: 10.1056/NEJMoa0900863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 12.Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, Krudsood S, Looareesuwan S, John CC, Liles WC, Kain KC. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS ONE. 2009;4:e4912. doi: 10.1371/journal.pone.0004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shantha Kumara HM, Hoffman A, Kim IY, Feingold D, Dujovny N, Kalady M, Luchtefeld M, Whelan RL. Colorectal resection, both open and laparoscopic-assisted, in patients with benign indications is associated with proangiogenic changes in plasma angiopoietin 1 and 2 levels. Surg Endosc. 2009;23:409–415. doi: 10.1007/s00464-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 14.Debrah AY, Mand S, Toliat MR, Marfo-Debrekyei Y, Batsa L, Nurnberg P, Lawson B, Adjei O, Hoerauf A, Pfarr K. Plasma vascular endothelial growth Factor-A (VEGF-A) and VEGF-A gene polymorphism are associated with hydrocele development in lymphatic filariasis. Am J Trop Med Hyg. 2007;77:601–608. [PubMed] [Google Scholar]
- 15.Esterre P, Plichart C, Huin-Blondey MO, Nguyen LN. Soluble cellular adhesion molecules, selectins, VEGF and endothelin-1 in patients with Wuchereria bancrofti infection and association with clinical status. Parasite Immunol. 2005;27:9–16. doi: 10.1111/j.1365-3024.2005.00732.x. [DOI] [PubMed] [Google Scholar]
- 16.Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 17.Bruno E, Cooper RJ, Wilson EL, Gabrilove JL, Hoffman R. Basic fibroblast growth factor promotes the proliferation of human megakaryocyte progenitor cells. Blood. 1993;82:430–435. [PubMed] [Google Scholar]
- 18.Wilson EL, Rifkin DB, Kelly F, Hannocks MJ, Gabrilove JL. Basic fibroblast growth factor stimulates myelopoiesis in long-term human bone marrow cultures. Blood. 1991;77:954–960. [PubMed] [Google Scholar]
- 19.Mandriota SJ, Pepper MS. Vascular endothelial growth factor-induced in vitro angiogenesis and plasminogen activator expression are dependent on endogenous basic fibroblast growth factor. J Cell Sci. 1997;110:2293–2302. doi: 10.1242/jcs.110.18.2293. [DOI] [PubMed] [Google Scholar]
- 20.Hasebe T, Imoto S, Ogura T, Mukai K. Significance of basic fibroblast growth factor and fibroblast growth factor receptor protein expression in the formation of fibrotic focus in invasive ductal carcinoma of the breast. Jpn J Cancer Res. 1997;88:877–885. doi: 10.1111/j.1349-7006.1997.tb00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohshima K, Sugihara M, Suzumiya J, Haraoka S, Kanda M, Shimazaki K, Katoh K, Kumagawa M, Kikuchi M. Basic fibroblast growth factor and fibrosis in Hodgkin's disease. Pathol Res Pract. 1999;195:149–155. doi: 10.1016/S0344-0338(99)80027-2. [DOI] [PubMed] [Google Scholar]
- 22.Ebos JM, Lee CR, Bogdanovic E, Alami J, Van Slyke P, Francia G, Xu P, Mutsaers AJ, Dumont DJ, Kerbel RS. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008;68:521–529. doi: 10.1158/0008-5472.CAN-07-3217. [DOI] [PubMed] [Google Scholar]
- 23.Herold-Mende C, Steiner HH, Andl T, Riede D, Buttler A, Reisser C, Fusenig NE, Mueller MM. Expression and functional significance of vascular endothelial growth factor receptors in human tumor cells. Lab Invest. 1999;79:1573–1582. [PubMed] [Google Scholar]
- 24.Pallares J, Rojo F, Iriarte J, Morote J, Armadans LI, de Torres I. Study of microvessel density and the expression of the angiogenic factors VEGF, bFGF and the receptors Flt-1 and FLK-1 in benign, premalignant and malignant prostate tissues. Histol Histopathol. 2006;21:857–865. doi: 10.14670/HH-21.857. [DOI] [PubMed] [Google Scholar]
- 25.Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Circulating serum levels of angiogenic factors and vascular endothelial growth factor receptors 1 and 2 in melanoma patients. Melanoma Res. 2006;16:405–411. doi: 10.1097/01.cmr.0000222598.27438.82. [DOI] [PubMed] [Google Scholar]
- 26.Hu Q, Dey AL, Yang Y, Shen Y, Jilani IB, Estey EH, Kantarjian HM, Giles FJ, Albitar M. Soluble vascular endothelial growth factor receptor 1, and not receptor 2, is an independent prognostic factor in acute myeloid leukemia and myelodysplastic syndromes. Cancer. 2004;100:1884–1891. doi: 10.1002/cncr.20187. [DOI] [PubMed] [Google Scholar]
- 27.Faderl S, Do KA, Johnson MM, Keating M, O'Brien S, Jilani I, Ferrajoli A, Ravandi-Kashani F, Aguilar C, Dey A, Thomas DA, Giles FJ, Kantarjian HM, Albitar M. Angiogenic factors may have a different prognostic role in adult acute lymphoblastic leukemia. Blood. 2005;106:4303–4307. doi: 10.1182/blood-2005-03-1010. [DOI] [PubMed] [Google Scholar]
- 28.Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, Kalayanarooj S, Libraty DH, Green S, Ennis FA, Rothman AL. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic fever. J Virol. 2007;81:1592–1600. doi: 10.1128/JVI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng JJ, Chou MM, Hsieh YT, Wen MC, Ho ES, Hsu SL. Differential expression of vascular endothelial growth factor, placenta growth factor and their receptors in placentae from pregnancies complicated by placenta accreta. Placenta. 2006;27:70–78. doi: 10.1016/j.placenta.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Deprimo SE, Bello CL, Smeraglia J, Baum CM, Spinella D, Rini BI, Michaelson MD, Motzer RJ. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–4713. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strumberg D, Schultheis B, Adamietz IA, Christensen O, Buechert M, Kraetzschmar J, Rajagopalan P, Ludwig M, Frost A, Steinbild S, Scheulen ME, Mross K. Phase I dose escalation study of telatinib (BAY 57-9352) in patients with advanced solid tumours. Br J Cancer. 2008;99:1579–1585. doi: 10.1038/sj.bjc.6604724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 35.Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999;79:213–223. [PubMed] [Google Scholar]
- 36.Pizurki L, Zhou Z, Glynos K, Roussos C, Papapetropoulos A. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br J Pharmacol. 2003;139:329–336. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 38.Fainaru O, Adini I, Benny O, Bazinet L, Pravda E, D'Amato R, Folkman J. Doxycycline induces membrane expression of VE-cadherin on endothelial cells and prevents vascular hyperpermeability. FASEB J. 2008;22:3728–3735. doi: 10.1096/fj.08-110494. [DOI] [PubMed] [Google Scholar]
- 39.Eberhard ML, Hightower AW, Addiss DG, Lammie PJ. Clearance of Wuchereria bancrofti antigen after treatment with diethylcarbamazine or ivermectin. Am J Trop Med Hyg. 1997;57:483–486. doi: 10.4269/ajtmh.1997.57.483. [DOI] [PubMed] [Google Scholar]
- 40.Ismail MM, Jayakody RL, Weil GJ, Nirmalan N, Jayasinghe KS, Abeyewickrema W, Rezvi Sheriff MH, Rajaratnam HN, Amarasekera N, de Silva DC, Michalski ML, Dissanaike AS. Efficacy of single dose combinations of albendazole, ivermectin and diethylcarbamazine for the treatment of bancroftian filariasis. Trans R Soc Trop Med Hyg. 1998;92:94–97. doi: 10.1016/s0035-9203(98)90972-5. [DOI] [PubMed] [Google Scholar]
- 41.Nicolas L, Plichart C, Nguyen LN, Moulia-Pelat JP. Reduction of Wuchereria bancrofti adult worm circulating antigen after annual treatments of diethylcarbamazine combined with ivermectin in French Polynesia. J Infect Dis. 1997;175:489–492. doi: 10.1093/infdis/175.2.489. [DOI] [PubMed] [Google Scholar]
- 42.Weil GJ, Ramzy RM, El Setouhy M, Kandil AM, Ahmed ES, Faris R. A longitudinal study of Bancroftian filariasis in the Nile Delta of Egypt: baseline data and one-year follow-up. Am J Trop Med Hyg. 1999;61:53–58. doi: 10.4269/ajtmh.1999.61.53. [DOI] [PubMed] [Google Scholar]
- 43.Weil GJ, Sethumadhavan KV, Santhanam S, Jain DC, Ghosh TK. Persistence of parasite antigenemia following diethylcarbamazine therapy of bancroftian filariasis. Am J Trop Med Hyg. 1988;38:589–595. doi: 10.4269/ajtmh.1988.38.589. [DOI] [PubMed] [Google Scholar]
- 44.Dreyer G, Addiss D, Williamson J, Noroes J. Efficacy of co-administered diethylcarbamazine and albendazole against adult Wuchereria bancrofti. Trans R Soc Trop Med Hyg. 2006;100:1118–1125. doi: 10.1016/j.trstmh.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Hussein O, El Setouhy M, Ahmed ES, Kandil AM, Ramzy RM, Helmy H, Weil GJ. Duplex Doppler sonographic assessment of the effects of diethylcarbamazine and albendazole therapy on adult filarial worms and adjacent host tissues in Bancroftian filariasis. Am J Trop Med Hyg. 2004;71:471–477. [PubMed] [Google Scholar]
- 46.Noroes J, Dreyer G, Santos A, Mendes VG, Medeiros Z, Addiss D. Assessment of the efficacy of diethylcarbamazine on adult Wuchereria bancrofti in vivo. Trans R Soc Trop Med Hyg. 1997;91:78–81. doi: 10.1016/s0035-9203(97)90405-3. [DOI] [PubMed] [Google Scholar]
- 47.Ottesen EA, Duke BO, Karam M, Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- 48.Debrah AY, Mand S, Marfo-Debrekyei Y, Batsa L, Pfarr K, Buttner M, Adjei O, Buttner D, Hoerauf A. Macrofilaricidal effect of 4 weeks of treatment with doxycycline on Wuchereria bancrofti. Trop Med Int Health. 2007;12:1433–1441. doi: 10.1111/j.1365-3156.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 49.Bennuru S, Semnani R, Meng Z, Ribeiro JM, Veenstra TD, Nutman TB. Brugia malayi excreted/secreted proteins at the host/parasite interface: stage- and gender-specific proteomic profiling. PLoS Negl Trop Dis. 2009;3:e410. doi: 10.1371/journal.pntd.0000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hewitson JP, Harcus YM, Curwen RS, Dowle AA, Atmadja AK, Ashton PD, Wilson A, Maizels RM. The secretome of the filarial parasite, Brugia malayi: proteomic profile of adult excretory-secretory products. Mol Biochem Parasitol. 2008;160:8–21. doi: 10.1016/j.molbiopara.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Moreno Y, Geary TG. Stage- and gender-specific proteomic analysis of Brugia malayi excretory-secretory products. PLoS Negl Trop Dis. 2008;2:e326. doi: 10.1371/journal.pntd.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]


