Abstract
Neurognathostomiasis is a rare but severe form of human gnathostomiasis. Diagnosis of neurognathostomiasis is made presumably by using clinical manifestations. Serologic tests for neurognathostomiasis are not widely available and limited. We studied 12 patients with diagnoses of neurognathostomiasis at Srinagarind Hospital, Khon Kaen University, Thailand. There were three types of neurognathostomiasis (five patients with intracerebral hemorrhage, one patient with subarachnoid hemorrhage, and six patients with myelitis). All patients were tested for antibodies against Gnathostoma spinigerum by an immunoblotting technique. The sensitivity and specificity of the 21-kD and 24-kD diagnostic bands were 83.3% and 100%, and 91.7% and 100%, respectively. The sensitivity, specificity, positive predictive value, and negative predictive value for the 21-kD and 24-kD diagnostic bands were all 100%. Both diagnostic bands are a helpful diagnostic tool for neuro gnathostomiasis and show good diagnostic properties.
Gnathostomiasis, which is caused by Gnathostoma spinigerum, is commonly found in Japan, Thailand, and Mexico.1,2 It also has been reported worldwide. The most common manifestation is the cutaneous form. Neurologic complications of gnathostomiasis are quite rare but result in high morbidity and mortality. The diagnosis of neurognathostomiasis is made presumptively and clinically. However, chances of recovering the worm are extremely rare. The most useful diagnostic tool is a serologic test, such as immunoblotting against G. spinigerum antigen. In addition, there are several diagnostic bands available for the diagnosis of cutaneous or ocular gnathostomiasis. However, serologic tests for neurognathostomiasis are still limited. Therefore, we studied the use of immunoblotting for diagnosis of neurognathostomiasis.
We prospectively enrolled patients who were suspected of having neurognathostomiasis at Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, Thailand, during 2003–2007. A presumptive diagnosis of neurognathostomiasis used clinical criteria adapted from a previous report.3 The major criteria were the evidence of possible exposure to G. spinigerum larvae by eating raw meat from a disease-endemic area and acute-onset neurologic symptoms. The minor criteria were migratory swelling, radicular pain, blood eosinophilia > 500 cells/mm3, eosinophils in cerebrospinal fluid, or suggestive results by neuroimaging. A presumptive diagnosis of neurognathostomiasis was made when a person satisfied both major criteria and one of the minor criteria.
History of exposure was ingestion of intermediate or transport hosts, including chicken, fish, or freshwater shrimp. There are three types of suggestive neurologic symptoms: unexplained intracranial bleeding, unexplained subarachnoid hemorrhage, and myelitis with eosinophilia. Unexplained intracranial bleeding was defined as hemorrhage at uncommon sites with hypertensive hemorrhage, hemorrhage suggestive for gnathostomiasis by radiographic findings such as linear or tract-like hemorrhage, or multiple bleeding sites without blood dyscrasia. Unexplained subarachnoid hemorrhage was defined as subarachnoid hemorrhage with negative results for intracranial aneurysm or arteriovenous malformation by cerebral angiogram.4–10 In addition, all patients were required to have negative serologic results for Angiostrongylus cantonensis (rodent lungworm), Paragonimus heterotremus (lung fluke), Fasciola gigantica (cattle liver fluke), and Cysticercus cellulosae (pork tapeworm cysticerci).
Immunoblotting analysis for detection of specific IgG against antigenic components of G. spinigerum was performed at the time of hospitalization as described below.11,12 The same method was also used to test 15 healthy adults (controls) from Thailand.
Pooled serum samples from persons with proven cutaneous gnathostomiasis and healthy controls were used as controls of this study. Briefly, G. spinigerum advanced third-stage larval protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrotransfer blotting onto nitrocellulose membrane strips. Antigen-blotted nitrocellulose strips were blocked with optimum dilutions of skimmed milk, probed with each serum sample, and tested with peroxidase-conjugated monoclonal antibody to human IgG. Strips were washed and incubated with diaminobenzidine sulfate until a dark brown band appeared. Control pooled negative and positive serum samples were also tested under the same conditions. Identical patterns were observed as in a previous report.12
Twelve patients were enrolled in our study: 5 patients with intracranial bleeding, 1 patient with subarachnoid hemorrhage, and 6 patients with myelitis and eosinophila. Eight of the 12 patients were male. The mean age (SD) of the patients was 41.9 (13.0) years. Five (50%) of 10 patients had migratory swelling, 7 (70%) of 10 had radicular pain, 8 (80%) of 10 had blood eosinophilia, 10 (91%) of 11 had eosinophils in cerebrospinal fluid, and 10 (83%) of 12 had suggestive neuroimaging results (Table 1).
Table 1.
Clinical variables and results of immunoblotting test for Gnathostoma spinigerum antibody for 12 patients*
| Variable | Patient | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Age, years | 45 | 50 | 24 | 64 | 46 | 26 | 35 | 62 | 36 | 32 | 49 | 34 |
| Sex | M | F | M | F | F | M | M | M | F | M | M | M |
| Weakness type | C | C | C | C | C | C | S | S | S | S | S | S |
| Eating raw meat | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Migration swelling | Y | N | NA | N | Y | N | Y | N | Y | Y | N | NA |
| Radicular pain | Y | N | NA | N | Y | N | Y | Y | Y | NA | Y | Y |
| Blood eosinophilia > 500/mm3 | Y | N | NA | Y | Y | Y | Y | N | NA | Y | Y | Y |
| CSF eosinophilia | Y | N | NA | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Neuroimaging | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | N |
| Antibody to 24-kD antigen† | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Antibody to 21-kD antigen† | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | Y |
C = cerebral (hemiplegia or hemiparesis); S = spinal (paraplegia or paraparesis); Y = yes/positive; N = no/negative; NA = not applicable; CSF = cerebrospinal fluid.
By immunoblotting test.
Examples of immunoblotting patterns for detection of IgG against crude somatic advanced third-stage larvae G. spinigerum antigen are shown in Figure 1. Immunoblotting detected antigenic bands with molecular masses ranging from 21-kD to > 110-kD. There were 13 major diagnostic bands (Table 2): ≥ 110-kD (found in 12 patients [100%]), 92-kD (12 patients [100%]), 88-kD (12 patients [100%]), 85-kD (12 patients [100%]), 60-kD (5 patients [41.7%]), 52-kD (12 patients [100%]), 49-kD (12 patients [100%]), 44.5-kD (12 patients [100%]), 33-kD (10 patients [83.3%]), 31-kD (11 patients [91.7%]), 29-kD (4 patients [33.3%]), 24-kD (11 patients [91.7%]), and 21-kD (10 patients [83.3%]). In control group, two antigenic bands were found in two persons: 92-kD (found in 1 person [6.7%]) and 44.5-kD (1 person [6.7%]).
Figure 1.
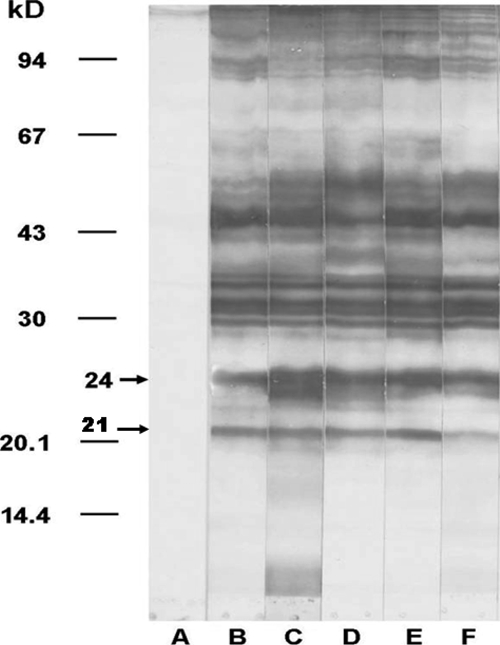
Representative patterns of immunoblot analysis for detection of serum IgG against crude somatic Gnathostoma spinigerum advanced third-stage larval antigen in persons with gnathostomiasis. Blots were developed with samples from pooled negative serum (lane A), pooled serum from persons with gnathostomiasis (lane B), and sera from persons with cerebrospinal gnathostomiasis (lanes C–F).
Table 2.
Patients with neurognathostomiasis and control groups with positive results for various antigenic bands against Gnathostoma spinigerum antigen
| Groups | Molecular weight (kD) of G. spinigerum antigenic component recognized by individual gnathostomiasis serum | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| > 110 | 92 | 88 | 85 | 60 | 52 | 49 | 44.5 | 33 | 31 | 29 | 24 | 21 | |
| Neurognathostomiasis | 12 | 12 | 12 | 12 | 5 | 12 | 12 | 12 | 10 | 11 | 4 | 11 | 10 |
| Control | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
All patients had positive results for the 21-kD or 24-kD diagnostic bands (Table 1). The sensitivity and specificity of the 21-kD and 24-kD diagnostic bands were 83.3% and 100%, and 91.7% and 100%, respectively (Table 3). Positive predictive values for these two diagnostic bands were 100%, and negative predictive values for these two diagnostic bands were 88.2% and 93.8%, respectively.
Table 3.
Diagnostic properties of diagnostic bands for neurognathostomiasis by immunoblotting
| Diagnostic band, kD | Sensitivity, % | Specificity, % | Positive predictive value, % | Negative predictive value, % |
|---|---|---|---|---|
| 21 | 83.3 | 100 | 100 | 88.2 |
| 24 | 91.7 | 100 | 100 | 93.8 |
| 21 or 24 | 100 | 100 | 100 | 100 |
The 21-kD and 24-kD diagnostic bands detected by immunoblotting are useful for diagnosis of neurognathostomiasis. The 24-kD diagnostic band has somewhat higher sensitivity than the 21-kD diagnostic band. These results are similar to those of previous reports of sensitivities of these bands for the diagnosis of cutaneous and ocular gnathostomiasis.11,12 The specificities of these two bands for the diagnosis of neurognathostomiasis were 100% (Table 3).
Several bands were found in all persons by immunoblotting (e.g., 85-kD, 88-kD, and 92-kD). However, the bands specific for a diagnosis of gnathostomiasis were 21-kD and 24-kD.13 Although clinical neurognathostomiasis is unique, the positive serologic test is useful for diagnosis. The diagnostic properties of both diagnostic bands were excellent for neurognathostomiasis.
We propose presumptive criteria for neurognathostomiasis, which have been modified from those in a previous report.3 All patients with a diagnosis of neurognathostomiasis must satisfy the major criteria and any of the minor criteria. The minor criteria included migratory swelling, radicular pain, blood eosinophilia > 500 cells/mm3, eosinophils in cerebrospinal fluid, or suggestive neuroimaging results. In clinical practice, patients with radicular pain may have a history of eating raw meat, which might leads to a false diagnosis of gnathostomiasis if they also have motor weakness. The presumptive diagnosis of neurognathostomiasis may be more convincing if other minor criteria are satisfied (Table 1). We recommend using either the 21-kD or 24-kDa bands as a supportive tool for diagnosis of patients with neurognathostomiais or gnathostomiasis that involves the central nervous system.
Acknowledgments
We thank the Office of the Higher Education Commission, the Ministry of Education and Research, and the Diagnosis Center for Emerging Infectious Disease, Khon Kaen University, Thailand for their assistance.
Footnotes
Authors' addresses: Pewpan M. Intapan and Wanchai Maleewong, Department of Parasitology, Faculty of Medicine, and Research and Diagnosis Center for Emerging Infectious Disease, Khon Kaen University, Khon Kaen, 40002, Thailand, E-mails: pewpan@kku.ac.th and wanch_ma@kku.ac.th. Piyarat Khotsri, Department of Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen, 40002, Thailand, E-mail: bewocto@yahoo.com. Jaturat Kanpittaya, Department of Radiology, Faculty of Medicine, and Diagnosis Center for Emerging Infectious Disease, Khon Kaen University, Khon Kaen, Thailand, E-mail: jatkan@gmail.com. Verajit Chotmongkol and Kittisak Sawanyawisuth, Department of Medicine, Faculty of Medicine, and Research and Diagnosis Center for Emerging Infectious Disease, Khon Kaen University, Khon Kaen, Thailand, E-mails: chotmongkolverajit@yahoo.com and kittisak@kku.ac.th.
References
- 1.Nawa Y. Historical review and current status of gnathostomiasis in Asia. Southeast Asian J Trop Med Public Health. 1991;22:217–219. [PubMed] [Google Scholar]
- 2.Ogata K, Nawa Y, Akahane H, Diaz Camacho SP, Lamothe-Argumedo R, Cruz-Reyes A. Gnathostomiasis in Mexico. Am J Trop Med Hyg. 1998;58:316–318. doi: 10.4269/ajtmh.1998.58.316. [DOI] [PubMed] [Google Scholar]
- 3.Kraivichian K, Nuchprayoon S, Sitichalernchai P, Chaicumpa W, Yentakam S. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004;71:623–628. [PubMed] [Google Scholar]
- 4.Brant-Zawadzki M, Wofsy CB, Schechter G. CT-evidence of subarachnoid hemorrhage due to presumed gnathostomiasis. West J Med. 1982;137:65–67. [PMC free article] [PubMed] [Google Scholar]
- 5.Sawanyawisuth K, Chlebicki MP, Pratt E, Kanpittaya J, Intapan PM. Sequential imaging studies of cerebral gnathostomiasis with subdural hemorrhage as its complication. Trans R Soc Trop Med Hyg. 2009;103:102–104. doi: 10.1016/j.trstmh.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Sithinamsuwan P, Chairangsaris P. Images in clinical medicine. Gnathostomiasis—neuroimaging of larval migration. N Engl J Med. 2005;353:188. doi: 10.1056/NEJMicm040795. [DOI] [PubMed] [Google Scholar]
- 7.Sawanyawisuth K, Tiamkao S, Nitinavakarn B, Dekumyoy P, Jitpimolmard S. MR imaging findings in cauda equina gnathostomiasis. AJNR Am J Neuroradiol. 2005;26:39–42. [PMC free article] [PubMed] [Google Scholar]
- 8.Sawanyawisuth K, Tiamkao S, Kanpittaya J, Dekumyoy P, Jitpimolmard S. MR imaging findings in cerebrospinal gnathostomiasis. AJNR Am J Neuroradiol. 2004;25:446–449. [PMC free article] [PubMed] [Google Scholar]
- 9.Boongird P, Phuapradit P, Siridej N, Chirachariyavej T, Chuahirun S, Vejjajiva A. Neurological manifestations of gnathostomiasis. J Neurol Sci. 1977;31:279–291. doi: 10.1016/0022-510x(77)90113-7. [DOI] [PubMed] [Google Scholar]
- 10.Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English language literature. Clin Infect Dis. 1993;16:33–50. doi: 10.1093/clinids/16.1.33. [DOI] [PubMed] [Google Scholar]
- 11.Anantaphruti MT, Nuamtanong S, Dekumyoy P. Diagnostic values of IgG4 in human gnathostomiasis. Trop Med Int Health. 2005;10:1013–1021. doi: 10.1111/j.1365-3156.2005.01478.x. [DOI] [PubMed] [Google Scholar]
- 12.Laummaunwai P, Sawanyawisuth K, Intapan PM, Chotmongkol V, Wongkham C, Maleewong W. Evaluation of human IgG class and subclass antibodies to a 24 kDa antigenic component of Gnathostoma spinigerum for the serodiagnosis of gnathostomiasis. Parasitol Res. 2007;101:703–708. doi: 10.1007/s00436-007-0538-3. [DOI] [PubMed] [Google Scholar]
- 13.Tapchaisri P, Nopparatana C, Chaicumpa W, Setasuban P. Specific antigen of Gnathostoma spinigerum for immunodiagnosis of human gnathostomiasis. Int J Parasitol. 1991;21:315–319. doi: 10.1016/0020-7519(91)90033-4. [DOI] [PubMed] [Google Scholar]


