Abstract
Radiation sensitization is significantly increased by proteotoxic stress, such as a heat shock. We undertook an investigation, seeking to identify natural products that induced proteotoxic stress and then determined if a compound exhibited radiosensitizing properties. The hydroxychalcones, 2′,5′-dihydroxychalcone (D-601) and 2,2′-dihydroxychalcone (D-501), were found to activate heat shock factor 1 (Hsf1) and exhibited radiation sensitization properties in colon and pancreatic cancer cells. The radiosensitization ability of D-601 was blocked by pretreatment with α-napthoflavone (ANF), a specific inhibitor of cytochrome P450 1A2 (CYP1A2), suggesting that the metabolite of D-601 is essential for radiosensitization. The study demonstrated the ability of hydroxychalcones to radiosensitize cancer cells and provides new leads for developing novel radiation sensitizers.
Dietary polyphenols are present in fruits, vegetables, herbal medicines, wines, and spices. These compounds possess antioxidant anti-inflammatory and anticancer activities. Chalcones are compounds with two aromatic rings and an unsaturated side chain, i.e., phenyl ketone analogs of cinnamic acid. Hydroxychalcones are abundant in plant material and have been shown to have antitumor activities, which has been attributed to prooxidant phenoxyl radical formation rather than antioxidant properties 1. It is the proteotoxic and genotoxic oxidative stress induced by hydroxychalcones that provide a rationale for their use as cancer chemotherapy. Radiation therapy represents an important tool for the treatment of colorectal cancer 2, 3. Its efficacy is a consequence of genotoxic oxidative stress. Current technology allows precise conformal 3-dimensional irradiation of a tumor, resulting in significant normal tissue dose sparing. Yet, even with this outstanding ability to physically target tumor tissue while sparing surrounding normal tissues, there are many instances in which tumors do not respond to irradiation. Furthermore, 8-10% of tumors that exhibit an initial response, recur and the median overall survival for these patients is approximately 2 years from the time of recurrence4, 5. The failure of radiation therapy to yield definitive local-regional control underscores the urgent need for development of radiation sensitizers.
Heat shock (temperatures > 42°C) is one of the most potent radiation sensitizers identified to date6, 7. Recent phase III clinical trials have demonstrated that hyperthermia can increase complete response rates and overall survival of patients with cervical tumors, as well as squamous and soft tissue sarcomas 8-11. Hyperthermia, however, is currently limited clinically because of technical problems of heat delivery 12. In order to overcome this problem, we designed and synthesized several novel indole analogs that enhance the degree of radiosensitization produced by a moderate heat shock (41°C)13, 14. The molecular mechanisms involved in thermal radiosensitization involve a proteotoxic stress that initiates mitotic catastrophe and a late apoptosis13. We have also designed and synthesized several analogs that function as heat mimetics that produce radiosensitization at physiological temperatures15. In our continuing effort to develop heat mimetics that function as radiosensitizers at physiological temperature, we searched the scientific literature for proteotoxic compounds. In the present study two hydroxychalcones were selected for study. These compounds exhibit prooxidant properties, which are known to induce proteotoxic stress and initiate mitochondrial membrane depolarization1. The selection process also included commercial availability.
Hydroxychalcones, 2′,5′-dihydroxychalcone (D-601) and 2,2′-dihydroxychalcone (D-501), were purchased from Indofine Chemical Company, Inc., NJ. Colony formation assays15 of a human colon adenocarcinoma cell line (HT-29) and a human pancreatic carcinoma cell line (Panc-1) were used to estimate drug toxicity and radiation sensitivity. Cancer cells were treated with DMSO/drug for 2 hr to determine non-toxic drug concentrations, defined as a plating efficiency16 greater than 80%. After 10 to 14 days, cells were fixed for 15 minutes with 3:1 methanol/acetic acid and stained for 15 minutes with 0.5% crystal violet (Sigma) in methanol. Colonies were counted to estimate the percentage of survival. D-601 did not exhibit any toxicity (plating efficiency > 80%) at 25 μM. D-501 was used at 10 μM (Plating efficiency > 80%).
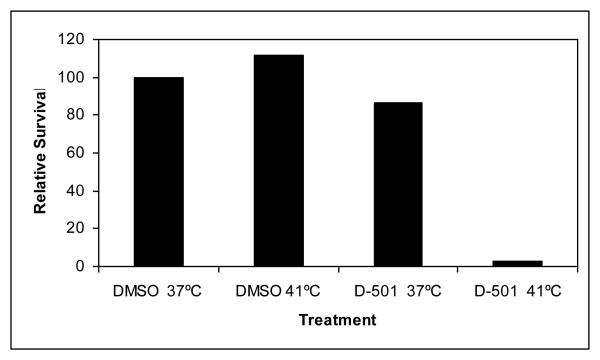
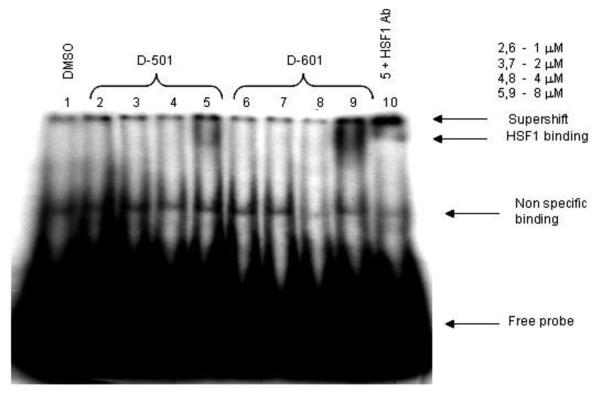
D-501 (10 μM) was initially tested for its ability to sensitize HT-29 cells to mild heat shock (41°C) (Fig. 2). D-501 exhibited robust thermal sensitization of HT-29 cells. Destabilization of protein conformation by heat represents a common underlying biophysical mechanism for heat induced cell killing and thermal radiosensitization16-19. Enhanced Hsf1 DNA-binding activity is also observed with increased destabilization and unfolding of proteins 20. Senisterra et al.21 found that the minimum temperature that caused cellular proteins with intrinsically low conformational stability to unfold and denature is quantitatively the same minimum temperature required for Hsf1 activation. Hsf1 resides as a repressed monomer due to its association with Hsp90. In the presence of unfolded protein, Hsp90 disassociates from Hsf1, initiating Hsf1 trimerization and acquisition of DNA-binding activity 20. We therefore used Hsf1 activation as a surrogate for measurement of chalcone-mediated global protein denaturation. HT-29 cells were treated with various conceentrations of drug for 30 min and nuclear protein extracted. Gel shift assays were conducted using rat Hsp70 heat shock element (HSE) oligomer 22. The data presented in Fig. 3 illustrate significant enhancement of Hsf1 DNA binding activity upon addition of D-501 and D-601 (8 μM) to cells incubated for 2 hr at 37°C. HSF1 binding was not observed in control treatment. HSF1 supershift was conducted on Lane 10 using Hsf1 antibody confirming the presence of Hsf1 binding at the HSE. These results demonstrate that D-501 and D-601 destabilized protein conformation at physiological temperatures.
Fig.2.
HT-29 cells were treated with D-501/DMSO and heat shocked at 41°C for 2 hr. Medium was changed and cells incubated for 10-14 days and then stained. Colonies were counted. Four flasks were used for each condition.
Fig.3.
Gel mobility shift assay. HT-29 cells were treated with D-501 and D-601 at various concentrations and nuclear protein was probed for binding to 32P labeled heat shock element (HSE).
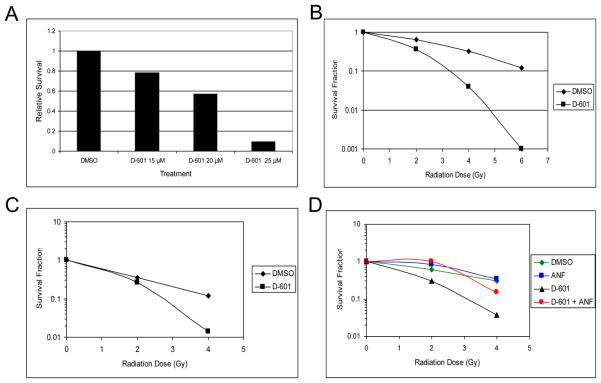
We determined if hydroxychalcones D-601 and D-501 (Data not shown) enhanced the radiation sensitivity of HT-29 and Panc-1 cells. Cells were exposed to drug/DMSO for 30 min/37°C, during irradiation at room temperature and additional 90 min at 37°C. Irradiation was accomplished using a Cs137 source at 2 Gy per min. Cell survival was measured by clonogenic assay as described above. D-601 exhibited a concentration dependent radiosensitization of HT-29 cells (Fig. 4A). Full radiation dose response curves for HT-29 and Panc-1 cells are shown in Fig. 4B and 4C. D-601 synergistically radiosensitized both cancer cell lines.
Fig. 4.
Radiosensitization of HT-29 and Panc 1 cells by D-601. A) Concentration effect of D-601 on HT-29 cell survival following 4 Gy. Survival following 4 Gy (DMSO control) was 0.25 and is expressed as a relative survival of 1.0; B) Survival of HT-29 cells at 25 μM of D-601 and various radiation doses; C) Effect of D-601 and radiation on the survival of Panc1 cells; D) Effect of α-napthoflavone (ANF) on radiosensitization by D-601.
We also tested the potency of D-501. HT-29 cells were exposed to a dose of 10 μM D-501 and administered 4 Gy. Survival relative to the DMSO control was 0.43. Inspection of Fig 4A indicates that a dose of 20 μM D-601 was required to produce the same degree of radiation sensitivity. Thus, D-501 is twice as potent as D-601.
At the clinically relevant dose of 2 Gy, D-501 increased radiation sensitivity 2 fold. In HT-29 a dose of 2 Gy did not produce a statistically significant decrease in survival compared to non-irradiated cells (survival was 1.0 +/− 2 Gy; p > 0.05; Student's t test). In the presence of 10 μM D-501, 2 Gy reduced survival to 0.53 (p < 0.05; Student's t test).
It is not known whether these chalcones exert their effects directly or through metabolites that may have formed due to P450 actions. Hydroxychalcones have been reported to possess cytotoxic properties1. Since P450 enzymes add hydroxy group to the hydroxychalcones23, 24, it is hypothesized that metabolites of these chalcones could be the active substance. To test this hypothesis, we have used α-naphthoflavone (ANF) which is a specific inhibitor of CYP1A2 in combination with D-601. The results are presented in Fig. 4D. Pretreatment of HT-29 cells with ANF partially reversed the effects of D-601 dependent radiosensitization. Pretreatment with a P450 3A inhibitor, miconazole did not affect the radiosensitization induced by D-601 (data not shown). These results support the hypothesis that a metabolite of D-601 formed due to the actions of cytochrome P450 1A2 is responsible for partial radiosensitization of HT-29 cells. We predict that D-501 also produces active metabolites due to its structural similarity to D-601. For example, hydoxylated metabolite of the catechol butein [2′,4′3,4-tetrahydroxychalcone] is more potent antioxidant, electron donor, and hydrogen donor than isoliquiritigenin [2,4,4′-trihydroxychalcone] due to the presence of additional hydroxy group25. We hypothesize that the increased potency of D-501 is due to multiple hydroxylations of D-501 (due to activation of both rings by hydroxy groups present in the molecule) by P450 enzyme(s). This could lead to formation of diquinone product as shown with diphenolic compounds26 and ultimately generate oxidative stress.
Cell cycle analysis was performed in the presence of D-601 and radiation. Cells were treated with DMSO/drug for 2 hr and were harvested. The cells were suspended in 500 μl of PBS containing 0.1% glucose and kept at 4°C. Cold 70% EtOH (5 ml) was immediately added and mixed. Prior to FACS analysis, the cells were washed with PBS, added 300 μl of propidium iodide solution and 20 μl of 10 mg/ml RNase. The contents were incubated at 37° C for 30-45 min. The cells were transferred to FACS tubes and analyzed. The analysis demonstrated that exposure to D-601 plus radiation induced polyploidy formation that was followed by an apoptotic response, as measured by sub-G0 cells 48 hrs after irradiation (data not shown).
In the present study, we have demonstrated the ability of hydroxychalcones to induce proteotoxicity at physiological temperatures. Based on our previous studies13, we hypothesize that radiosensitization is a consequence of denaturation of critical proteins by D-601 that leads to mitotic catastrophe and a late apoptotic response. Chalcones are known to exhibit a wide variety of activities including glutathione depletion which leads to Phase II enzyme induction through activation of transcription factor, Nrf2. This is the basis of cancer prevention studies. In this case, chalcones act as Michael Reaction Acceptors due to the presence of α,β-unsaturated ketone27. We have shown that glutathione depletors activate heat shock factor 1 (Hsf1) and induce HSP70 protein expression28. Thermal sensitization was not observed when indole based chalcones were tested (data not shown). Thus, we predict the thermal sensitization property of hydroxychalcones is not completely based on their glutathione depletion properties. Further study is needed to identify the exact mechanism by which hydroxychalcones function as radiation sensitizers. In addition, we have shown that P450 enzyme inhibitor partially blocks radiosensitization generated by D-601 treatment. Guo et al.24 have shown that hydroxychalcone, isoliquiritigenin, generated several metabolites which include hydroxylated product (2′4,4′,5′-tetrahydroxychalcone), cyclized products (4,4′-dihydroxyflavanone), and cis- and trans 6,4′-dihydroxyaurone. In the present study we have not studied which metabolites formed from D-601. We predict a minimum of hydroxylated product and cyclized flavanone formation with this drug. Further study needed to identify active metabolite. In conclusion, these compounds provide new leads for the development of novel thermal radiosensitizers that function at physiological temperature.
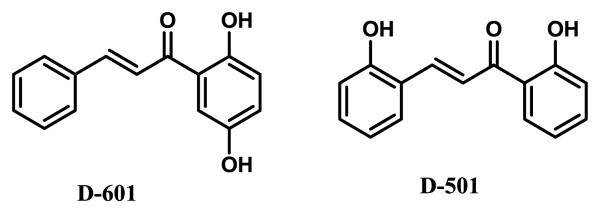
Fig.1.
Structures of hydroxychalcones (D-601 and D-501) used in the present study. Compound numbers are their catalog number from Indofine Chemical Company.
Acknowledgements
This work was supported by NIH/National Cancer Institute grant PO1 CA104457 and Pilot Project support from P50 CA95103.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sabzevari O, Galati G, Moridani MY, Siraki A, O'Brien PJ. Chemico-biological interactions. 2004;148:57. doi: 10.1016/j.cbi.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Gunnlaugsson A, Anderson H, Fernebro E, Kjellen E, Bystrom P, Berglund K, Ekelund M, Pahlman L, Holm T, Glimelius B, Johnsson A. Eur J Cancer. 2009;45:807. doi: 10.1016/j.ejca.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW, van de Velde CJ. Annals of surgery. 2007;246:693. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]
- 4.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. The New England journal of medicine. 2006;355:1114. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 5.Yu TK, Bhosale PR, Crane CH, Iyer RB, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Eng C, Wolff RA, Janjan NA, Delclos ME, Krishnan S, Das P. International journal of radiation oncology, biology, physics. 2008;71:1175. doi: 10.1016/j.ijrobp.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kampinga HH, Dikomey E. International journal of radiation biology. 2001;77:399. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- 7.Raaphorst GP, Azzam EI. British journal of cancer. 1983;48:45. doi: 10.1038/bjc.1983.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitamura K, Ishida M, Kimura Y, Saeki H, Maehara Y, Sugimachi K. Hepato-gastroenterology. 2002;49:1560. [PubMed] [Google Scholar]
- 9.Tanaka H, Ostapenko VV, Miyano M, Nishide T, Sonobe M, Toda K, Nishide I, Mune M, Yukawa S. Hepato-gastroenterology. 2002;49:1666. [PubMed] [Google Scholar]
- 10.van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Lancet. 2000;355:1119. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 11.Schlemmer M, Wendtner CM, Lindner L, Abdel-Rahman S, Hiddemann W, Issels RD. Int J Hyperthermia. 2010;26:127. doi: 10.3109/02656730903335995. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen OS, Horsman M, Overgaard J. Eur J Cancer. 2001;37:1587. doi: 10.1016/s0959-8049(01)00193-9. [DOI] [PubMed] [Google Scholar]
- 13.Sekhar KR, Sonar VN, Muthusamy V, Sasi S, Laszlo A, Sawani J, Horikoshi N, Higashikubo R, Bristow RG, Borrelli MJ, Crooks PA, Lepock JR, Roti Roti JL, Freeman ML. Cancer research. 2007;67:695. doi: 10.1158/0008-5472.CAN-06-3212. [DOI] [PubMed] [Google Scholar]
- 14.Sonar VN, Thirupathi Reddy Y, Sekhar KR, Sasi S, Freeman ML, Crooks PA. Bioorganic & medicinal chemistry letters. 2007;17:6821. doi: 10.1016/j.bmcl.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy YT, Sekhar KR, Sasi N, Reddy PN, Freeman ML, Crooks PA. Bioorganic & medicinal chemistry letters. 2010;20:600. doi: 10.1016/j.bmcl.2009.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laszlo A, Davidson T, Harvey A, Sim JE, Malyapa RS, Spitz DR, Roti Roti JL. Int J Hyperthermia. 2006;22:43. doi: 10.1080/02656730500394296. [DOI] [PubMed] [Google Scholar]
- 17.Lepock JR. Int J Hyperthermia. 2005;21:681. doi: 10.1080/02656730500307298. [DOI] [PubMed] [Google Scholar]
- 18.Lepock JR, Frey HE, Ritchie KP. The Journal of cell biology. 1993;122:1267. doi: 10.1083/jcb.122.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stege GJ, Brunsting JF, Kampinga HH, Konings AW. Journal of cellular physiology. 1995;164:579. doi: 10.1002/jcp.1041640316. [DOI] [PubMed] [Google Scholar]
- 20.Westerheide SD, Morimoto RI. The Journal of biological chemistry. 2005;280:33097. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- 21.Senisterra GA, Huntley SA, Escaravage M, Sekhar KR, Freeman ML, Borrelli M, Lepock JR. Biochemistry. 1997;36:11002. doi: 10.1021/bi9711590. [DOI] [PubMed] [Google Scholar]
- 22.McDuffee AT, Senisterra G, Huntley S, Lepock JR, Sekhar KR, Meredith MJ, Borrelli MJ, Morrow JD, Freeman ML. Journal of cellular physiology. 1997;171:143. doi: 10.1002/(SICI)1097-4652(199705)171:2<143::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 23.Guo J, Liu A, Cao H, Luo Y, Pezzuto JM, van Breemen RB. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:2104. doi: 10.1124/dmd.108.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo J, Liu D, Nikolic D, Zhu D, Pezzuto JM, van Breemen RB. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:461. doi: 10.1124/dmd.107.018721. [DOI] [PubMed] [Google Scholar]
- 25.Nerya O, Musa R, Khatib S, Tamir S, Vaya J. Phytochemistry. 2004;65:1389. doi: 10.1016/j.phytochem.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Bensasson RV, Zoete V, Dinkova-Kostova AT, Talalay P. Chemical research in toxicology. 2008;21:805. doi: 10.1021/tx7002883. [DOI] [PubMed] [Google Scholar]
- 27.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3404. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman ML, Sierra-Rivera E, Voorhees GJ, Eisert DR, Meredith MJ. Radiation research. 1993;135:387. doi: 10.2307/3578879. [DOI] [PubMed] [Google Scholar]