Abstract
Multiplex real-time polymerase chain reaction (PCR) was developed for differential detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii. Specific primers were designed for all three species, and then differentiation of E. histolytica and E. dispar was achieved simultaneously using a hybridization probe and melting curve analysis, whereas E. moshkovskii was detected with a separate probe under the same condition. This assay detected as little as 0.2 pg of E. histolytica DNA and 2 pg each for E. dispar and E. moshkovskii DNA. Thirty-five clinical samples suspected to be E. histolytica infection by microscopy were tested. The results showed 32 positive samples; four samples were E. histolytica and 28 samples were E. dispar. Interestingly, one E. dispar positive sample showed a mixed infection with E. moshkovskii. This is the first report of E. moshkovskii infection from Thailand and this assay is currently the most rapid and sensitive method to differentiate these human amoebas.
Introduction
Traditionally, the laboratory detection of Entamoeba species in human feces has relied on the microscopic examination of fresh or fixed stool samples.1 This procedure is cheap and simple, but it has several limitations, such as being incapable of distinguishing between the cysts and trophozoites of the disease-causing species Entamoeba histolytica, the nonpathogenic Entamoeba dispar, and the free-living amoeba Entamoeba moshkovskii. A high prevalence and association of E. moshkovskii with E. histolytica and E. dispar has been reported in young children from Bangladesh2,3 and E. moshkovskii was recently detected in patients between 31 and 50 years of age presenting with gastrointestinal symptoms in Australia4; therefore, humans may be a true host for this amoeba. This observation has made the differentiation of the three species in clinical samples of great importance, both for diagnosis and for epidemiological studies.
A number of protein and DNA detection systems have been developed in recent years to distinguish E. histolytica from E. dispar in clinical specimens.5–9 Nevertheless, polymerase chain reaction (PCR) is still the method of choice for clinical and epidemiological studies of amoebiasis in the developed countries and has been strongly endorsed by the World Health Organization (WHO).10 Most PCR assays for differential detection of E. histolytica and E. dispar target either the small-subunit ribosomal RNA (rRNA) (18S rRNA) gene8 or species-specific episomal repeats.11 The sensitivity and specificity of PCR assays exceed what can be accomplished with microscopy and are comparable to those of the antigen test.6,8
However, most studies that have investigated the prevalence of E. histolytica and E. dispar have not considered the possible presence of E. moshkovskii, due partly to a lack of tools to detect E. moshkovskii other than cultivation,12 which is labor-intensive, not always successful, and problematic in the case of mixed infections. Recently, conventional PCR assays for E. moshkovskii in clinical diagnosis of amoebiasis have been performed3,4,13,14 but most of the protocols reported so far require further processing of the amplicon, which is time-consuming and prone to false-positive results caused by possible cross-contamination.
Real-time PCR allows specific detection of the amplicon by using fluorescence-labeled probes with continuous monitoring of PCR product formation so that post-PCR processing is not needed.15,16 This method reduces the time required and minimizes the risk of contamination from the environment, and the closed reaction tube should also prevent cross-contamination. To date, a few real-time PCR assays for specific detection of E. histolytica had been published and evaluated.17–19 Stool real-time PCR is a highly sensitive and specific technique for this infection compared with serological and microscopic methods.20 However, a real-time PCR assay for detection of E. moshkovskii has not yet been developed and it is very important to differentiate all three species in clinical samples to avoid unnecessary treatment of E. dispar- or E. moshkovskii-infected patients with antiamoebic drugs.
Therefore, a real-time PCR assay for sensitive and rapid detection and differentiation of E. moshkovskii, E. histolytica, and E. dispar directly from clinical specimens was developed and evaluated for its high sensitivity and specificity.
Materials and Methods
DNA samples.
In this study genomic DNAs from E. histolytica HM-1:IMSS, E. dispar SAW 760, and E. moshkovskii Laredo cells grown in axenic culture were used as positive controls, and were provided by Dr. Graham Clark of the London School of Hygiene and Tropical Medicine.
Clinical samples.
Thirty-five clinical samples, including both 33 fecal and two liver aspirate specimens, were collected from individuals who sought medical attention for different reasons at the Phramongkutklao hospital in Bangkok, Thailand, and used to evaluate the assay developed. All suspected of E. histolytica, positive samples are fresh samples collected from patients, and wet mounts were prepared for examination of E. histolytica cyst or trophozoite by microscopy. Entamoeba histolytica cysts in stool samples were determined on the basis of their shape and size and the number of nuclei observed. The DNA was extracted directly from fresh samples using the QIAamp Stool DNA Extraction Kit (Qiagen, Hilden, Germany) and then stored at −20°C until used.
Design of primers and probes.
The primers and probes were designed on the basis of an alignment of E. histolytica, E. dispar, and E. moshkovskii small-subunit rRNA gene sequences (GenBank accession nos. X64142, Z49256, and AF149906, respectively). All primers and probes were designed and constructed in cooperation with TIB MOLBIOL (Berlin, Germany).
The forward (EhdmF: 5′-CgA AAg CAT TTC ACT CAA CTg-3′) and reverse (EhdmR: 5′-TCC CCC TgA AgT CCA TAA ACTC-3′) primers are conserved in the three Entamoeba species' SSU rRNA sequences and produce a product 222 bp in length. Three hybridization probes, which include one universal fluorescein labeled probe (Ehdm-FL: 5′-ACT ATA AAC gAT gTC AAC CAA ggA TTg gAT gAAA-FITC-3′) and two different LCRed labeled probes (Ehd-640: 5′-TCA gAT gTA CAA AgA TAg AgA AgC ATT gTT TCTA-phosphate-3′and Em-705: 5′-AAg AAA TTC gCg gAT gAA gAA ACA TTg TTT-phosphate-3′), were specifically designed to bind internal to the amplification primers to identify and differentiate E. histolytica, E. dispar, and E. moshkovskii from other organisms.
Entamoeba moshkovskii is identified and differentiated from E. histolytica/E. dispar by Em-705 probe binding, monitored in channel 3 (F3/F1). In contrast, E. histolytica and E. dispar are both detected by the Ehd-640 labeled probe monitored in channel 2 (F2/F1) and are differentiated by melting curve analysis (−d (F2/F1)/dT). All primers, probes, and reaction conditions were optimized accordingly to a standard protocol described for the LightCycler System (Roche Molecular Biochemicals, Mannheim, Germany) in cooperation with TIB MOLBIOL.
Real-time PCR assay.
Real-time PCR was performed using the LightCycler System (Roche Molecular Biochemicals). Basic reagents for the LightCycler PCR were purchased from Roche Diagnostics. Real-time hot-start PCR was performed with LightCycler FastStart DNA Master Hybridization Probes Kit in a LightCycler Instrument (Roche Diagnostics).
Reaction conditions for the assay were chosen according to a standard LightCycler protocol in our laboratories. This assay was performed in a single tube and the reaction mixture contained 4 μL of LightCycler FastStart DNA MasterPLUS HybProbe, 1 μL (0.5 μM) of each PCR primer, 2 μL (0.2 μM) of each hybridization probe, 9 μL of H2O, and 1 μL of DNA extract. The amplification program included an initial denaturation step at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 10 s, annealing at 50°C for 10 s, and extension at 72°C for 10 s. The temperature transition rate was 20°C/s except for the annealing step, where the rate was 5°C/s. Fluorescence was measured at the end of each extension step.
A melting curve analysis was performed after the last amplification cycle by heating the product at 20°C/s to 95°C, cooling it at 20°C/s to 45°C, keeping it at 45°C for 30 s, and then slowly heating it at 0.1°C/s to 85°C. Fluorescence was measured through the slow heating phase. Melting curve analysis was used to identify the specific PCR products of E. histolytica and E. dispar. A sample was regarded as positive when the LightCycler software (version 5.32) determined a crossing point in the quantification analysis screen. The PCR product produced a characteristic melting curve with a discernible peak in the melting curve at 59.5 to 60.8°C (Tm average = 60.00 ± 0.53°C) for E. histolytica and at 57.2 to 57.5°C (Tm average = 57.3 ± 0.1°C) for E. dispar. Amplification was also confirmed in all reactions by gel electrophoresis.
Sensitivity and specificity of real-time PCR.
The sensitivity of the real-time PCR assay was determined by 10-fold serial dilution of genomic DNA of each Entamoeba species from 2 ng to 0.2 pg using the protocols described previously. The specificity of the real-time PCR assay was tested against several different control samples, which included DNA from humans, and several protozoa and pathogenic bacteria known to infect humans: Escherichia coli, Salmonella spp., Shigella spp., Vibrio cholerae, Blastocystis hominis, Giardia lamblia, and Cryptosporidium spp. The DNA extracted from a parasite-free fecal sample was used as a negative control. A mixture of DNA template from E. histolytica, E. dispar, and E. moshkovskii was also tested for the presence of any cross-reaction or cross-amplification between the three Entamoeba species.
Results
Sensitivity and specificity of the real-time PCR assay.
Amplification was specific when all primers and probes were mixed together in a single reaction and produced a 222 bp fragment from the control genomic DNA prepared from E. histolytcia, E. dispar, and E. moshkovskii. No interference or cross-hybridization was observed between each of the hybridization probes (Ehd-640 for E. histolytica and E. dispar and Em-705 for E. moshkovskii) and the 222 bp product amplified from each DNA. The probes specifically detect each of the target PCR products, giving an increased fluorescence signal as the PCR progressed. Detection and differentiation of E. histolytica and E. dispar was achieved by melting curve analysis. Melting curve analysis showed that the specific PCR products for E. histolytica or E. dispar produced a characteristic melting curve with a discernible peak at 59.5 to 60.80°C for E. histolytica and 57.2 to 57.5°C for E. dispar (Figure 1A).
Figure 1.
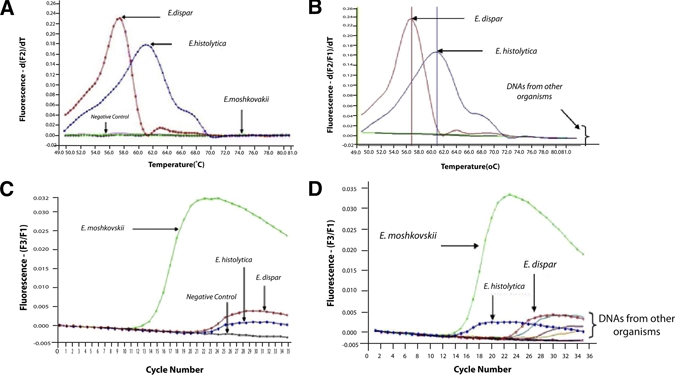
(A and C) Amplification in real-time mode for real-time polymerase chain reaction (PCR) and melting curves generated by LightCycler software and differentiation of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii. (B and D) Specificity of the assay was demonstrated by using human DNA and DNA extracted from pure or axenic cultures of several protozoa and pathogenic bacteria known to infect humans, including Entamoeba coli, Salmonella spp., Shigella spp., Vibrio cholerae, Blastocystis hominis, Giardia lamblia, and Cryptosporidium spp. This figure appears in color at www.ajtmh.org.
The specificity of the real-time PCR was evaluated using each of the control DNA and also on DNA extracted from a variety of sources. No cross-amplification or fluorescence signal was observed when the assay was tested against human DNA or against any genomic or infected stool DNA sample with different bacterial or protozoan pathogens (Figure 1B). For detection of E. moshkovskii no melting curve analysis was needed as the presence of these amoebae can be detected and differentiated from other Entamoeba by the Em-705 hybridization probe alone (Figure 1C). Melting curve analysis permitted the clear identification of E. histolytica and E. dispar as shown in Figure 1A. The Tm values for the melting curves were highly reproducible in three replicates. The specificity of E. moshkovskii assay using the Em-705 hybridization probe is shown in Figure 1D.
The sensitivity of the assay was determined using a series of 10-fold serially diluted genomic DNA samples containing known concentrations of each control DNA. The results show that the assay is able to detect as little as 0.2 pg for E. histolytica (Figure 2A) and 2 pg each for both E. dispar (Figure 2B) and E. moshkovskii DNA (Figure 2C). This minimum detection level suggests that it is very sensitive for detection of each amoeba species. In the specificity test of E. moshkovskii, DNA of other organisms could produce some noise lower than 0.005 of fluorescence (Figure 1D), however, it was not significant because it was certainly below the detectable level of this test (2 pg) as shown in Figure 2C.
Figure 2.
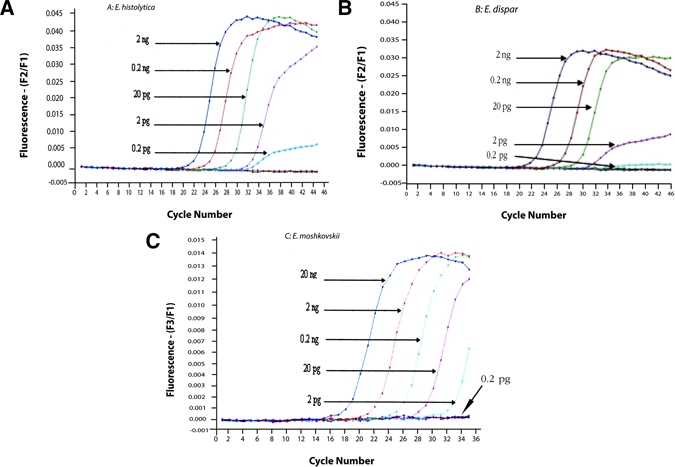
Detection limit of real-time polymerase chain reaction (PCR) assay for (A) Escherichia histolytica, (B) Entamoeba dispar, and (C) Entamoeba moshkovskii. Ten-fold serial dilution of DNA consists of 1:10 = 2 ng; 1:100 = 0.2 ng; 1:1000 = 20 pg; 1:10 000 = 2 pg; 1:100 000 = 0.2 pg. This figure appears in color at www.ajtmh.org.
Evaluation of the real-time PCR assay on fecal samples from Thai patients.
A total of 35 clinical samples were used for evaluation of the real-time PCR assay developed in this study. Melting curve analysis showed that four samples produced the characteristic melting curves that were specific for E. histolytica, whereas the other 28 samples produced a discernible peak that is specific for E. dispar. In addition, one specimen, which was positive for E. dispar, was also found to be positive for E. moshkovskii using the Em-705 hybridization probe, whereas the other 34 samples produced no fluorescein signals for the presence of E. moshkovskii.
This is the first report of the existence of E. moshkovskii, and its coexistence with E. dispar, in fecal sample in the Thai population. The other three negative samples by real-time PCR assay showed positive bands when tested by using the genus-specific PCR assay.18 Microscopic examination showed that these three samples were positive for Entamoeba coli however a smaller size of a few cysts were observed. On the basis of the real-time PCR, these three samples were not mixed infections with E. histolytica or E. dispar or E. moshkovskii as suspected.
Discussion
To date, a few real-time PCR assays for the specific detection of E. histolytica have been published17,21,22 however none of them is designed for E. moshkovskii infection. In this study, we describe the development of a real-time PCR for differential diagnosis of the three species of Entamoeba that share identical morphology in clinical specimens, as both cysts and trophozoites. This technique requires only one PCR step, compared with at least two steps in the traditional nested PCR,3 it is performed in a closed system where post-PCR handling is not required, and it is highly sensitive and could be used for quantitative purposes.
The assay successfully amplified positive controls from E. histolytica- and E. dispar-infected individuals that had been confirmed by other PCR assays.5,13 We also showed that the assay is the most sensitive method for differential detection of E. histolytica, E. dispar, and E. moshkovskii because it is able to detect as little as 0.2 pg for E. histolytica and 2 pg each for both E. dispar DNA and E. moshkovskii, whereas a single round PCR assay can detect 9.5 pg of E. dispar and 19 pg of E. histolytica or E. moshkovskii.12 Comparing our assay with conventional PCR assays strongly suggests that its sensitivity, specificity, and reliability are more than sufficient for accurate identification of three species in clinical specimens.
In the melting curve analysis for differentiation of E. histolytica and E. dispar, we observed a slight shift to a higher Tm (up to 1.4°C for E. histolytica and 0.5°C for E. dispar) in the amplicons generated by the control genomic DNA compared with the other specimens tested. The cause for this shift is currently unknown, although we assume sequence differences in the genomic DNA or variable salt concentrations in the DNA aliquots are responsible. Therefore, taking into consideration the Tm values for the genomic control DNA and 10 selected patient samples, we propose two broad non-overlapping Tm ranges to identify the E. histolytica and E. dispar in any blinded patient specimens using real-time PCR. Specimens are regarded as positive when PCR products produce a characteristic melting curve temperature (Tm) with a discernible peak at 59.5 to 60.8°C (Tm average = 60.0 ± 0.5°C) for E. histolytica and 57.2 to 57.5°C (Tm average = 57.3 ± 0.1°C) for E. dispar.
We showed that this real-time PCR assay was capable of detecting nearly all of (32/35) the suspected E. histolytica cases and showed that most of them were actually positive for E. dispar, four cases of E. histolytica, and only one case of E. moshkovskii. This shows that our real-time PCR is highly sensitive, capable of detecting target DNA at a copy number that the conventional PCR unable to detect.
On the basis of our real-time PCR assay, the number of E. dispar positive cases found in stool samples is about 14 times higher than E. histolytica because there were only two positive stool samples and two positive liver abscess samples for E. histolytica, but 28 stool samples were positive for E. dispar. This result clearly indicates that the method used in diagnosis of amoebiasis could significantly affect estimates of the actual number of Entamoeba infections and this study in Thailand supports that E. dispar infection is, in general, much more common than E. histolytica worldwide.23–25
Interestingly, our real-time PCR assay was able to identify for the first time an E. moshkovskii infection in Thailand and it is a mixed infection with E. dispar. The coexistance of E. moshkovskii with E. dispar in stool sample has also been reported in Bangladesh using another PCR assay.3 Because this is the first study ever conducted in Thailand to detect the presence of E. moshkovskii, it is not possible to estimate its prevalence. Therefore, more samples need to be studied before the prevalence of E. moshkovskii in the Thai population can be clearly determined. Certainly, it will be useful to include the E. moshkovskii set of primers in any future epidemiological studies in Thailand. The three samples previously suspected as mixed infection cases of Entamoeba coli with E. histolytica by microscopy and positive by genus specific but negative by real-time PCR were confirmed that they were E. coli infections. Therefore, further development of molecular diagnosis for detection of other nonpathogenic Entamoeba species commonly found in humans, such as E. coli and E. hartmanni, will lead to specific identification and provide the true prevalence of these amoebae in epidemiological studies.
In conclusion, because of the excellent specificity and sensitivity of the real-time PCR assay developed in this study, we propose its application as an alternative tool in routine diagnosis and in epidemiological studies of amoebiasis. This method will provide more accurate epidemiological data and a greater understanding of infections with these three amoebae in humans.
Acknowledgments
We thank Kanthanit Thima and Somsri Kajorndechakiate, Department of Protozoology, Faculty of Tropical Medicine, Mahidol University for their technical assistance in this study, and Graham Clark (London School of Hygiene and Tropical Medicine) for providing the control DNA.
Footnotes
Financial support: This research grant was supported by Mahidol University, Bangkok, Thailand.
Authors' address: Zulhainan Hamzah, Ipoh Public Health Laboratory, Ministry of Health Malaysia, Perak, Malaysia, E-mail: zulhainan@hotmail.com. Songsak Petmitr, Department of Tropical Nutrition and Food Science, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mail: tmspm@mahidol.ac.th. Mathirut Mungthin and Saovanee Leelayoova, Department of Parasitology, Phramongkutklao College of Medicine, Bangkok, Thailand, E-mails: mathirut@hotmail.com and s_leelayoova@scientist.com. Porntip Chavalitshewinkoon-Petmitr, Department of Protozoology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mail: tmppm@mahidol.ac.th.
References
- 1.Gonzalez-Ruiz A, Haque R, Aguirre A, Castanon G, Hall A, Guhl F, Ruiz-Palacios G, Miles MA, Warhurst DC. Value of microscopy in the diagnosis of dysentery associated with invasive Entamoeba histolytica. J Clin Pathol. 1994;47:236–239. doi: 10.1136/jcp.47.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haque R, Ali IK, Clark CG, Petri WA., Jr A case report of Entamoeba moshkovskii infection in a Bangladeshi child. Parasitol Int. 1998;47:201–202. [Google Scholar]
- 3.Ali IK, Hossain MB, Roy S, Ayeh-Kumi PF, Petri WA, Jr, Haque R, Clark CG. Entamoeba moshkovskii infections in children, Bangladesh. Emerg Infect Dis. 2003;9:580–584. doi: 10.3201/eid0905.020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. PCR detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii in stool samples from Sydney, Australia. J Clin Microbiol. 2007;45:1035–1037. doi: 10.1128/JCM.02144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tachibana H, Kobayashi S, Takekoshi M, Ihara S. Distinguishing pathogenic isolates of Entamoeba histolytica by polymerase chain reaction. J Infect Dis. 1991;164:825–826. doi: 10.1093/infdis/164.4.825. [DOI] [PubMed] [Google Scholar]
- 6.Mirelman D, Nuchamowitz Y, Stolarsky T. Comparison of use of enzyme-linked immunosorbent assay-based kits and PCR amplification of rRNA genes for simultaneous detection of Entamoeba histolytica and E. dispar. J Clin Microbiol. 1997;35:2405–2407. doi: 10.1128/jcm.35.9.2405-2407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque R, Ali IK, Akther S, Petri WA., Jr Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J Clin Microbiol. 1998;36:449–452. doi: 10.1128/jcm.36.2.449-452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evangelopoulos A, Spanakos G, Patsoula E, Vakalis N, Legakis N. A nested, multiplex, PCR assay for the simultaneous detection and differentiation of Entamoeba histolytica and Entamoeba dispar in faeces. Ann Trop Med Parasitol. 2000;94:233–240. doi: 10.1080/00034980050006401. [DOI] [PubMed] [Google Scholar]
- 9.Nunez YO, Fernandez MA, Torres-Nunez D, Silva JA, Montano I, Maestre JL, Fonte L. Multiplex polymerase chain reaction amplification and differentiation of Entamoeba histolytica and Entamoeba dispar DNA from stool samples. Am J Trop Med Hyg. 2001;64:293–297. doi: 10.4269/ajtmh.2001.64.293. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Amebiasis. WHO Wkly Epidemiol Rec. 1997;72:97–100. [Google Scholar]
- 11.Clark CG, Diamond LS. The Laredo strain and other Entamoeba histolytica-like amoebae are Entamoeba moshkovskii. Mol Biochem Parasitol. 1991;46:11–18. doi: 10.1016/0166-6851(91)90194-b. [DOI] [PubMed] [Google Scholar]
- 12.Hamzah Z, Petmitr S, Mungthin M, Leelayoova S, Chavalitshewinkoon-Petmitr P. Differential detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii by a single-round PCR assay. J Clin Microbiol. 2006;44:3196–3200. doi: 10.1128/JCM.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khairnar K, Parija SC. A novel nested multiplex polymerase chain reaction (PCR) assay for differential detection of Entamoeba histolytica, E. moshkovskii and E. dispar DNA in stool samples. BMC Microbiol. 2007;7:47. doi: 10.1186/1471-2180-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wittwer CT, Herrmann MG, Moss AA, Rasmussen RP. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques. 1997;22:130–138. doi: 10.2144/97221bi01. [DOI] [PubMed] [Google Scholar]
- 15.Wittwer CT, Ririe KM, Andrew RV, David DA, Gundry RA, Balis UJ. The LightCycler: a microvolume multisample fluorimeter with rapid temperature control. Biotechniques. 1997;22:176–181. doi: 10.2144/97221pf02. [DOI] [PubMed] [Google Scholar]
- 16.Blessmann J, Buss H, Nu PA, Dinh BT, Ngo QT, Van AL, Alla MD, Jackson TF, Ravdin JI, Tannich E. Real-time PCR for detection and differentiation of Entamoeba histolytica and Entamoeba dispar in fecal samples. J Clin Microbiol. 2002;40:4413–4417. doi: 10.1128/JCM.40.12.4413-4417.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qvarnstrom Y, James C, Xayavong M, Holloway BP, Visvesvara GS, Sriram R, Da Silva AJ. Comparison of real-time PCR protocols for differential laboratory diagnosis of amebiasis. J Clin Microbiol. 2005;43:5491–5497. doi: 10.1128/JCM.43.11.5491-5497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verweij JJ, Polderman AM, Clark GC. Genetic variation among human isolates of uninucleated cyst-producing Entamoeba species. J Clin Microbiol. 2001;39:1644–1646. doi: 10.1128/JCM.39.4.1644-1646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy S, Kabir M, Mondal D, Petri WA, Jr, Haque R. Real-time PCR assay for the diagnosis of Entamoeba histolytica infection. J Clin Microbiol. 2005;43:2168–2172. doi: 10.1128/JCM.43.5.2168-2172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A, Houpt E, Petri WA. Rapid diagnosis of intestinal parasitic protozoa, with a focus on Entamoeba histolytica. Interdiscip Perspect Infect Dis 547090. 2009. Epub 2009 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verweij JJ, Vermeer J, Brienen EA, Blotkamp C, Laeijendecker D, van Lieshout L, Polderman AM. Entamoeba histolytica infections in captive primates. Parasitol Res. 2003;90:100–103. doi: 10.1007/s00436-002-0808-z. [DOI] [PubMed] [Google Scholar]
- 22.Verweij JJ, Blange RA, Templeton K, Schinkel J, Brienen EA, van Rooyen MA, van Lieshout L, Polderman AM. Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J Clin Microbiol. 2004;42:1220–1223. doi: 10.1128/JCM.42.3.1220-1223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leiva B, Lebbad M, Winiecka-Krusnell J, Altamirano I, Tellez A, Linder E. Overdiagnosis of Entamoeba histolytica and Entamoeba dispar in Nicaragua: a microscopic, triage parasite panel and PCR study. Arch Med Res. 2006;37:529–534. doi: 10.1016/j.arcmed.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Pillai DR, Keystone JS, Sheppard DC, MacLean JD, MacPherson DW, Kain KC. Entamoeba histolytica and Entamoeba dispar: epidemiology and comparison of diagnostic methods in a setting of nonendemicity. Clin Infect Dis. 1999;29:1315–1318. doi: 10.1086/313433. [DOI] [PubMed] [Google Scholar]
- 25.Petri WA, Jr, Haque R, Lyerly D, Vines RR. Estimating the impact of amebiasis on health. Parasitol Today. 2000;16:320–321. doi: 10.1016/s0169-4758(00)01730-0. [DOI] [PubMed] [Google Scholar]


