Abstract
TNF receptor-associated factor 2 (TRAF2) is a key component in NF-κB signaling triggered by TNF–α 1,2. Genetic evidence indicates that TRAF2 is necessary for polyubiquitination of receptor interacting protein 1 (RIP1) 3 that then serves as a platform for recruitment and stimulation of IκB kinase (IKK) leading to activation of the transcription factor NF-κB. Although TRAF2 is a RING domain ubiquitin ligase, direct evidence that TRAF2 catalyzes the ubiquitination of RIP1 is lacking. TRAF2 binds to sphingosine kinase 1 (SphK1) 4, one of the isoenzymes that generates the pro-survival lipid mediator sphingosine-1-phosphate (S1P) inside cells. Here we show that SphK1 and production of S1P is necessary for Lys 63-linked polyubiquitination of RIP1, phosphorylation of IKK and IκBα, and IκBα degradation, leading to NF-κB activation. Surprisingly, these responses were mediated by intracellular S1P independently of its cell surface G protein-coupled receptors. S1P specifically binds to TRAF2 at the N-terminal RING domain and stimulates its E3 ligase activity. S1P, but not dihydro-S1P, dramatically increased recombinant TRAF2-catalyzed Lys 63- but not Lys 48-linked polyubiquitination of RIP1 in vitro in the presence of the ubiquitin conjugating enzymes (E2) UbcH13 or UbcH5a. Our data reveal that TRAF2 is a novel intracellular target of S1P, and that S1P is the missing co-factor for TRAF2 E3 ubiquitin ligase activity, suggesting a new paradigm for regulation of Lys 63-linked polyubiquitination. These results also highlight the key role of SphK1 and its product S1P in TNF-α signaling and the canonical NF-κB activation pathway important in inflammatory, anti-apoptotic, and immune processes.
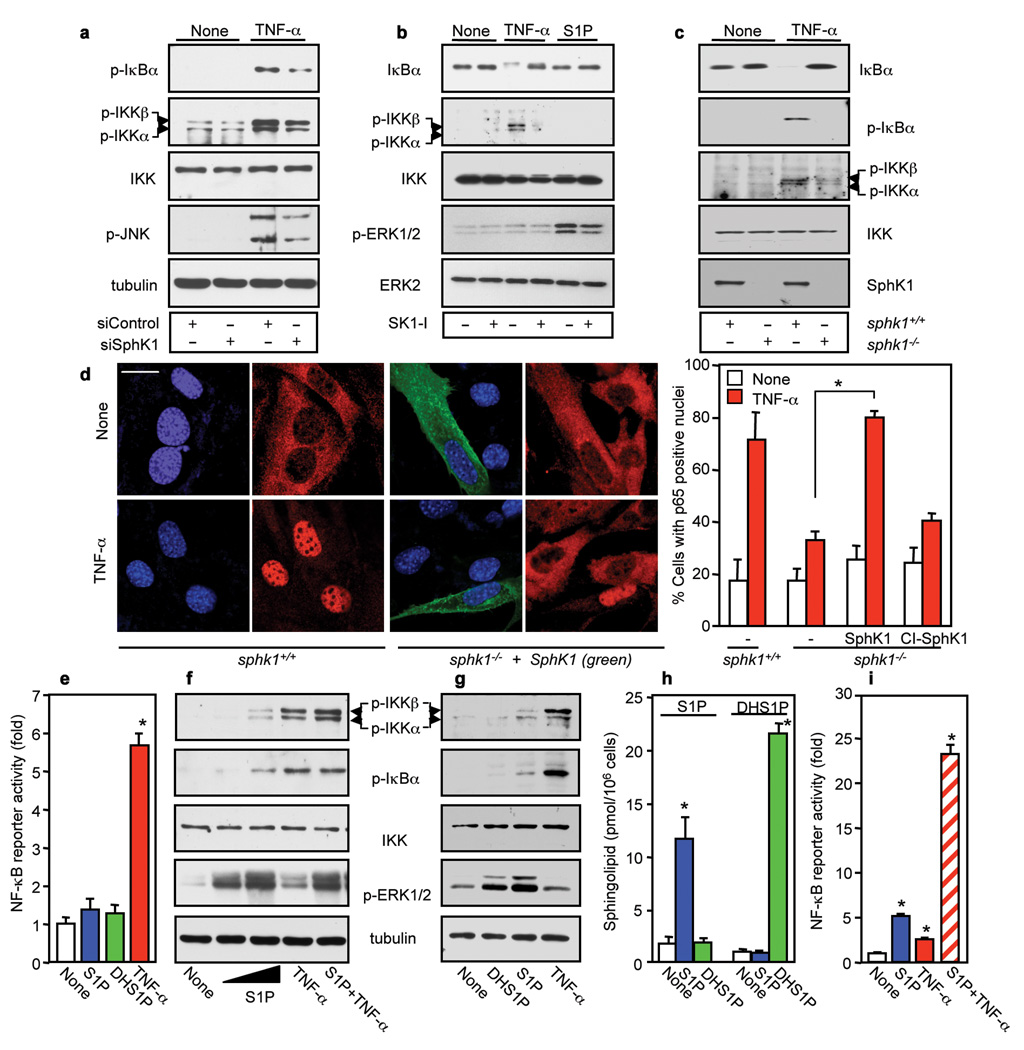
Engagement of the TNF receptor results in assembly of multi-component receptor-associated signaling complexes by adaptors including TNFR1-associated death domain (TRADD), the RING domain ubiquitin ligases, such as TRAF2, and RIP1, which activate the IκB kinase (IKK) complex, composed of two highly homologous kinase subunits, IKKα/IKK1 and IKKβ/IKK2, and a regulatory subunit NEMO/IKKγ. Phosphorylation of IκBα by the IKK complex leads to its Lys 48-linked polyubiquitination and subsequent proteasomal degradation liberating the NF-κB dimer, a transcription factor consisting of p65 and p50 subunits, which enters the nucleus and regulates transcription of target genes 1,2,5. It has previously been demonstrated that the interaction of SphK1 with TRAF2 and subsequent activation of SphK1 links TNF-α signals to activation of NF-κB 4, yet the mechanism of the involvement of SphK1 in the canonical NF-κB pathway has not been elucidated. To this end, expression of SphK1 was downregulated with small interfering RNA (siRNA), which reduced its levels by more than 70% without affecting SphK2 (Supplementary Fig. 1a). Depletion of SphK1 significantly decreased TNF-α–stimulated phosphorylation of IKKα, IKKβ, ανδ IκBα (Fig. 1a), and NF-κB DNA binding and reporter activities (Supplementary Fig. 1b,c,d). In contrast, depletion of SphK2 had no significant effects (Supplementary Fig. 1a,d). To exclude off-target effects, SphK1 expression was also downregulated with siRNAs targeted to two other regions of the SphK1 sequence and both inhibited TNF-α-induced phosphorylation of IκBα and IKKα/β (Supplementary Fig. 2a). Similar results were obtained in several other cell types (Supplementary Fig. 2b), suggesting that SphK1 has a general role in the canonical NF-κB pathway.
Figure 1. SphK1 and intracellular S1P are necessary for NF-κB activation by TNF-α independently of S1P receptors.
a, HEK 293 cells transfected with siControl or siSphK1 were treated with TNF-α and analyzed by immunoblotting. b, A7 cells were pretreated with SK1-I (10 µM) and stimulated with TNF-α or S1P (100 nM). c, sphk1+/+ and sphk1−/− MEFs were stimulated with TNF-α. d, sphk1+/+ or sphk1−/− MEFs transfected with V5-SphK1 or catalytically-inactive SphK1G82D (CI-SphK1) were treated with TNF-α, stained with Hoechst (blue), and p65 (red) and V5 (green) antibodies, and visualized by confocal microscopy. Bar, 20 µm. Percentages of cells with p65 positive nuclei are shown. * P < 0.01. e, NF-κB reporter activity was determined in A7 cells stimulated with TNF-α, 100 nM S1P or dihydro-S1P (DHS1P). f, A7 cells were stimulated with TNF-α (1 ng/ml) or S1P (100 nM or 10 µM) for 10 min. g, HeLa cells were stimulated with TNF-α (1 ng/ml), 10 µM S1P or DHS1P. h, Sphingolipids were analyzed by LC-ESI-MS/MS. i, NF-κB reporter activity was determined in HeLa cells stimulated with TNF-α (1 ng/ml), S1P (10 µM), or both. * P < 0.01 compared to None. n=3.
To conclusively demonstrate the involvement of SphK1, we also used pharmacological and genetic approaches. The specific SphK1 inhibitor, SK1-I, which decreases intracellular S1P levels 6, reduced TNF-α-induced IκBα degradation and phosphorylation of IKKα/β (Fig. 1b) and in a dose-dependent manner that correlated with inhibition of SphK1 activity (Supplementary Fig. 3a,c), yet did not have a significant effect on S1P-induced ERK1/2 phosphorylation (Fig. 1b), as expected for S1P triggered GPCR signaling pathways 7. Activation of c-Jun N-terminal kinase (JNK) by TNF-α was also diminished by depletion of SphK1 (Fig. 1a) or its inhibition (Supplementary Fig. 3b). Likewise, SK1-I reduced activation of p38 (Supplementary Fig. 3a). Finally, upon TNF-α treatment, phosphorylation of IκBα and IKKα/β was absent in sphk1−/− mouse embryonic fibroblasts (MEFs) compared with that in sphk1+/+ MEFs (Fig. 1c). Immunofluorescence and western blotting revealed that SphK1 depletion diminished TNF-α-induced translocation of p65 and p50 from the cytosol to the nucleus (Supplementary Fig. 4a,b). Similarly, sphk1−/− MEFS were also impaired in TNF-α-induced translocation of p65 to the nucleus and transfection of SphK1 rescued this defect, whereas catalytically inactive SphK1 did not (Fig. 1d). Thus, SphK1 is required for TNF-α-induced activation of IKKα/β, IκBα phosphorylation, and nuclear translocation of NF-κB. Moreover, CD40-induced phosphorylation of IKK and IκBα in a B lymphoid cell line was attenuated by SK1-I, which reduced intracellular levels of S1P (Supplementary Fig. 5a,b). Likewise, downregulation of SphK1 also reduced phosphorylation of IKK and IκBα by phorbol 12-myristate 13-acetate (PMA) and ionomycin stimulation (Supplementary Fig. 5c), supporting the general importance of SphK1 in NF-κB activation.
Activation of SphK1 typically results in spatially restricted formation and secretion of S1P that acts in an autocrine or paracrine manner to activate its GPCRs on the cell surface 7. As S1P1 and S1P3 receptors may activate NF-κB through G protein signaling 8,9, it was important to determine whether “inside-out signaling” by S1P could be responsible for SphK1-dependent activation of NF-κB. In agreement with previous studies 4,10,11, TNF-α activated SphK1 (Supplementary Fig. 6a,b), increased intracellular mass levels of S1P and enhanced its transport out of cells (Supplementary Fig. 6c). Surprisingly however, exogenously added 100 nM S1P or dihydro-S1P, which only lacks the double bond in S1P and binds to and activates all of the S1P receptors 7, did not have any detectable effects on phosphorylation of IκBα or its degradation, nor did they stimulate IKK phosphorylation in A7, HEK 293, or HeLa cells (Fig. 1b,f,g, Supplementary Fig. 6d,e). Yet, this concentration of S1P and dihydro-S1P, which is much greater than the Kd values for the S1P receptors, activated ERK1/2 in these cells (Fig. 1b,f,g, Supplementary Fig. 6d), indicating that the lack of effect on the NF-κB pathway is not due to an inability of S1P to signal through its cell surface receptors. Moreover, the S1P1/S1P3 antagonist VPC23019 did not affect TNF-α-induced phosphorylation of IKK and IκBα or its degradation in these cells (Supplementary Fig. 6e). Furthermore, in contrast to TNF-α, neither S1P nor dihydro-S1P stimulated NF-κB reporter activity (Fig. 1e). To corroborate the notion that the actions of SphK1 were due to intracellular generated S1P, we took advantage of our previous observation that only high concentrations of exogenously added S1P significantly increase its intracellular levels 12. While S1P at 100 nM had no significant effects (Fig. 1e,f), treatment with 10 µM S1P, which increased the total intracellular pools of S1P by 7-fold (Fig. 1h), enhanced phosphorylation of IKKα/β and IκBα (Fig. 1f,g) and NF-κB reporter activity (Fig. 1i). This high concentration of S1P also enhanced the response to a suboptimal dose of TNF-α (Fig. 1f,i). Importantly, however, a high concentration of dihydro-S1P, which has the same rate of uptake as S1P 13 and increases its intracellular level to a similar or even greater extent (Fig. 1h), did not mimic the effects of S1P on activation of the NF-κB pathway (Fig. 1g). Thus, intracellular S1P generated by the activation of SphK1 specifically regulates TNF-α-induced NF-κB in a S1P receptor-independent manner.
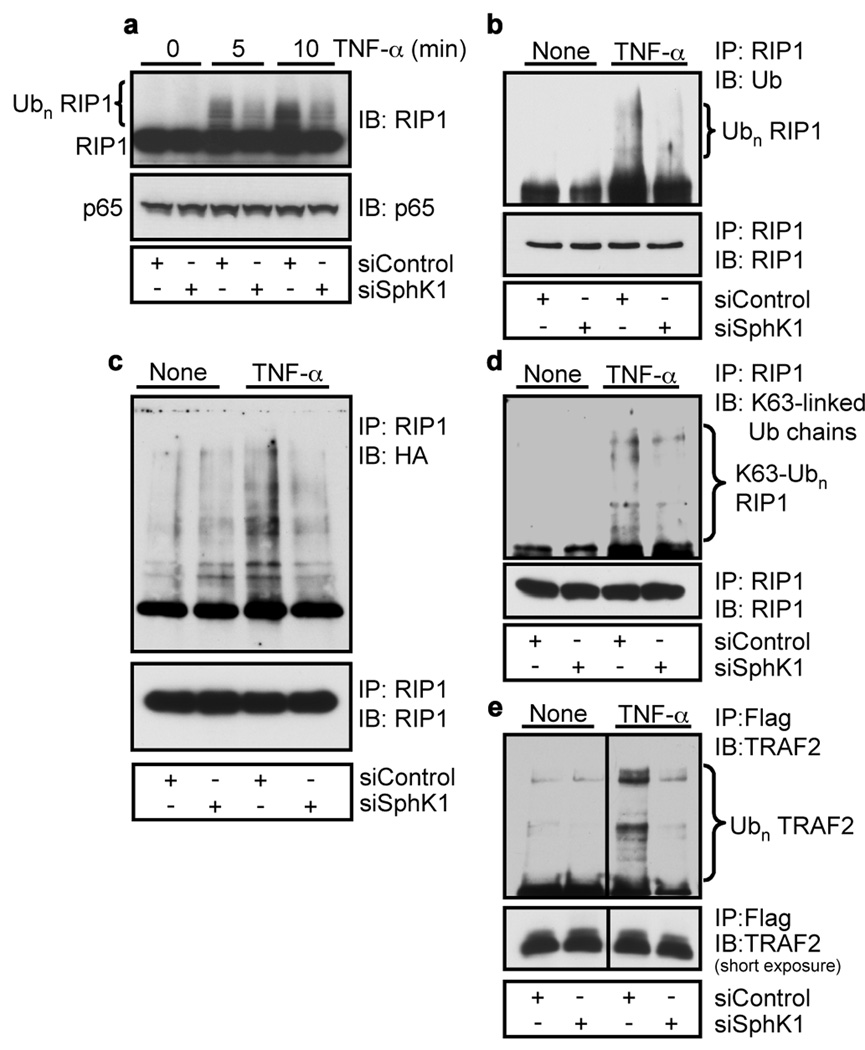
Unlike Lys 48-linked polyubiquitination, which targets proteins for proteasomal degradation, abundant evidence has demonstrated that Lys 63-linked or regulatory ubiquitination plays a critical role in signaling activation 5. One of the best characterized examples is the non-degradative Lys 63 polyubiquitination of RIP1 that serves as a scaffold to recruit proteins containing specific ubiquitin binding domains, resulting in recruitment and phosphorylation of the IKK complex leading to the activation of NF-κB 5,14,15. Figure 2a shows that the high molecular weight species of RIP1 rapidly formed in response to TNF-α were greatly diminished by depletion of SphK1. Analysis of immunoprecipitated RIP1 by immunoblotting with an ubiquitin-specific antibody verified that knockdown of SphK1 reduced polyubiquitination of RIP1 (Fig. 2b). Furthermore, siSphK1 markedly reduced TNF-α-induced conjugation of ectopically expressed ubiquitin with RIP1 (Fig. 2c). An antibody that specifically recognizes Lys 63-linked ubiquitin chains 16 revealed that endogenous Lys 63-linked polyubiquitination of RIP1 was stimulated by TNF-α, consistent with a previous study 17, which was greatly attenuated by SphK1 depletion (Fig. 2d). As was previously reported 18–20, following TNF-α stimulation, TRAF2 itself was polyubiquitinated and this was dramatically reduced in cells depleted of SphK1 (Fig. 2e). Taken together, these results suggest that SphK1 and intracellular generated S1P are important for Lys 63-linked polyubiquitination of RIP1 leading to recruitment and activation of the IKK complex.
Figure 2. SphK1 is required for TNF-α-induced Lys 63-linked polyubiquitination of RIP1.
a, A7 cells transfected with siControl or siSphK1 were stimulated with TNF-α (10 ng/ml). Proteins were immunoblotted with anti-RIP1 or anti-p65 antibodies. b, Lysates were immunoprecipitated with anti-RIP1 antibody and analyzed with anti-ubiquitin antibody. c, Lysates from cells transfected with HA-Ub and stimulated with TNF-α were immunoprecipitated with anti-RIP1 antibody and analyzed with HA antibody. d, Lysates were immunoprecipitated with anti-RIP1 antibody and proteins analyzed with Lys 63-specific polyubiquitin antibody. e, HEK 293 cells transfected with siControl or siSphK1 were transfected with Flag-TRAF2 and stimulated with TNF-α. Proteins were pulled down with anti-Flag beads and analyzed with anti-TRAF2 antibody.
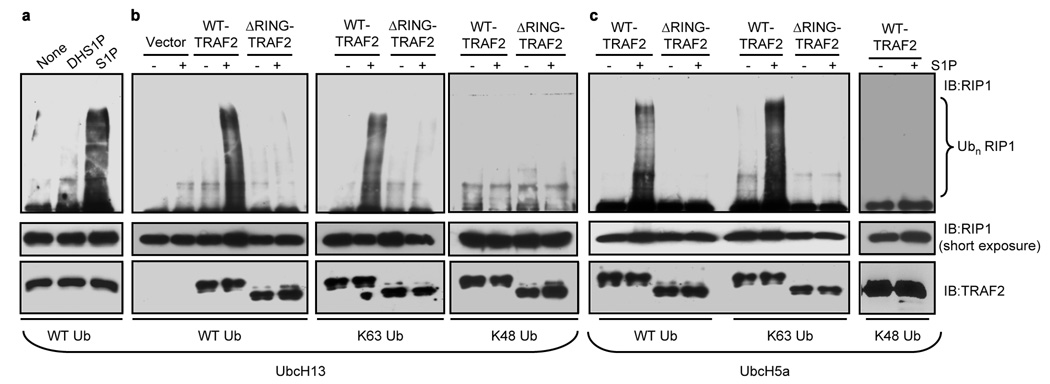
Although it has been shown that RIP1 ubiquitination is dependent on TRAF2 expression 3, evidence that TRAF2 directly catalyzes the ubiquitination of RIP1 is still lacking. Because intracellularly generated S1P was required for optimal TRAF2 and RIP1 polyubiquitination following stimulation by TNF-α, and SphK1 interacts with TRAF2 4, we examined whether S1P is required for direct RIP1 ubiquitination by TRAF2 in vitro. In agreement with previous studies 20,21, incubation of purified RIP1 with recombinant TRAF2, ubiquitin, the ubiquitin-activating enzyme E1, and Ubc13/Uev1a, an E2 that facilitates Lys 63 polyubiquitination, failed to produce ubiquitinated RIP1. Remarkably however, addition of S1P induced efficient TRAF2-mediated ubiquitination of RIP1 (Fig. 3a). In sharp contrast, dihydro-S1P did not mimic the effect of S1P (Fig. 3a). Neither sphingosine nor LPA, lipids structurally related to S1P, enhanced in vitro ubiquitination of RIP1 by TRAF2 (Supplementary Fig. S7a). Furthermore, TRAF2 with a deletion of the N-terminal 87 amino acids containing the RING domain (ΔRING-TRAF2), which cripples its E3 ligase activity, failed to ubiquitinate RIP1 in the absence or presence of S1P (Fig. 3b,c), underscoring the importance of the E3 ligase activity of TRAF2. To determine if the ubiquitin conjugated to RIP1 in vitro was Lys 63 linked, we examined TRAF2-mediated polyubiquitination of RIP1 with wild type ubiquitin and its mutants containing only one lysine at either position 48 (Lys 48) or 63 (Lys 63). S1P enhanced incorporation of wild type and Lys 63 only ubiquitin into RIP1, whereas there was little or no incorporation of the Lys 48 only mutant in the presence of S1P (Fig. 3b,c). Even with the promiscuous E2 enzyme Ubc5a, S1P was still capable of stimulating TRAF2-mediated polyubiquitination of RIP1 with wild type and Lys 63 only ubiquitin and not with Lys 48 only ubiquitin. This effect was also dependent on the presence of the RING domain of TRAF2 (Fig. 3c).
Figure 3. S1P is required for TRAF2-mediated Lys 63-linked polyubiquitination of RIP1 in Vitro.
a, In vitro ubiquitination of purified RIP1 was carried out with ATP, E1, Ubc13/Uev1a, ubiquitin, and TRAF2 with the indicated lipids (100 nM) and examined with anti-RIP1 antibody. b,c, Ubiquitination reactions were carried out with purified WT-TRAF2 or ΔRING-TRAF2 in the presence of UbcH13/Uev1a (b) or UbcH5a/Uev1a (c) as E2s and ubiquitin proteins (WT, Lys 63 only, or Lys 48 only), without or with 100 nM S1P. RIP1 ubiquitination was determined with anti-RIP1 antibody and TRAF2 input with anti-TRAF2 antibody.
Although TRAF2 can act as an adaptor for cIAP1 and cIAP2 that can themselves serve as E3 ligases for RIP1 21–23, neither cIAPs nor TRAF5 were associated with purified TRAF2 used for in vitro ubiquitination assays (Supplementary Fig. 7b). Moreover, S1P also enhanced the E3 ligase activity of recombinant TRAF2 purified from Sf9 insect cells (Supplementary Fig. 7c), further demonstrating that TRAF2 itself is the target of S1P.
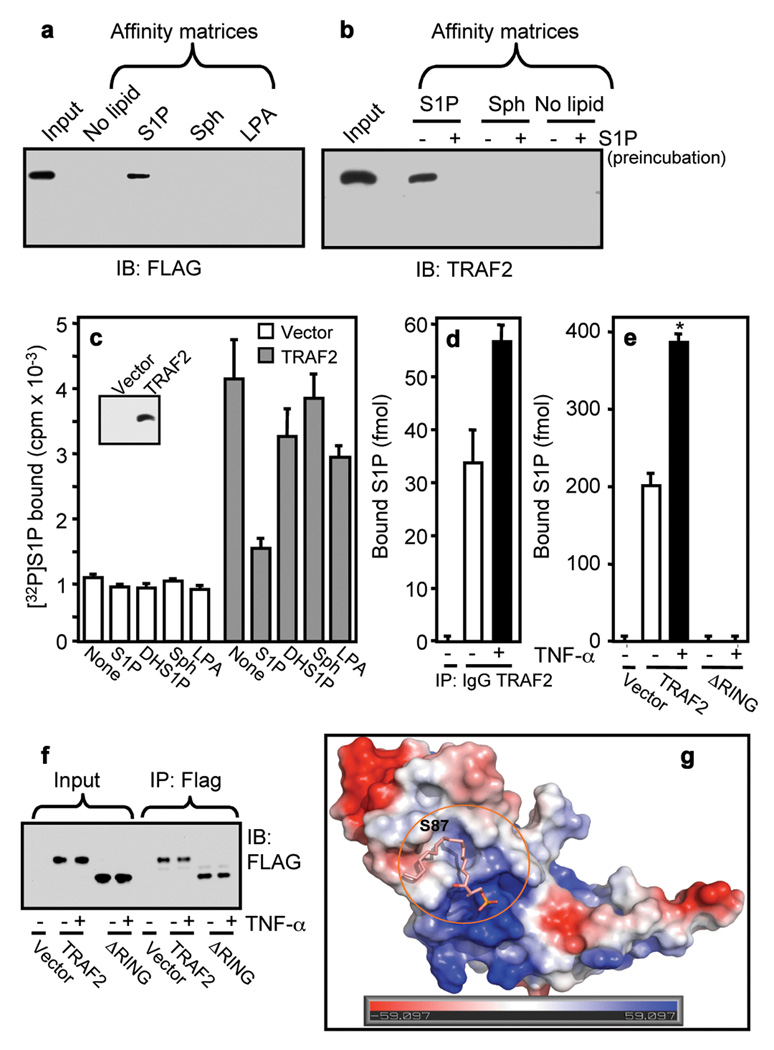
Because our results show that S1P is required for the in vitro E3 ligase activity of TRAF2, it was important to confirm that TRAF2 is a direct target of intracellularly produced S1P. To this end, we first examined binding of TRAF2 to S1P immobilized on agarose beads. Both ectopically expressed Flag-TRAF2 (Fig. 4a) and endogenous TRAF2 (Fig. 4b) were pulled down by matrices carrying S1P but not by control, sphingosine, or LPA matrices. Pre-incubation with exogenous S1P abolished binding of endogenous TRAF2 to the S1P affinity beads (Fig. 4b). Moreover, recombinant TRAF2 purified from Sf9 insect cells also bound to S1P beads, whereas RIP1 and ΔRING-TRAF2 did not (Supplementary Fig. 7d,e,f).
Figure 4. Specific binding of S1P to TRAF2.
a, Lysates from HEK 293 cells transfected with Flag-TRAF2 were incubated with control (no lipid), S1P, LPA, or sphingosine affinity matrices. b, Lysates from naïve cells were pre-treated with 10 µM S1P, followed by pulldown with control (no lipid), S1P, or sphingosine affinity matrices and bound proteins analyzed by immunoblotting. c, Lysates from vector or Flag-TRAF2 transfected cells were incubated with anti-Flag agarose beads. Beads were washed and incubated with [32P]S1P (0.1 nM) in the absence or presence of 1 µM unlabeled S1P, dihydro-S1P, sphingosine or LPA, and [32P]S1P bound to TRAF2 was eluted with Flag peptide and radioactivity determined. Insert, blot of eluted TRAF2. d, Naïve cells were stimulated with TNF-α. Lysates were immunoprecipitated with anti-TRAF2 antibody or control IgG and bound sphingolipids determined by LC-ESI-MS/MS. Of all of the sphingolipids present in these cells (Supplementary Table 1), only S1P was detected in the immunocomplexes. e,f, Cells transfected with vector, Flag-TRAF2, or Flag-ΔRING-TRAF2 were stimulated with TNF-α. Lysates were immunoprecipitated with anti-Flag antibody. (e) Bound S1P determined by LC-ESI-MS/MS. (f) Immunoblot with anti-Flag antibody. g, Docking of S1P into the pocket of the RING domain of TRAF2. Surface contour of the binding site with S1P was colored by electrostatic potential.
Next, direct binding of S1P to TRAF2 was evaluated. 32P-labeled S1P specifically bound to Flag-tagged TRAF2 that was eluted from anti-Flag agarose beads with Flag peptide. Of note, binding to TRAF2 was abolished by addition of excess cold S1P but not by dihydro-S1P, LPA, or sphingosine (Fig. 4c), in agreement with the specific requirement for S1P to activate the E3 ligase activity of TRAF2 (Fig. 3a). Binding of 32P-S1P to TRAF2 was reduced by 50% at a concentration of 0.5 µM unlabeled S1P (Supplementary Fig. 8a). Finally, we sought to examine whether endogenous S1P is bound to TRAF2 in vivo and whether this interaction is influenced by TNF-α, which activates SphK1. Cells were treated with TNF-α and the sphingolipids in TRAF2 immunoprecipitates were measured by mass spectrometry (LC-ESI-MS/MS). Of all the sphingolipids present in cells, only S1P was bound to TRAF2 (Fig. 4d, Supplementary Table 1), and this association was significantly increased by TNF-α (Fig. 4d). Moreover, as expected from a specific interaction, much more S1P was bound to ectopically expressed TRAF2 and this association was also further enhanced by treatment with TNF-α. However, there was no detectable S1P associated with ΔRING-TRAF2 as measured by LC-ESI-MS/MS (Fig. 4e,f), which also did not bind to S1P affinity beads (Supplementary Fig. 7f), suggesting that the binding site for S1P is within the RING domain of TRAF2.
Molecular modeling of S1P into the RING domain of the crystal structure of TRAF2 24 revealed that it docked remarkably well in a 16 Å long binding cavity consisting of a hydrophobic region (F45, L58, A59, L62, A90, F91 and F92) and positively charged region (R43 and R97), which may stabilize the phosphate group of S1P (Fig. 4g, Supplementary Fig. 9). Further molecular dynamic simulation and free energy calculation indicates the binding for S1P is −8.07 kcal/mol while dihydro-S1P shows instability in binding with TRAF2 during the dynamic simulation process, consistent with the inability of dihydro-S1P to bind or activate TRAF2. Indeed, estimated Ki values by AutoDock 25 for S1P and dihydro-S1P are 8.74×10−7 and 5.37×10−4, respectively (T=298.15 K). In contrast, no binding was detected to TRAF3 (Supplementary Fig. 8b,c). In agreement, sequence alignment and homology modeling did not identify an obvious binding pocket near residue Lys97 of TRAF3 (corresponding to Ser87 in TRAF2). S1P also stimulated in vitro autoubiquitination of recombinant TRAF2 (Supplementary Fig. 8d).
Hence, S1P is a missing cofactor for TRAF2, an E3 ligase that plays an important role in Lys 63-linked polyubiquitination of RIP1 and consequent regulation of NF-κB activation and the anti-apoptotic program initiated by TNF-α 1,2,5. Increased RIP1 Lys 63 ubiquitination prevents switching of RIP1 from a prosurvival to proapoptotic adaptor protein (Supplementary Fig. 10). These findings also resolve the puzzle associated with the cytoprotective effects of SphK1 and S1P 7. Although S1P and dihydro-S1P are equally potent ligands for the five S1P receptors, only S1P suppresses apoptosis as only S1P and not dihydro-S1P binds to and activates TRAF2. Our study also provides a mechanistic explanation for the numerous observations of the importance of SphK1 in inflammatory, anti-apoptotic, and immune processes.
METHODS SUMMARY
For details of expression plasmids, proteins, antibodies, cell lines, and methods for immunoprecipitations, immunofluorescence, pulldowns, S1P binding, and mass spectrometry, see the online-only Methods. In vitro ubiquitination assays were performed as described previously 22, with some modifications, as described in Online Methods.
Supplementary Material
Acknowledgements
We thank Dr. Z.J. Chen, Dr. B. Darney, and Dr. M. Karin for HA-ubiquitin and TRAF constructs, and Dr. R. Proia for the sphk1−/− mice. This work was supported by grants from the National Institute of Health (R37GM043880, R01CA61774, R01AI50094, U19AI077435 to S.S.) and in part by the Ministry of Scientific and Technology of China, 2009CB918502 to C.L.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions S.E.A. and K.B.H. planned and performed most experiments, with assistance from N.C.H., G.M.S., E.Y.K., J.A., and M.M. C.L. and H.J. performed molecular docking, T.K. contributed to the planning of the experiments, S.M. and S.S. conceived the study, contributed to planning of the experiments and wrote the manuscript.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References
- 1.Karin M, Gallagher E. TNFR signaling: ubiquitin-conjugated TRAFfic signals control stop-and-go for MAPK signaling complexes. Immunol. Rev. 2009;228:225–240. doi: 10.1111/j.1600-065X.2008.00755.x. [DOI] [PubMed] [Google Scholar]
- 2.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Lee TH, Shank J, Cusson N, Kelliher MA. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J. Biol. Chem. 2004;279:33185–33191. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- 4.Xia P, et al. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J. Biol. Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 5.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 6.Paugh SW, et al. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112:1382–1391. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 8.Kimura T, et al. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high-density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J. Biol. Chem. 2006;281:37457–37467. doi: 10.1074/jbc.M605823200. [DOI] [PubMed] [Google Scholar]
- 9.Ki SH, Choi MJ, Lee CH, Kim SG. Galpha 12 specifically regulates COX-2 induction by sphingosine 1-phosphate: Role for JNK-dependent ubiquitination and degradation of Ikappa Balpha. J. Biol. Chem. 2007;282:1938–1947. doi: 10.1074/jbc.M606080200. [DOI] [PubMed] [Google Scholar]
- 10.Pitson SM, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: Role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J. Biol. Chem. 2007;282:14165–14177. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Brocklyn JR, et al. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled orphan receptor edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giussani P, et al. Sphingosine-1-phosphate phosphohydrolase regulates endoplasmic reticulum-to-Golgi trafficking of ceramide. Mol. Cell Biol. 2006;26:5055–5069. doi: 10.1128/MCB.02107-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat. Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, et al. Analysis of nondegradative protein ubiquitylation with a monoclonal antibody specific for lysine-63-linked polyubiquitin. Proc. Natl. Acad. Sci. U.S.A. 2008;105:20197–20202. doi: 10.1073/pnas.0810461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton K, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Shi CS, Kehrl JH. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2) J. Biol. Chem. 2003;278:15429–15434. doi: 10.1074/jbc.M211796200. [DOI] [PubMed] [Google Scholar]
- 19.Habelhah H, et al. Ubiquitination and translocation of TRAF2 is required for activation of JNK but not of p38 or NF-kappaB. EMBO J. 2004;23:322–332. doi: 10.1038/sj.emboj.7600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Wang L, Dorf ME. PKC phosphorylation of TRAF2 mediates IKKalpha/beta recruitment and K63-linked polyubiquitination. Mol. Cell. 2009;33:30–42. doi: 10.1016/j.molcel.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertrand MJ, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Varfolomeev E, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J. Biol. Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu M, Skaug B, Zeng W, Chen ZJ. A ubiquitin replacement strategy in human cells reveals distinct mechanisms of IKK activation by TNFalpha and IL-1beta. Mol. Cell. 2009;36:302–314. doi: 10.1016/j.molcel.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin Q, Lamothe B, Darnay BG, Wu H. Structural basis for the lack of E2 interaction in the RING domain of TRAF2. Biochemistry. 2009;48:10558–10567. doi: 10.1021/bi901462e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris GM, et al. Automated docking using a Lamarckian genetic algorithm and empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. [Google Scholar]
- 26.Hait NC, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maceyka M, Alvarez SE, Milstien S, Spiegel S. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Mol. Cell Biol. 2008;28:5687–5697. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allende ML, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 29.Paugh BS, et al. EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKCdelta, and sphingosine kinase 1 in glioblastoma cells. FASEB J. 2008;22:455–465. doi: 10.1096/fj.07-8276com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theiss AL, et al. Prohibitin inhibits tumor necrosis factor alpha-induced nuclear factor-kappa B nuclear translocation via the novel mechanism of decreasing importin alpha3 expression. Mol. Biol. Cell. 2009;20:4412–4423. doi: 10.1091/mbc.E09-05-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.