Abstract
Recent shifts in global health policy have led to the implementation of mass drug administration (MDA) for neglected tropical diseases. Here we show how population genetic analyses can provide vital insights into the impact of such MDA on endemic parasite populations. We show that even a single round of MDA produced a genetic bottleneck with reductions in a range of measures of genetic diversity of Schistosoma mansoni. Phylogenetic analyses and indices of population differentiation indicated that schistosomes collected in the same schools in different years were more dissimilar than those from different schools collected within either of the study's 2 years, in addition to distinguishing re-infection from non-clearance (that might indicate putatively resistant parasites) from within those children infected at both baseline and follow-up. Such unique results illustrate the importance of genetic monitoring and examination of long lived multi-cellular parasites such as these under novel or increased chemotherapeutic selective pressures.
Introduction
Recent shifts in global health policy have led to the implementation of mass drug administration (MDA) programs against a range of neglected tropical diseases (NTDs).1 Such large-scale programs exert prolonged novel selection pressures on parasites.2,3 Schistosomiasis is one such NTD of profound medical importance across many regions worldwide.4,5 Schistosomes, the causative agents, are digenean trematodes whose life-cycle alternates sexual reproduction in a mammalian host with asexual reproduction in a molluscan host. Transmission between hosts occurs by larval cercariae (infective to the mammalian host) and miracidia (infective to the molluscan host). Schistosomes are highly unusual in the class of Trematoda being dioecious, enabling the potential for increased genetic exchange within parasite populations. Indeed, genetic diversity is thought to be vital for schistosomes' ability to survive the pressures of their complex life-cycles,6 and their ability to adapt to changing environmental conditions. Although only a few population genetic studies have been performed on schistosomes to date, they have identified significant genetic diversity in adult worms within both human and rodent hosts.7–11
There is currently no effective vaccine against schistosomiasis and interruption of the transmission cycle through mollusciciding, biological control of the intermediate snail hosts and health education have generally proved insufficient control methods on their own.2 Chemotherapy with praziquantel (PZQ) is therefore the mainstay for schistosomiasis control12 and PZQ will remain the only drug of choice for several years.12 One major control program, the Schistosomiasis Control Initiative (SCI), was established in 2002 to assist selected sub-Saharan African countries to establish sustainable schistosomiasis control. Within the first 5 years alone, over 40 million courses of PZQ treatment have successfully been delivered to over 20 million individuals.13
One key issue inherent in such programs is, however, the potential evolution or establishment of drug resistance and/or tolerance, which has been a major problem in veterinary anthelmintics. This is of particular importance for schistosomiasis given that PZQ is the only readily available drug. To date, there is no evidence to suggest that PZQ resistance has become established under field conditions, even within China and Egypt, despite its widespread use for over 20 years.2,14,15 There is speculation that untreated parasites or refugia may partly explain the lack of establishment of PZQ resistance, with those parasites present in snails, non-human mammals, and untreated humans providing a pool of susceptible genes that “dilute” any resistance genes in endemic populations.3 Subdivision in schistosome parasite populations, and the possibility of inherent costs of resistance, have also been suggested as additional explanations for the lack of spread of PZQ resistance.3 Nevertheless, evidence arising from clinical investigations primarily within Egypt and Senegal,16 reports of individual failures of PZQ treatment in infected travelers,17 and results from a range of laboratory studies and artificial-selection of resistant lines,15 give cause for concern regarding potential future changes in the PZQ susceptibility status of schistosome populations in natural foci.
It is important, therefore, that the effects of PZQ treatment on schistosome population genetics be monitored for any changes, not only those potentially related to drug resistance (for which there are currently no linked genetic markers), but also for changes in genetic diversity and population structure. Reductions in diversity resulting from chemotherapy may indicate that the population will be less able to adapt to a range of environmental pressures (including further chemotherapy). Conversely, increases in diversity or genotypic change may indicate increased genetic exchange of a population of parasites with other populations (in different geographical areas and/or host species). Either of these outcomes would be key indicators of the potential impact of MDA on current and future schistosome epidemiology. One may predict, however, that a single round of MDA within primary school-aged children would have no such effect on schistosome population genetics caused by the large refugia present in untreated adults and preschool children, the long generation time of these parasites and the relatively high efficacy of PZQ treatment.
This study, using data gathered from children within two schools in a Lake Victoria region of Tanzania, therefore aimed to elucidate, for the first time, the potential impact of the first round of MDA on the population genetic structure of a PZQ-naive Schistosoma mansoni, causative agent of intestinal schistosomiasis in sub-Saharan Africa, the Caribbean, and Yemen.
Methods
Study design and schools sampled.
This study was carried out in two schools; Kisorya (Bunda district, Mara region) and Bukindo (Ukerewe district, Mwanza region) both situated close to Lake Victoria in Tanzania. At the start of this study (April 2005) children from neither of these schools had previously been treated with PZQ, and PZQ was not readily available in local health centers. The entire population was therefore assumed to be PZQ naive. Parasite samples collected in April 2005 were therefore termed baseline and samples collected in April 2006 (after MDA) were termed follow-up. The MDA with PZQ (for the treatment of schistosomiasis) and albendazole (for soil transmitted helminthiasis) was performed in November 2005 targeting all primary school-aged children (enrolled and not enrolled, 7–11 years of age) in 11 regions of Tanzania, including the neighboring Mwanza and Mara regions. Although coverage data for Bunda district and the individual schools was not retrieved by the program, coverage in Ukerewe district was high at 90%. Local populations, including school enrolment, remained stable over this time period (with no large migrations) (Lwambo N, unpublished observation). Environmental and meteorological conditions were similar between years (National Institute for Medical Research, unpublished data).
In most SCI-associated National control programs, the impact of annual treatments on the prevalence and intensity of schistosome infection has been impressive.13 Unpublished SCI data from this first round of mass chemotherapy in Kisorya and Bukindo indicated, however, no significant changes in parasite prevalence or intensity in Kisorya (2005, 91% prevalence, 418 epg; 2006, 92% prevalence, 432 epg) but a reduction in infection intensity in Bukindo (2005, 60% prevalence, 135 epg; 2006, 77% prevalence, 66 epg). This may be caused by high levels of transmission and therefore re-infection in these Lake Victoria highly endemic areas.
Baseline miracidial samples were collected from randomly selected children 7–11 years of age identified to be infected by positive Kato-Katz smears (up to 60 miracidia per child were collected) from both Kisorya and Bukindo schools. One year follow-up miracidial samples were again collected from infected children 7–11 years of age from the same schools (only a small number of which were from the same children, as detailed below). Samples from an additional subsection of the children collected from Kisorya at follow-up were from 7 year olds who were new entries to the school and hence had not been previously treated in the first round of MDA (because of not being of school age) and whose infections were therefore presumed to be PZQ naive. Data from these individuals thereby provide an insight into the effect of MDA the previous year on parasite infections in children who had not yet been treated, and hence the potential impact of PZQ on parasite genetics at the population level.
Recently developed techniques for parasite collection, storage, and amplification were used enabling large numbers of individual schistosome miracidia to be analyzed directly from children without the associated genetic bottlenecks and selection biases inherent in laboratory passage18 (because of the inability to sample adult schistosomes from their location in the mesenteric system previous studies have generally relied on infecting snails with miracidia, exposing laboratory mammals to subsequent cercariae from these field isolates, and performing analyses on the subsequent adult worms).
Collection and storage of miracidia.
Infected children were identified by positive Kato-Katz smears. Stool samples from each infected child were prepared separately for miracidial hatching with individual miracidia pipetted directly onto Whatman FTA cards for storage as described by Gower and others.18 At baseline (2005) miracidia were collected from 38 children from Bukindo primary school and 42 children from Kisorya primary school. At follow-up (2006) miracidia were collected from 18 children at Bukindo primary school and 29 children from Kisorya primary school. Only a few of these follow-up miracidial samples (1 child from Bukindo, 8 children from Kisorya) were collected from the same children as at baseline as many of those children from which samples were taken at baseline were Kato-Katz negative at follow-up. All children, regardless of infection status, were treated with PZQ under MDA in November 2005 and September 2007.
Molecular analyses.
Molecular analyses were carried out on up to 20, randomly selected, miracidia per child per time (baseline versus follow-up) point. Pilot studies revealed that 20 larvae may provide a representative sample of the total genetic variability of all miracida/cercaria shed per individual on a certain day, although this is likely to vary per individual and epidemiological setting.10,18–20 The DNA preparation was carried out on the Whatman cards as per the manufacturer's protocol (Whatman FTA cards). Polymerase chain reaction (PCR) was carried out using a previously published multiplex allowing genotyping of seven microsatellite loci (Table 1).10,18–20 Small modifications were made to the fluorescent labels because of the use of an ABI Prism 3730 Genetic Analyser (Applied Biosystems, UK) for genotyping rather than the ABI 377 automated sequencer for which the assay was developed. Forward primers were labeled using 6-FAM, PET, VIC, and NED dyes. The PCR reactions were performed on Gene Amp PCR system 9700 (Applied Biosystems, Cheshire, UK), incorporating positive and negative controls on all plates.
Table 1.
Details of microsatellite loci used in this study
Population genetic analyses.
Allele sizes were calculated in Genemapper (version 4.0). Analysis was restricted to miracidia with allele calls for at least three loci. A total of 1,706 miracidia were analyzed. A parasite population was defined as the population from a single child at one time point. Observed (Ho) and expected (He) heterozygosity for each population were calculated in powermarker24 and the allelic richness (Ar) of each population was calculated in fstat version 2.9.3.2.25 Differences in the mean Ho, He, and Ar between baseline and follow-up populations were investigated using a general linear model (GLM) in Minitab Statistical Software (Minitab Inc., State College, PA) with child age, infection intensity (eggs per gram) and miracidial samples size as covariates. Differences in mean Ho, He, and Ar between PZQ-naive 7 year olds at baseline and follow-up were investigated using a GLM with infection intensity and miracidial sample size as covariates. Summary statistics for Ho, He, and Ar across all miracidia collected at each time point were created in fstat version 2.9.3.225 and differences between time points were tested for using a permutation model with 15,000 permutations. Differences between all miracidia in each time point were also visualized using the Bayesian clustering program structure versus 2.2.25 A Markov Chain Monte Carlo (MCMC) burn-in of 10,000 steps and 1,000,000 steps after burn-in was used to assign samples to four putative populations. As a measure of genetic distance between populations, a matrix of Cavalli-Sforza and Edwards' chord distances26 was calculated using POPULATIONS 1.2.2827 and visualized using a neighbor-joining tree, with bootstrapping with 10,000 replications. Measures for population differentiation (Fst) were calculated between schools within years and between years within schools using GDA (Genetic Data Analysis: version 1.0).28 Analyses were bootstrapped over loci with 30,000 replications to achieve 95% confidence intervals.
Ethics statement.
Ethical approval was obtained from the Ethical Review Board of National Institute of Medical Research (clearance number NIMR/HQ/R.8a/Vol.IX/379), Tanzania and from the Imperial College Research Ethics Committee (ICREC), Imperial College London, UK in combination with the ongoing Schistosomiasis Control Initiative activities. Written consent for the schoolchildren to participate in longitudinal monitoring of the national control program for schistosomiasis and soil-transmitted helminth (STH) was given by head teachers because in African schools, written consent of the child's guardian is very difficult to obtain (because of the associated impoverished conditions and often low literacy). The parents/guardians verbal consent was recorded at school committees including parents, teachers, and community leaders after they received satisfactory information about the study. Each individual child also gave verbal consent before recruitment.
Results
The GLMs showed that the populations of parasites within individual children at each of the two schools had significantly reduced levels of allelic richness (Bukindo, 3.9% reduction, F1,43 = 12.49, P = 0.001; Kisorya, 3.3% reduction, F1,62 = 10.41, P = 0.002) and expected heterozygosity (Bukindo, 7.6% reduction, F1,40 = 4.86, P = 0.033; Kisorya, 8.7% reduction, F1,52 = 19.81, P < 0.001) post-treatment in comparison to baseline (Figure 1), even when controlling for intensity of infection.
Figure 1.
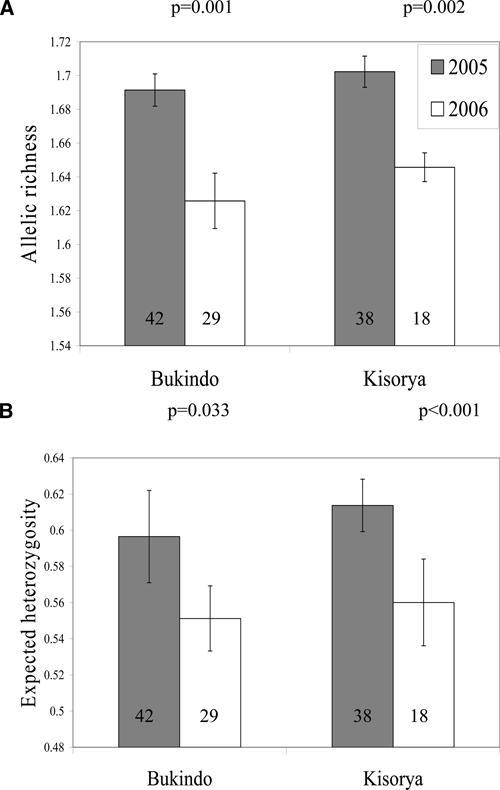
(A, B) Genetic diversity of Schistosoma mansoni populations within children. Mean (±SEM) allelic richness and expected heterozygosity of the S. mansoni populations of individual children from two schools (Kisorya and Bukindo) collected from 80 children in 2005 (baseline) and 47 children in 2006 (post-praziquantel (PZQ) mass chemotherapy follow-up). Number of children in each group indicated within bars.
To determine whether this within-child reduction in genetic diversity was in fact indicative of a general reduction in diversity across the whole parasite population, we further investigated the results for parasites from all children combined across each year. The overall diversity of the parasites collected from all children within each school was thus compared between the time points. A reduction in genetic diversity between baseline and follow-up samples was identified (Table 2), and a non-parametric permutation test indicated the reduction in allelic diversity approached statistical significance (P = 0.084). Results from Structure were consistent with these results indicating greater diversity across all parasites at baseline (with parasites divided between four putative populations at k = 4) in comparison to follow-up (with parasites categorized mainly into only two of the four putative populations) indicating a lower level of genetic diversity (Figure 2). Analysis at the school level (across all children in the cohort), showed that this reduction in genetic diversity observed within individual children was indeed indicative of a general reduction in parasite genetic diversity across all these children and therefore potentially in the parasite community itself.
Table 2.
Mean allelic richness and expected heterozygosity across all Schistosoma mansoni samples analyzed pre-treatment and post-PZQ treatment
| Allelic richness | Expected heterozygosity | |
|---|---|---|
| 2005 (baseline) | 10.353 | 0.720 |
| 2006 (follow-up) | 9.432 | 0.668 |
| P value obtained after 15,000 permutations in Fstat | 0.085 | 0.167 |
Figure 2.
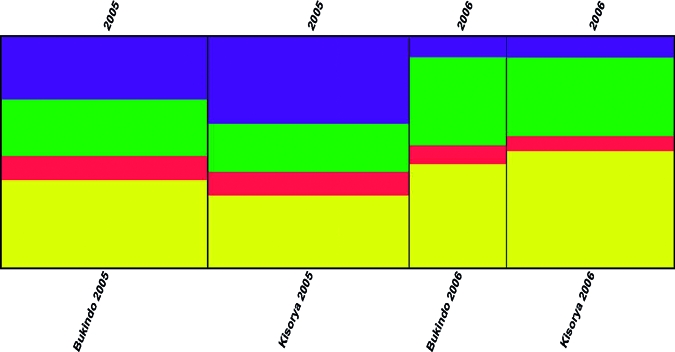
Clustering output of Schistosoma mansoni populations. A clustering output produced by distruct visualizing the outputs of data analyses from structure (a software program implementing a Bayesian clustering algorithm to determine how many putative separate populations are present in the genetic data) showing the allocation of miracidia populations by school and year to each of four putative populations. Results indicated greater diversity across all parasites at 2005 baseline (with parasites divided approximately equally between all four putative populations at k = 4, indicated by the colors blue, red, yellow and green) in comparison to the 2006 follow-up (where parasites were categorized mainly into only two, indicated by green and yellow here, of the four putative populations).
A key comparison was made between those parasite samples collected from the newly recruited 7 year olds at follow-up in 2006, who had not been previously treated with PZQ (as they were not yet of school age during baseline sampling and therefore essentially act as a control group for non-chemotherapy-induced changes between the 2 years) and parasites from 7 year olds collected at baseline in 2005. A reduction in allelic richness was observed, with miracidia collected from 7 year olds at follow-up showing lower allelic richness than those collected at baseline (4.3% reduction, F1,16 = 4.14, P = 0.05) from GLM (Figure 3). The statistical significance of this result was likely limited by the small sample size of 7 year olds.
Figure 3.
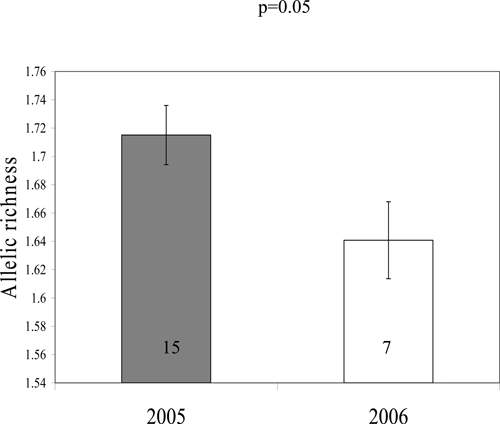
Genetic diversity of Schistosoma mansoni populations within 7 year olds. Mean (±SEM) allelic richness of parasite populations collected from praziquantel naive 7-year-old children in 2005 (baseline) and 2006 (follow-up) for Kisorya school. Number of children in each group indicated within bars.
In addition to impacting the diversity of schistosome infections, MDA might also be predicted to change the frequency of specific alleles and genotypes in the population. We therefore calculated the genetic disparity between the parasite populations of individual children using Cavalli-Sforza and Edwards' chord distances and visualized the similarity of parasite samples collected at baseline and post-treatment using a neighbor-joining clustering algorithm. The resulting phenogram (Figure 4) showed clear separation between samples from children at baseline and follow-up (although lack of bootstrap support indicates that this separation is small) with some clustering but no clear separation between schools at each of these time points. Three populations of parasites from children clustered within the opposite time point, and this tree was used to confirm that none of the samples from nine children, who were infected and sampled at both time points (indicated in blue and red on Figure 4) clustered together, suggesting that these may be reinfections rather than treatment failures (and hence not potentially resistant parasites). The results from this neighbor-joining tree were confirmed with Fst analyses, which indicated between-year within-school population separation (although small) to be significant and greater than between school within year population separation (Figure 5).
Figure 4.
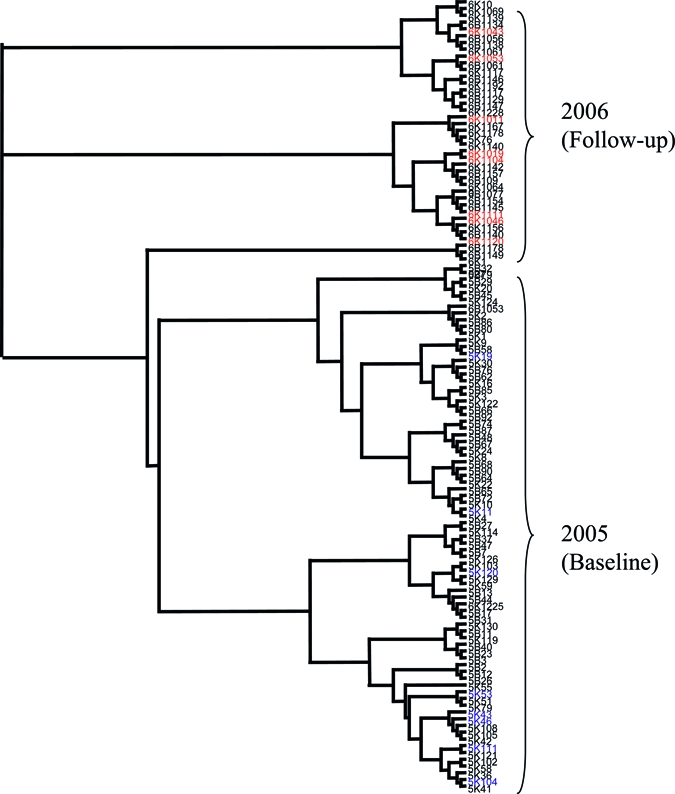
Neighbor-joining tree of parasite populations per child. Neighbor-joining tree displaying genetic distance calculated using Cavalli-Sforza and Edwards' chord distances23 for all the populations of miracidia collected (from each child at each time point). Populations labeled blue and red are those parasites collected from the same child at both baseline and follow-up (blue at baseline and red at follow-up). Populations are coded by year (5 = 2005, 6 = 2006), school (B = Bukindo, K = Kisorya) and child ID number (all individuals at follow-up have “10” in front of their two digit ID number in comparison to baseline or a “1” in front of their three digit ID number in comparison to baseline).
Figure 5.
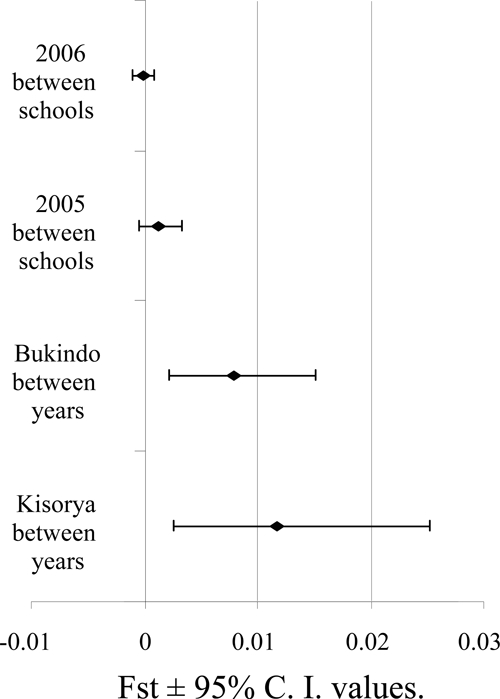
Population differentiation between schools within each year and between years within each school as represented by Fst ± 95% confidence interval (CI) values.
Discussion
One may have predicted, because of the large parasite refugia in snails, untreated children and adults, and potentially also untreated non-human mammals such as rodents and other primates in the case of S. mansoni (each of which can be considered as a “reservoir” for re-infection), that one MDA treatment of school-aged children would have very little, if any, impact on the genetic composition and characteristics of the schistosome population. Indeed, because those parasites killed by treatment would only represent a small proportion of the circulating parasite population, multiple years worth of treatment might be expected to be necessary before impacts on parasite genetic diversity and genotype would be apparent (as observed after up to 15 years of ivermectin treatment as part of the onchocerciasis control program29). In contrast, we report here a significant reduction in parasite genetic diversity after a single PZQ MDA treatment.
One might propose, therefore, that the observed reductions in parasite genetic diversity within each child could simply reflect the short time over which children were able to acquire those infections measured at follow-up (i.e. only 6 months presuming treatment had cleared any prior infections) relative to those measured at baseline, which might have been acquired over several years. However, indication that this may not be a sufficient explanation is provided by the results from the new 2006, previously untreated, cohort of 7-year-old children, and their comparison with the matched pre-MDA cohort of untreated 7 year olds in 2005, because similar reductions in allelic richness were observed in these “control” 7 year olds at follow-up. Indeed, this result tends to indicate that not only did one round of MDA affect parasite diversity within each child treated and across all children treated; it may also have affected parasite diversity within untreated children and therefore the broader S. mansoni population as a whole.
These observations have important implications for our understanding of population genetics of parasites in general and potential implications for the implementation of NTD MDA campaigns. The unpredicted apparent “bottleneck” imposed by one round of MDA on schistosome population genetics has several putative explanations, all of which require further investigation. It may indicate that not all parasites present in refugia at the time of treatment contribute to the re-infection of children in this age group (7–11), and hence the “effective reservoir” may be smaller than previously thought. One could speculate that parasite genetic differentiation may occur between human hosts of different ages caused by immunological differences, with the untreated pre-school children, teenagers, and adults not treated in this study harboring different parasite genotypes, which may be less likely to re-infect children in the study group ages (7–11). This reduction in genetic diversity as a result of one MDA treatment may therefore result from a large number of the genotypes of parasites currently adapted to infecting humans of the group 7–11 years of age having been killed by chemotherapy, with not all parasites present in the “reservoir” adapted to re-infect these children or being present in the specific transmission foci that these children frequent. Further investigation would be necessary to confirm or disprove this hypothesis.
An alternative/additional potential hypothesis is that the presence of chemotherapy-induced acquired immunity, whereby the host immune system is exposed to parasite antigens through the damage and subsequent killing of adult schistosome worms by the action of PZQ,30 may compound the reduction in the “effective reservoir.” The resulting chemotherapy-induced “vaccination” of children to those parasites, which they were harboring and treated for may potentially lead to a reduction in genetic diversity of infections as children may be resistant to genotypes closely related to those that they were infected with pre-treatment. This may in turn explain the phylogenetic separation between parasites collected from baseline and follow-up. With repeated treatments (especially community-wide treatments occurring in areas of high endemicity), continued reduction in genetic diversity may be predicted. Furthermore, as the aforementioned natural diversity in schistosome populations is thought to be essential to complete their complex life-cycles and respond to changing environmental pressures, continued significant reductions in such diversity may therefore reduce their ability to adapt and survive any future novel environmental selective pressures to which they may be exposed.
However, a non-biological explanation for the decreased diversity is that multiplex PCR from low yields of miracidial DNA results in null amplification, although there is little reason to suspect a systematically higher rate of null alleles in the post-treatment collections because all data collection and processing techniques remained constant. An alternative and less optimistic explanation for the observed reduction in diversity is that chemotherapy-imposed selection is occurring. The reduction in genetic diversity may represent the increased success of a small number of alleles, although this may be unlikely after only one treatment event. While population genetic studies with neutral microsatellite markers do not have the power to directly identify selection, careful monitoring of changes in microsatellite allele frequency over time and treatment success (aided by the identification of re-infections versus treatment failures with phenograms) may enable selection for PZQ resistance to be detected even with the current absence of specific genetic markers for resistance. Continued and expanded monitoring of schistosome population genetics over several treatments is now important, especially where epidemiological data suggests non-clearances. Such techniques should ideally be extended to all the NTDs covered in the new and expanding human MDA programs, particularly as this study has shown that currently available tools can provide important markers to elucidate the impact of selective pressures such as chemotherapy on parasite population genetics and hence potentially, disease epidemiology.
Acknowledgments
We thank the field and technical staff of the National Institute for Medical Research, Mwanza for their collaboration and Fiona Allan, Sarah Hogan, and James Rudge for their assistance with field work. We are particularly grateful to the head teachers, staff, and children for their willingness to participate in the survey. We also thank Sam Loker, David Rollinson, and Bill Hanage for helpful discussion and/or comments on the manuscript.
Footnotes
Financial support: Field work, analyses and/or authors were supported by grants from the Bill and Melinda Gates Foundation, the European Union (CONTRAST EU/INCO.Dev contract no.: 032203), the Wellcome Trust (grant number WT063774), and the Royal Society (JPW as a Royal Society University Research Fellow, Charlotte Gower as a Dorothy Hodgkin Fellow).
Authors' addresses: Alice J. Norton, Charlotte M. Gower, Poppy H. L. Lamberton, Lynsey Blair, Alan Fenwick, and Joanne P. Webster, Department of Infectious Disease Epidemiology, Imperial College, Faculty of Medicine, London, UK, E-mails: a.norton@wellcome.ac.uk, charlotte.gower@imperial.ac.uk, poppy.lamberton@imperial.ac.uk, lynsey.blair@imperial.ac.uk, alan.fenwick@imperial.ac.uk, and joanne.webster@imperial.ac.uk. Alice J. Norton current address: The Wellcome Trust, London, UK. Bonnie L. Webster, Department of Zoology, The Natural History Museum, London, UK, E-mail: b.webster@nhm.ac.uk. Nicholas J. S. Lwambo, Mwanza Research Centre, National Institute for Medical Research, Mwanza, Tanzania, E-mail: lwambon@live.co.uk.
References
- 1.Hotez P, Raff S, Fenwick A, Richards F, Molyneux D. Recent progress in integrated neglected tropical disease control. Trends Parasitol. 2007;23:511–514. doi: 10.1016/j.pt.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Fenwick A, Webster JP. Schistosomiasis: challenges for control, treatment and drug resistance. Curr Opin Infect Dis. 2006;19:577–582. doi: 10.1097/01.qco.0000247591.13671.6a. [DOI] [PubMed] [Google Scholar]
- 3.Webster JP, Gower CM, Norton AJ. Application of evolutionary concepts to predicting and evaluating the impact of mass-chemotherapy schistosomiasis control programmes. Evolutionary Applications. 2008;1:66–83. doi: 10.1111/j.1752-4571.2007.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 5.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 6.Rollinson D, Kaukas A, Johnston DA, Simpson AJ, Tanaka M. Some molecular insights into schistosome evolution. Int J Parasitol. 1997;27:11–28. doi: 10.1016/s0020-7519(96)00169-5. [DOI] [PubMed] [Google Scholar]
- 7.Agola LE, Mburu DN, DeJong RJ, Mungai BN, Muluvi GM, Njagi EN, Loker ES, Mkoji GM. Microsatellite typing reveals strong genetic structure of Schistosoma mansoni from localities in Kenya. Infect Genet Evol. 2006;6:484–490. doi: 10.1016/j.meegid.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Barral V, Morand S, Pointier JP, Theron A. Distribution of schistosome genetic diversity within naturally infected Rattus rattus detected by RAPD markers. Parasitology. 1996;113:511–517. doi: 10.1017/s003118200006755x. [DOI] [PubMed] [Google Scholar]
- 9.Curtis J, Minchella D. Schistosome population genetic structure: when clumping worms is not just splitting hairs. Parasitol Today. 2000;16:68–71. doi: 10.1016/s0169-4758(99)01553-7. [DOI] [PubMed] [Google Scholar]
- 10.Rollinson D, Webster JP, Nyakanna S, Stothard AJ., Jr Genetic diversity of schistosomes and snails: implications for control. Parasitology. 2009;136:1801–1811. doi: 10.1017/S0031182009990412. [DOI] [PubMed] [Google Scholar]
- 11.Stothard JR, Webster BL, Weber T, Nyakaana S, Webster JP, Kazibwe F, Kabatereine NB, Rollinson D. Molecular epidemiology of Schistosoma mansoni in Uganda: DNA barcoding reveals substantive genetic diversity within Lake Albert and Lake Victoria populations. Parasitology. 2009;136:1813–1824. doi: 10.1017/S003118200999031X. [DOI] [PubMed] [Google Scholar]
- 12.Colley DG, LoVerde PT, Savioli L. Infectious disease. Medical helminthology in the 21st century. Science. 2001;293:1437–1438. doi: 10.1126/science.1060733. [DOI] [PubMed] [Google Scholar]
- 13.Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, Zhang Y, Garba A, Stothard JR, Gabrielli AF, Clements AC, Kabatereine NB, Toure S, Dembele R, Nyandindi U, Mwansa J, Koukounari A. The Schistosomiasis Control Initiative (SCI): rational, development and implementation from 2002–2008. Parasitology. 2009;136:1719–1730. doi: 10.1017/S0031182009990400. [DOI] [PubMed] [Google Scholar]
- 14.Doenhoff MJ, Kusel JR, Coles GC, Cioli D. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans R Soc Trop Med Hyg. 2002;96:465–469. doi: 10.1016/s0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]
- 15.Doenhoff MJ, Pica-Mattoccia L. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Rev Anti Infect Ther. 2006;4:199–210. doi: 10.1586/14787210.4.2.199. [DOI] [PubMed] [Google Scholar]
- 16.Danso-Appiah A, De Vlas SJ. Interpreting low praziquantel cure rates of Schistosoma mansoni infections in Senegal. Trends Parasitol. 2002;18:125–129. doi: 10.1016/s1471-4922(01)02209-7. [DOI] [PubMed] [Google Scholar]
- 17.Alonso D, Munoz J, Gascon J, Valls ME, Corachan M. Failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am J Trop Med Hyg. 2006;74:342–344. [PubMed] [Google Scholar]
- 18.Gower CM, Shrivastava J, Lamberton PH, Rollinson D, Emory A, Webster BL, Kabatereine NB, Webster JP. Development and application of an ethical and epidemiologically appropriate assay for the multi-locus microsatellite analysis of Schistosoma mansoni. Parasitology. 2007;134:523–536. doi: 10.1017/S0031182006001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrivastava J, Gower CM, Balolong E, Jr, Wang TP, Qian BZ, Webster JP. Population genetics of multi-host parasites—the case for molecular epidemiological studies of Schistosoma japonicum using naturally sampled larval stages. Parasitology. 2005;131:617–626. doi: 10.1017/S0031182005008413. [DOI] [PubMed] [Google Scholar]
- 20.Wang T-P, Shrivastava J, Johansen MV, Zhang ZK, Webster JP. Does multiple hosts mean multiple parasites? Population genetic structure of Schistosoma japonicum between definitive host species. Int J Parasitol. 2006;36:1317–1325. doi: 10.1016/j.ijpara.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Curtis J, Sorensen RE, Minchella DJ. Schistosome genetic diversity: the implications of population structure as detected with microsatellite markers. Parasitology. 2002;125:S51–S59. doi: 10.1017/s0031182002002020. [DOI] [PubMed] [Google Scholar]
- 22.Durand P, Sire C, Theron A. Isolation of microsatellite markers in the digenetic trematode Schistosoma mansoni from Guadeloupe Island. Mol Ecol. 2000;9:997–998. doi: 10.1046/j.1365-294x.2000.00939-4.x. [DOI] [PubMed] [Google Scholar]
- 23.Blair L, Webster JP, Barker GC. Isolation and characterization of polymorphic microsatellite markers in Schistosoma mansoni from Africa. Mol Ecol Notes. 2001;1:93–95. [Google Scholar]
- 24.Lui K, Muse S. PowerMarker: integrated analysis environment for genetic marker data. Bioinformatics. 2005;21:2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- 25.Goudet J. Fstat version 1.2: a computer program to calculate F-statistics. J Hered. 1995;86:485–486. [Google Scholar]
- 26.Cavalli-Sforza LL, Edwards AW. Phylogenetic analysis. Models and estimation procedures. Am J Hum Genet. 1967;19:233–257. [PMC free article] [PubMed] [Google Scholar]
- 27.Langella O. Populations 1.2.28 genetic software. CNRS CNRS UPR9034. 1999. www.cnrs-gif.fr/pge Available at.
- 28.Lewis PO, Zaykin D. Genetic data analysis: computer program for the analysis of allelic data, version 1.1. 2001.
- 29.Ardelli BF, Guerriero SB, Pritchard RK. Ivermectin imposes selection pressure on P-glycoprotein from Onchocerca volvulus: linkage disequilibrium and genotype diversity. Parasitology. 2006;132:370–386. doi: 10.1017/S0031182005008991. [DOI] [PubMed] [Google Scholar]
- 30.Correa-Oliveira R, Caldas IR, Martins-Filho OA, Queiroz CC, Lambertucci JR, Cunha-Melo JR, Silveira AS, Prata A, Wilson A, Gazzinelli G. Analysis of the effects of treatment of human Schistosoma mansoni infection on the immune response of patients in endemic areas. Acta Trop. 2000;77:141–146. doi: 10.1016/s0001-706x(00)00127-3. [DOI] [PubMed] [Google Scholar]


