Abstract
Background
Agents that target pro-inflammatory cytokines may be useful in pulmonary sarcoidosis.
Objective
To determine effectiveness of a non-selective cyclic nucleotide phosphodiesterase (PDE) inhibitor, pentoxifylline (POF).
Design
Randomized, double-blind, placebo-controlled trial
Setting
Clinical Research Center, National Institutes of Health.
Patients
27 patients with biopsy-confirmed pulmonary sarcoidosis receiving prednisone.
Intervention
Placebo or POF (1200-2000 mg/day) for 10 months, as prednisone was tapered.
Measurements
Primary endpoints: sustained improvement in two or more pulmonary function parameters, or a combination of one pulmonary function parameter and dyspnea.
Results
Except for one patient, primary endpoints were not reached in POF-treated patients. Therefore, a post hoc analysis was performed. The observed relative risk reduction for flares associated with POF treatment was 54.9% (95% CI 0.21, 0.89) and the absolute risk reduction was 50.6% (95% CI 0.22, 0.80). Compared to placebo treatment, in the POF group, the mean prednisone dose was lower at 8 and 10 months (p = 0.007 and 0.01 respectively), and there was a trend towards less prednisone usage over the entire study period (p = 0.053), as determined by cumulative change analysis.
Conclusions
Although our exploratory post hoc analysis suggested that POF reduced flares and had steroid-sparing effects, given the study limitations, definitive conclusions cannot be drawn regarding the efficacy of POF in pulmonary sarcoidosis. In addition, gastrointestinal side-effects, at the doses used, would seem to limit the use of POF in treating pulmonary sarcoidosis. Overall, however, this trial may provide a basis for using more specific, better-tolerated, PDE inhibitors in future clinical trials.
Keywords: pentoxifylline, phosphodiesterase, sarcoidosis, steroid-sparing, pulmonary function
Introduction
Sarcoidosis is a multi-system inflammatory disease of unknown etiology, in which affected organs, most commonly the lungs, are infiltrated with well-formed, non-caseating granulomas composed of monocytes, macrophages, epithelioid and multinucleated giant cells, and CD4+T helper-1(Th-1) lymphocytes (1, 2). Since the pathogenesis of granuloma formation in sarcoidosis involves the production and release of inflammatory chemokines, such as macrophage inflammatory protein 1 alpha (MIP-1α) (3) and MIP-1β (4, 5), regulated on activation normal T expressed and secreted (RANTES) (6) and other cytokines (7, 8), including interferon gamma (INF-γ), tumor necrosis factor alpha (TNF) (9), interleukin-2 (IL-2) (10), and interleukin-12 (IL-12) (11), therapies directed against one or more of these mediators might be effective in ameliorating the granulomatous inflammatory process, which is often intractable and debilitating.
The second messenger, adenosine-3′, 5′-cyclic monophosphate (cAMP) is known to inhibit inflammatory responses (12). Agents that increase cAMP, including inhibitors of cyclic nucleotide phosphodiesterases (PDEs) (13), such as pentoxifylline (POF) (14), could serve as therapeutic options to corticosteroids, which are the current mainstay of therapy for sarcoidosis (2), and have numerous undesirable side effects which can lead to a decreased quality of life.
Pentoxifylline (POF), a xanthine derivative, is a non-specific PDE inhibitor which exhibits anti-inflammatory properties and has been used for the treatment of peripheral vascular disease (15-20). POF inhibited interleukin-2 receptor (IL-2R) expression (21), and production of TNF (22), IL-2, and IFNγ by human peripheral blood monocytes and T-lymphocytes (15). Administration of POF reduced plasma TNF and decreased TNF and IL-12 mRNA expression by peripheral blood monocytes isolated from subjects with relapsing-remitting multiple sclerosis (18), a Th-1 polarized inflammatory disease. In addition, POF inhibited TNF release by alveolar macrophages isolated from patients with sarcoidosis (23, 24) and extrinsic allergic alveolitis (25). Since TNF plays a pivotal role in granuloma formation and maintenance (26, 27), and since the clinical course of pulmonary sarcoidosis may correlate with increased IL-2 and TNF cytokine production (10), POF, by its inhibition of TNF release, might be useful as a steroid-sparing agent in diseases such as pulmonary sarcoidosis.
Since some reported benefits of POF in an open-label study (28) could have been related to spontaneous remission of pulmonary sarcoidosis, we designed a randomized, double-blind, placebo-controlled trial with POF in patients with pulmonary sarcoidosis who required corticosteroid therapy, to determine whether POF could provide an alternative to prednisone. However, recruitment goals were not met, and there were no differences in primary endpoints, that is, sustained improvement in two or more pulmonary function parameters, or a combination of one pulmonary function parameter and dyspnea. Therefore, a post hoc exploratory analysis was performed for hypothesis generation, and an analysis of the study methodology was conducted to provide guideposts for design of future trials. As reported here, this analysis indicated that POF-treated patients experienced significantly fewer flares, or recurrence of disease, and suggested that POF might have had steroid-sparing effects.
Methods
Patient Selection and Eligibility Criteria
Subjects between ages 18 and 70 were enrolled if they had pulmonary sarcoidosis, which was diagnosed by a compatible clinical history and supported by a lung or intrathoracic lymph node biopsy, and, in addition to other criteria, if they were prednisone-requiring as determined by their pulmonologist or primary medical doctor.
Protocol Design
After telephone and clinical screening of recruited subjects, informed consent was obtained from eligible subjects, who were then enrolled and randomized, in a double-blind fashion, to receive POF or placebo, as their baseline prednisone was systematically tapered and discontinued as tolerated. If the dose of prednisone, at enrollment, was greater than or equal to 40 mg per day, prednisone was reduced bi-weekly according to the following regimen: 40 mg daily, 30 mg daily, 20 mg daily, 15 mg daily, 10 mg daily, then 10 mg alternating with 7.5 mg, 10 mg alternating with 5 mg, 10 mg every other day, 7.5 mg every other day, 5 mg every other day, following which prednisone was discontinued. For subjects taking less than 40 mg daily at the time of enrollment, prednisone was tapered to the next lowest dose and reduced in same stepwise regimen. If a subject experienced a pulmonary sarcoidosis flare, defined by worsening respiratory symptoms, CXR, or pulmonary function tests (PFTs), that is, >15% decline in FEV1, FVC, or a >20% decline in DLCO, during the study, the prednisone dose was increased to 40 mg daily for two weeks and then tapered as described above to their baseline dose. Chest radiographs were qualitatively reviewed by a pulmonologist and radiologist.
Subjects were randomized to receive, as study drug, either placebo or POF, 1600 mg/day if body weight was less than 70 kg, or 2000 mg/day if body weight exceeded 70 kg. Study subjects were clinically evaluated at enrollment and approximately every four to six weeks for a 10 month period. A dyspnea assessment (Appendix A) (29-31), PFTs, complete blood counts, and chemistries were performed at each visit. Subjects were randomized in blocks of 2, 4, and 6, and randomization was implemented by the NIH Pharmaceutical Development Service; patients and investigators were blinded to treatment assignment. Study drugs were administered orally, in divided doses, with food. The protocol (99-H-0057) was approved by the NHLBI Institutional Review Board, and registered with ClinicalTrials.gov (NCT00001877). Data were reviewed independently by the NHLBI Pulmonary DSMB.
Endpoints
Primary endpoints were defined as: (a) a significant improvement in two or more PFT parameters (a significant increase was defined as an increase of >15% from baseline in FEV1 or FVC, or an increase of >20% from baseline in DLCO (30, 32, 33), or (b) a significant increase in one PFT parameter combined with any improvement in the level of dyspnea, which was sustained at months 8-10 of the study. To achieve primary endpoint criteria, the change in PFTs and dyspnea could not be accompanied by an increase in prednisone at any time over the study.
Sample Size and Statistical Analysis
Sample size for this study was based on the primary endpoint. A projected sample size of 100 patients (50 in each group) was selected to have 85% power to detect a 75% or greater achievement of the primary endpoint criteria in the POF arm as compared to 45% in the placebo arm, with a two-sided α=0.05.
The planned analyses were performed using a Fisher’s exact test for frequency data and Wilcoxon rank test or permutation test for continuous data. Secondary and post hoc analyses involved, in addition, other parametric and non-parametric methods, such as the last rank carried forward (LRCF) (34) and cumulative change (CC) (34) analyses. The LR-CF allow evaluation of subjects with different durations of participation over the course of study. In the LRCF method, the rank of change from baseline among the pooled sample at the time of the last visit is carried forward for each non-completer. Then the two arms are compared at the end of the study using a Wilcoxon rank test. Cumulative change analysis was used to compare the prednisone dose between the two arms; at each visit and for each group, all available dose changes from consecutive visits were averaged, and then these averages were aggregated over time. All inferences made in subgroup, secondary, and post hoc analyses were considered exploratory.
Results
Study Overview and Patient Characteristics
In this trial of pentoxifylline in pulmonary sarcoidosis, 248 patients were initially screened via telephone; of these, after further clinical evaluation, 28 were enrolled in the study; one subject with severe disease was not included in the analysis (Figure 1). Twenty-seven had mild-to-moderate respiratory impairment (35), and few baseline differences (Table 1). Subjects in both treatment groups were of similar age and gender, although more women were enrolled overall. More African-American patients were randomized to the POF group (11 subjects) than placebo (6 subjects) (p < 0.05). Enrolled patients did not actively smoke cigarettes. At the time of enrollment, the majority of subjects had radiographic stage II or III disease. A majority of subjects in both arms reported dyspnea of mild-to-moderate severity, i.e., grades 1-3 (Table 1). Arterial oxygenation and PFT parameters were normal, except for a lower hemoglobin-adjusted DLCO in POF patients (p < 0.05). Prednisone doses at the time of randomization were similar in placebo (16 ± 3 mg) and POF-groups (15 ± 4 mg). At enrollment, subjects in the POF arm had significantly higher serum angiotensin converting enzyme (SACE) levels than those in the placebo arm (p < 0.05) (Table 1). There were no significant differences between the two treatment groups in bronchoalveolar lavage (BAL) macrophage counts, or in the mean duration of participation, i.e., 32 weeks for the placebo group vs. 31 weeks for the POF group (Table 2).
Fig. 1.
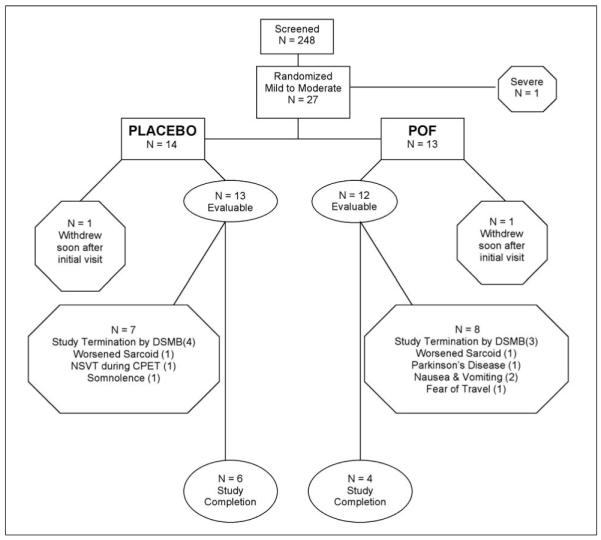
Patient Disposition.
Of the twenty-seven patients with mild-to-moderate disease, ten patients, [6] in the placebo group and [4] in the POF group completed the study. Seventeen patients were unable to complete the study, [8] placebo and [9] POF-treated patients. Four [4] placebo-treated subjects and [3] POF-treated subjects were unable to complete the study due to early protocol termination by the NHLBI Pulmonary DSMB. One [1] patient in each group, withdrew soon after their initial visit, and they were not included in the analysis. One [1] patient in each group, who had met flare criteria, voluntarily withdrew because of worsened sarcoid symptoms; they were included in the flare tabulations. Of the remaining placebo patients, one [1] was removed because of self-limited non-sustained ventricular tachycardia on a cardiac exercise stress test; the other subject [1] voluntarily withdrew because of worsened narcolepsy. Three [3] POF-treated subjects failed to complete the study due to voluntary withdrawal attributable to the following: [1], gastrointestinal side effects despite a dose reduction to 1200 mg/day; [1], Parkinson’s disease; and [1], fear of travel after the September 11th tragedy. Virtually all subjects adhered to their prescribed treatment regimen, as determined by patient history and pill counts.
Table 1.
Baseline Characteristics of Sarcoid Patients with Mild-to-Moderate Disease, and Treated with Placebo or POF
| Placebo | POF | Placebo | POF | ||
|---|---|---|---|---|---|
| AgeII | 44 ± 2 | 49 ± 3 | Dyspnea Scale | ||
| 0 | 3 | 4 | |||
| Gender | 1 | 6 | 5 | ||
| Male | 5 | 3 | 2 | 4 | 3 |
| Female | 9 | 10 | 3 | 1 | 1 |
| 4 | 0 | 0 | |||
| Ethnicity | |||||
| Caucasian | 8 | 2 | PFTsII (means) | ||
| African-American** | 6 | 11 | FEV1 % Predicted | 85 ± 6 | 90 ± 7 |
| FVC % Predicted | 86 ± 4 | 89 ± 6 | |||
| Smoking Status | TLC % Predicted | 86 ± 4 | 91 ± 5 | ||
| Never | 9 | 9 | DLCO % Predicted** | 91 ± 5 | 78 ± 6 |
| Ex-Smoker | 5 | 4 | |||
| Current Smoker | 0 | 0 | Arterial PaO2II(mean) (mmHg) and [kPa] |
84 ± 2 [11.17] |
80 ± 3 [10.64] |
| Initial Mean Prednisone | |||||
| Radiographic Stage++ | DoseII (mg/day) | 16 ± 3 | 15 ± 4 | ||
| 0 (Normal) | 1 | 2 | |||
| I (Hilar LN) | 2 | 2 | Mean SACEII,**(U/L) | 24 ± 3 | 44 ± 6 |
| II (Hilar LN & Infiltrates) | 3 | 4 | |||
| Mean BAL | |||||
| III (Infiltrates) | 7 | 4 | macrophages^ | 75.8% | 71.1% |
| IV (Fibrocystic Disease) | 1 | 1 | |||
Values represent means ± SEM.
P < 0.05 as determined by either a two-tailed Fisher’s exact test or Student’s t-test.
All patients had a history of radiographic stages equal to or greater than 1; LN = lymph node.
The BALF data was derived from 13 placebo - and 10 POF - treated subjects, respectively
Table 2.
Study Results
| Placebo n, (%) | POF n, (%) | P-Value* | |
|---|---|---|---|
| Patient distribution | |||
| Total Randomized Mild to Moderate Disease | 14 | 13 | NS |
| Successful Study Completions | 6 (43) | 4 (31) | NS |
| Temporary Prednisone Discontinuation | 9 (64) | 9 (69) | NS |
| Compliance with Medications | 14 (100) | 12 (92) | NS |
| No. Patients Participating at least 3 months | 13 (93) | 12 (92) | NS |
| No. Patients Participating at least 6 months | 9 (64) | 9 (69) | NS |
| No. Evaluable Patients Removed or Withdrawn | 7/13 (54) | 8/12 (67) | NS |
| Flares | |||
| Observed Flares (Entire Study) | 12/13 (92) | 5/12 (42) | 0.011 |
| Observed Flares (in study ≥ 6 months) | 9/9 (100) | 3/9 (33) | 0.009 |
| TheoreticalII + Observed Flares (up to 6 months) | 12/14 (86) | 6/13 (46) | 0.046 |
| Theoretical** + Observed Flares (up to 9 months) | 12/14 (86) | 9/13 (69) | 0.384 |
| Primary endpoints | |||
| Improvement ≥ 2 PFT Parameters | 0 | 0 | NS |
| Improvement 1 PFT Parameter & Dyspnea | 0 | 1 | NS |
| Durations Mean (SEM) | |||
| Duration of Study Participation (Weeks) | 31.85 (3.37) | 30.75 (3.22) | NS |
| Prednisone-Free Period (Weeks) | 6.3 (2.0) | 13.3 (3.1) | 0.071 |
| Prednisone++ Mean (SEM) mg/day | |||
| Prednisone at 6 months (N = 9 Placebo; N = 9 POF) |
12.13 (4.03) | 3.24 (1.86) | 0.059 |
| Prednisone at 8 months (N = 8 Placebo; N = 8 POF) |
11.21 (3.23) | 0.71 (0.61) | 0.007 |
| Prednisone at 10 months (N = 6 Placebo; N = 4 POF) |
9.36 (0.96) | 0.46 (0.36) | 0.010 |
| Prednisone Entire Study Period | 11.997 (1.67) | 7.537 (2.51) | 0.146 |
| Adverse events | |||
| Recurrent Mild Nausea | 0 (0) | 8 (62) | 0.003 |
| Repeated Mild Diarrhea | 0 (0) | 7 (54) | 0.003 |
| Abdominal Cramps | 0 (0) | 2 (15) | NS |
| Emesis | 0 (0) | 3 (23) | NS |
The incidence of flares was analyzed for the “entire study period”, which included evaluable subjects who were in the trial for less than 6 months. In order to avoid potential specious effects caused by drop outs,the analysis was then confined to subjects participating at least 6 months or more, as denoted by “(in study ≥ 6 months)”. Subsequently, flares were then analyzed by an intention-to-treat, worst case scenario method (51). In this method, removed subjects and drop outs who were in the treatment arm (POF), without a documented event (flare), were allocated as if they had an event (“theoretical” flare); drop outs/removed cases in the placebo arm, without a documented event, were allocated as if they would not have flared.
Theoretical Flares indicates that the analysis was confined to drops outs/removed subjects up to 6 months; for the POF arm, 1 drop out/removed patient was allocated to the flare state, and for the placebo arm, 1 drop out/removal was ascribed to the non-flare state.
Theoretical Flares indicates that the analysis was restricted to drop outs/removed subjects that occurred up to 9 months; for the POF arm, 3 drop outs/removed patients were allocated to the flare state, while in the placebo group, 1 drop out/removed subject was ascribed to the non-flare state as noted previously. Prednisone usage was determined by pill counts and patient history.
The mean prednisone dose for an individual subject was calculated by averaging the daily dose at a given visit, with the mean daily dose of the preceding 4 weeks. The results were then pooled within the treatment arms and are shown above.
P-values based on a permutation test for continuous outcomes and Fisher’s exact test for categorical or count data. NS represents p > 0.05
Because of a slow rate of recruitment, the DSMB recommended termination of the study. In each group, a significant number of subjects were removed or dropped out (Figure 1 and Table 2). This reduced the number of evaluable subjects by approximately 30% per arm (Figure 1) [subjects were considered evaluable if they participated for at least 3 months (± 1 week)]. In addition, one subject from each arm, who met flare criteria, voluntarily withdrew because of worsened disease; they were counted in the flare tabulations. The clinical course and treatment outcomes of the twenty-seven subjects with mild-to-moderate disease are summarized in Table 2. The mean duration of sarcoidosis prior to enrollment was 6.12 (SEM 1.47) years for the placebo group and 7.07 (SEM 2.66) years for the POF cohort (p = 0.81); the mean duration overall was 6.45 years. This reflects a population which was most likely affected with unremitting or chronic-relapsing sarcoidosis, which would be consistent with their steroid-requiring history, and further suggests that this population was appropriate for the study. The duration of disease was determined by the difference in time between the diagnostic biopsy (histopathology report) and the date of enrollment. Except for one POF-treated patient, primary endpoints were not achieved. Thus, a post hoc, exploratory analysis of the effects of POF on the incidence and risk of flares, as well as prednisone usage, was performed.
Risk Analysis of Flares
Flares were defined by a worsening in dyspnea, CXR, or PFTs. Overall, there were fewer observed flares in POF-treated subjects, i.e., 5/12 in the POF group vs. 12/13 in the placebo arm (p = 0.011) (Table 2). This corresponded to a relative risk reduction (RRR) of 55.4% (95% CI 0.21, 0.90), an absolute risk reduction (ARR) of 50.6%, and the number needed to treat (NNT) to prevent one flare was 2 patients (95% CI 1.11, 4.76) (Table 3) (36). This analysis included subjects who were in the trial for less than 6 months. For participants in the study for greater than or equal to 6 months, there were 3/9 flares in the POF group and 9/9 flares in the placebo group (p = 0.009). The corresponding RRR was 66.7% (95% CI 0.36, 0.97); ARR 66.7% and NNT = 2 (95% CI 1.03, 2.79). The data were also analyzed by an intention-to-treat, worst case scenario method (37). In this method, drop outs and removed subjects in the POF arm, without a documented flare, were considered to have developed a theoretical flare; in the placebo arm, drop outs and removed subjects who had not flared, were allocated to a non-flare state (Table 2). Using this method of analysis for handling drop outs and removed subjects up to six months, the RRR for POF treatment was 46.2%, ARR 39.6% and NNT 3; for drop outs and subject removals that occurred up to 9 months, the RRR was 19.2%, ARR 16.5%, and the NNT was 6 (Table 3).
Table 3.
Post Hoc Analysis: Flares
| Flares | POF | 95% Confidence Interval |
|---|---|---|
| A. Flares (Entire Study) | ||
| Relative Risk Reduction | 0.549 | (0.21, 0.89) |
| Absolute Risk Reduction | 0.506 | (0.22, 0.80) |
| Number Needed to Treat | 2 | (1.26, 4.61) |
| B. Flares (For subjects participating > 6 months) | ||
| Relative Risk Reduction | 0.667 | (0.36, 0.97) |
| Absolute Risk Reduction | 0.667 | (0.36, 0.97) |
| Number Needed to Treat | 2 | (1.03, 2.79) |
| C. Flares (Worst Case Scenario, drop outs up to 6 months) | ||
| Relative Risk Reduction | 0.462 | (0.08, 0.84) |
| Absolute Risk Reduction | 0.396 | (0.11, 0.68) |
| Number Needed to Treat | 3 | (1.46, 9.31) |
| D. Flares (Worst Case Scenario, drop outs up to 9 months) | ||
| Relative Risk Reduction | 0.192 | (−0.17, 0.55) |
| Absolute Risk Reduction | 0.165 | (−0.10, 0.43) |
| Number Needed to Treat | 6 | (2.31, infinity) |
Determination of the relative risk reduction (RRR), absolute risk reduction (ARR), and number needed to treat (NNT) (50), with pentoxifylline (POF), was performed for pulmonary sarcoid flares. (A) The analysis of flares for the “entire study period”, included evaluable subjects who were in the trial for less than six months. Flare risk analysis was then restricted to subjects participating at least 6 months or more (B). Thereafter, an intention-to-treat,worst case scenario method (51) was applied. In this method, drop outs and removed subjects in the POF arm, without a documented flare, were allocated as if they had developed a “theoretical” flare; drop outs and removed subjects in the placebo arm, who had not flared, were ascribed to a non-flare state. The worst case scenario risk analysis was confined to subject drop outs and removals that occurred up to 6 (C) and 9 (D) months, respectively. The four groups of post hoc flare analyses are shown above.
Analysis of Prednisone Usage
The mean prednisone dose (mg/day) was significantly lower at 8 and 10 months in the POF arm (p = 0.007 and 0.010), respectively (Table 2), but not at 6 months (p = 0.059). A four week average at each time point was used to avoid spurious results. In the POF-treated group, there was a trend toward a larger reduction from baseline of prednisone over the whole study period (p = 0.053), as determined by cumulative change analysis (34) (Figure 2). In accord with protocol design, prednisone was tapered and discontinued as tolerated, in 9 patients in each study arm (Table 2). As seen in Table 2, there was a trend toward a greater number of prednisone-free weeks in the POF-treated subjects than in those given placebo; the mean duration of prednisone-free weeks for the POF-treated patients was 13.3 ± 3.1 compared to 6.3 ± 2.0 for placebo-treated subjects (p = 0.07).
Fig. 2.
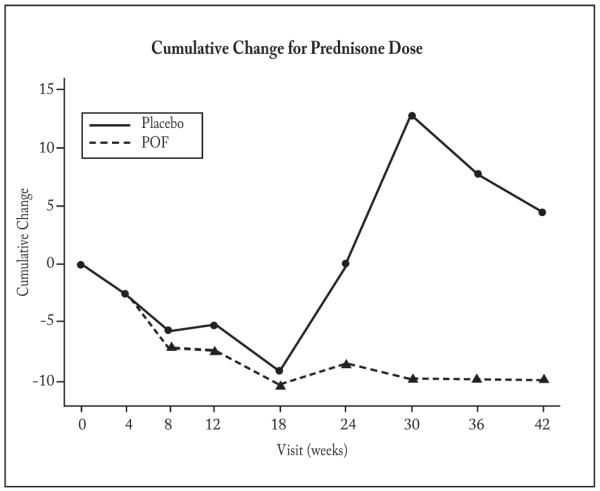
Cumulative Change Analysis of Prednisone Dose
There was a trend toward separation between the placebo and POF groups in prednisone dose over the course of the study (p=0.053), as was determined via cumulative change analysis (34). Cumulative change analysis was used to compare the prednisone dose between the two arms. At each visit and for each group, all available dose changes from consecutive visits were averaged, and then these averages were aggregated over time. We included patients who had at least 3 months, ± 1 week, of data. There were 13 patients in the placebo group and 12 in the POF group. Virtually all subjects adhered to their prescribed treatment regimen, as determined by patient history and pill counts.
Adverse Events
Adverse effects were reported in 12 of 13 POF-treated patients and 4 of 14 patients given placebo; gastrointestinal side effects, primarily mild nausea and diarrhea, were limited to POF-treated patients (p < 0.003) (Table 2). All subjects, who experienced gastrointestinal side effects, were initially instructed to reduce their study drug dosage. Three [3] of 5 subjects whose POF dose was reduced to 1200 mg/day had improvement in gastrointestinal symptoms, whereas two subjects discontinued study drug due to nausea and vomiting.
Discussion
This study attempted to determine the effectiveness of a non-selective cyclic nucleotide phosphodiesterase inhibitor, POF, in treating pulmonary sarcoidosis. Twenty-seven subjects with mild to moderate pulmonary sarcoidosis were randomized to placebo or POF. In the primary analysis, no significant difference in primary endpoints was demonstrated between the treatment arms. Consequently, a post hoc analysis of the results was performed, which demonstrated fewer flares in the POF-treated subjects, RRR 54.9% (95% CI 0.21, 0.89), ARR was 50.6% (95% CI 0.22, 0.80), and NNT = 2. These results were corroborated by analyzing subjects who remained in the trial for at least 6 months or more. The mean prednisone usage was significantly lower in the POF-treated group at 8 and 10 months (p = 0.007 and 0.010), respectively. Overall, there was a trend toward less prednisone use in the POF-treated group (p = 0.053), based on cumulative change analysis. In addition, there was a trend toward a longer steroid-sparing period in the POF arm (p = 0.07). Thus, the post hoc exploratory analysis suggested that POF-treated subjects had fewer flares and used less prednisone
Given the significant study limitations, such as slow recruitment and the relatively high dropout/removal rate in both arms, the apparent positive results from this trial should be interpreted with caution, since they were derived from a post hoc analysis, which cannot be considered confirmatory. In this setting, terminating the study before active participants completed the trial accentuated the attrition; 4 of 13 evaluable subjects in the placebo arm and 3 of 12 subjects in the POF arm were removed as a consequence of the DSMB decision. Two of 13 (15.4%) of POF-treated subjects withdrew prematurely because of side-effects (nausea and vomiting).
This current investigation may be compared to two other trials. In one trial, 24 subjects taking corticosteroids were randomized to methotrexate or placebo (38). Prednisone usage was significantly less in the methotrexate arm compared to the placebo arm (p < 0.05) during the second six months of the study. Similarly, in our study, in the POF treatment group, there was a statistically significant reduction in prednisone dose at 8 and 10 months, but not at 6 months, as determined by cumulative change analysis. In another trial, 37 sarcoidosis subjects were treated in an open-label fashion with prednisone alone or orally administered cyclosporine A with prednisone (39). No significant difference in primary endpoint was demonstrated between the two groups. In both the POF trial and the cyclosporine trial, the same clinical endpoints were utilized, that is, a 15% change from baseline in FVC or FEV1; and, in both trials, clinical improvement was defined as a significant increase in two PFT parameters, or a significant improvement in one PFT parameter and dyspnea score. Serial PFT measurements are affected by age-related changes, as well as random and systematic variation (40). In light of this, the ATS has considered a 15% change in FEV1 as significant (31); consequently, a 15% change in FEV1 or FVC was used in this POF study. Neither the cyclosporine study nor the POF investigation demonstrated a significant difference in the primary endpoint. With hindsight, the primary endpoint in our investigation may have been too stringent, given that no previous study has documented such large changes in PFTs in this population. In a more recent trial, 138 subjects were randomized to receive infliximab or placebo (41). A 2.5% increase from baseline in FVC percent predicted was observed in the infliximab group. Although this was considered statistically significant (p = 0.038), its clinical significance was unclear. In the setting of a steroid taper, as in our study, it might be difficult to demonstrate large increases in PFT parameters, which, in addition, have not been shown to be reliable clinical indicators of disease progression or severity in patients with pulmonary sarcoidosis. Taken together, the limitations of pulmonary function tests underscore the need for better metrics in this population.
The reason for the slow recruitment may have been multifactorial; the lack of an accessible clinical cohort appears to be the major cause. Developing a registry and a natural history protocol may help provide a pool of well-characterized subjects. Collaboration with extramural sites would likely facilitate recruitment as well.
In conclusion, this post hoc analysis suggested that POF reduced pulmonary sarcoidosis flares and had a steroid-sparing effect. Although fairly mild, the frequent gastrointestinal side-effects, at the doses used, would likely limit the routine use of POF in treating pulmonary sarcoidosis. While definitive conclusions cannot be made in terms of POF efficacy in pulmonary sarcoidosis, this trial, despite its shortcomings, was hypothesis generating, and provides a basis for using more specific, better-tolerated, PDE inhibitors in future clinical investigations. PDE4-selective inhibitors exhibit potent anti-inflammatory effects in preclinical and clinical studies, and one such inhibitor, roflumilast, has demonstrated some clinical benefit in recent trials for treatment of COPD (42) and asthma (43).
With respect to pulmonary sarcoidosis, no well-established steroid-sparing regimen has been validated by a randomized, double-blind, placebo-controlled clinical trial. Since steroids, the mainstay of therapy, have numerous untoward side-effects, including diabetes mellitus, hypertension, osteoporosis, weight gain, and possible increased cardiovascular risk, alternative therapies are needed. From this perspective, rigorous clinical trials are warranted to determine whether novel anti-inflammatory agents can provide steroid-sparing benefits and improve therapeutic outcomes in patients with sarcoidosis.
Acknowledgement
Nurses and Nurse Practitioners: Ruth Litzenberger, RN; Pauline Barnes, RN; Keith Jackson, RN; Pamela Brooks, MSN, CRNP; Rosamma DeCastro, MSN, CRNP; Carolyn Hedin MSN, CRNP; Antoinette Rabel, MSN, CRNP; Rose May MSN, CRNP; Maria Stagnitto, MSN, Eileen Lange, RN, and the 8E nursing staff. Respiratory Therapists: Mark Barton, Clara Jolley, and Peter McGraw. Referring Physicians: Octavius Polk, MD, Howard University School of Medicine and Provident Hospital, Washington, DC; Victor Jackson, MD and Claude Tellis, MD, Baton Rouge, LA. Technical assistance: Marie Weston, PhD, Young Choi, PhD, Farshid Rouhani, MS, and Yana Tsygansky, BS. Pharmacist: Judith M. Starling, PharmD. Pathologist: William D. Travis, MD, Memorial Sloan-Kettering Cancer Center. Data and Safety Monitoring Board: Joseph G.N. Garcia, MD (chairperson), The University of Chicago; Paul Albert, PhD, NCI, NIH; Peter Bitterman, MD, University of Minnesota; Evan DeRenzo, PhD. Grove Point, MD; Barry Fanburg, MD, New England Medical Center; Jay Ryu, MD, Mayo Clinic, MN; and Reverend Dr. Frederick Schmidt, Perkins School of Theology, Texas. We thank Martha Vaughan, MD, Stewart Levine, MD, Angelo Taveira-DaSilva, MD, PhD, and Bernadette Gochuico, MD, for reviewing this manuscript.
NIH Clinical Research Center (NHLBI) Protocol #99-H-0057
Research support: Division of Intramural Research, National Institutes of Health, National Heart, Lung, and Blood Institute
Glossary
Abbreviations
- ARR
absolute risk reduction
- BAL
bronchoalveolar lavage
- COPD
chronic obstructive pulmonary disease
- CXR
chest radiograph
- DLCO
diffusion capacity of carbon monoxide
- DSMB
Data and Safety Monitoring Board
- FEV1
forced expiratory volume in the first second
- FVC
forced vital capacity
- LN
lymph node
- PFTs
pulmonary function tests
- POF
pentoxifylline
- PDE
cyclic nucleotide phosphodiesterase
- NHLBI
National Heart, Lung, and Blood Institute
- NNT
number needed to treat
- RRR
relative risk reduction
- SACE
serum angiotensin-converting enzyme
- TLC
total lung capacity
- TNF
tumor necrosis factor alpha
APPENDIX A: Dyspnea Determination
Classification and Scoring of Dyspnea
| Modified Medical Research Council Dyspnea Scale (31) | ||
|---|---|---|
| Severity | Grade | Description |
| 0 | Not troubled with breathlessness except with strenuous exercise | |
| Mild | 1 | Troubled by shortness of breath when hurrying on the level or walking up a slight hill |
| Moderate | 2 | Walks slower than people of the same age on the level because of breathlessness or has to stop for breath when walking at own pace on the level |
| Severe | 3 | Stops for breath after walking about 100 yards or after a few minutes on the level |
| Very Severe | 4 | Too breathless to leave the house or breathless when dressing or undressing |
| The grade of dyspnea was determined by the response to the corresponding questions below, which were adapted from the American Medical Association ‘s Guides to the Evaluation of Permanent Impairment (30). |
|---|
| Question |
| Do you have to walk more slowly on the level than people of your age because of breathlessness? |
| Do you ever have to stop for breath when walking at your own pace? |
| Do you ever have to stop for breath after walking about 100 yards or for a few minutes on the level? |
| Are you too breathless to leave the house, or breathless after dressing or undressing? |
References
- 1.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003;361(9363):1111–8. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- 3.Ziegenhagen MW, Schrum S, Zissel G, Zipfel PF, Schlaak M, Muller-Quernheim J. Increased expression of proinflammatory chemokines in bronchoalveolar lavage cells of patients with progressing idiopathic pulmonary fibrosis and sarcoidosis. J Investig Med. 1998;46(5):223–31. [PubMed] [Google Scholar]
- 4.Capelli A, Di SA, Lusuardi M, Gnemmi I, Donner CF. Increased macrophage inflammatory protein-1alpha and macrophage inflammatory protein-1beta levels in bronchoalveolar lavage fluid of patients affected by different stages of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2002;165(2):236–41. doi: 10.1164/ajrccm.165.2.2106084. [DOI] [PubMed] [Google Scholar]
- 5.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260(5106):355–8. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 6.Petrek M, Pantelidis P, Southcott AM, et al. The source and role of RANTES in interstitial lung disease. Eur Respir J. 1997;10(6):1207–16. doi: 10.1183/09031936.97.10061207. [DOI] [PubMed] [Google Scholar]
- 7.Agostini C, Meneghin A, Semenzato G. T-lymphocytes and cytokines in sarcoidosis. Curr Opin Pulm Med. 2002;8(5):435–40. doi: 10.1097/00063198-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Gerke AK, Hunninghake G. The immunology of sarcoidosis. Clin.Chest Med. 2008;29(3):379–90. doi: 10.1016/j.ccm.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Baughman RP, Strohofer SA, Buchsbaum J, Lower EE. Release of tumor necrosis factor by alveolar macrophages of patients with sarcoidosis. J Lab Clin Med. 1990;115(1):36–42. [PubMed] [Google Scholar]
- 10.Ziegenhagen MW, Benner UK, Zissel G, Zabel P, Schlaak M, Muller-Quernheim J. Sarcoidosis: TNF-alpha release from alveolar macrophages and serum level of sIL-2R are prognostic markers. Am J Respir Crit Care Med. 1997;156(5):1586–92. doi: 10.1164/ajrccm.156.5.97-02050. [DOI] [PubMed] [Google Scholar]
- 11.Shigehara K, Shijubo N, Ohmichi M, et al. IL-12 and IL-18 are increased and stimulate IFN-gamma production in sarcoid lungs. J Immunol. 2001;166(1):642–9. doi: 10.4049/jimmunol.166.1.642. [DOI] [PubMed] [Google Scholar]
- 12.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 13.Sanz MJ, Cortijo J, Morcillo EJ. PDE4 inhibitors as new anti-inflammatory drugs: effects on cell trafficking and cell adhesion molecules expression. Pharmacol Ther. 2005;106(3):269–97. doi: 10.1016/j.pharmthera.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Lauterbach R, Pawlik D, Zembala M, et al. Pentoxyfylline in and prevention and treatment of chronic lung disease. Acta Paediatr Suppl. 2004;93(444):20–2. doi: 10.1111/j.1651-2227.2004.tb03043.x. [DOI] [PubMed] [Google Scholar]
- 15.Thanhauser A, Reiling N, Bohle A, et al. Pentoxifylline: a potent inhibitor of IL-2 and IFN-gamma biosynthesis and BCG-induced cytotoxicity. Immunology. 1993;80(1):151–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Schandene L, Vandenbussche P, Crusiaux A, et al. Differential effects of pentoxifylline on the production of tumour necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) by monocytes and T cells. Immunology. 1992;76(1):30–4. [PMC free article] [PubMed] [Google Scholar]
- 17.Prabhakar U, Lipshutz D, Truneh A. Inhibition of CD44, CD45 and LFA-3 mediated cytokine release from human monocytes by SK&F 86002 and pentoxifylline. Int J Immunopharmacol. 1993;15(2):205–9. doi: 10.1016/0192-0561(93)90096-h. [DOI] [PubMed] [Google Scholar]
- 18.Rieckmann P, Weber F, Gunther A, et al. Pentoxifylline, a phosphodiesterase inhibitor, induces immune deviation in patients with multiple sclerosis. J Neuroimmunol. 1996;64(2):193–200. doi: 10.1016/0165-5728(95)00176-x. [DOI] [PubMed] [Google Scholar]
- 19.Lauterbach R, Pawlik D, Kowalczyk D, Ksycinski W, Helwich E, Zembala M. Effect of the immunomodulating agent, pentoxifylline, in the treatment of sepsis in prematurely delivered infants: a placebo-controlled, double-blind trial. Crit Care Med. 1999;27(4):807–14. doi: 10.1097/00003246-199904000-00042. [DOI] [PubMed] [Google Scholar]
- 20.Haddad JJ, Land SC, Tarnow-Mordi WO, Zembala M, Kowalczyk D, Lauterbach R. Immunopharmacological potential of selective phosphodiesterase inhibition. I. Differential regulation of lipopolysaccharide-mediated proinflammatory cytokine (interleukin-6 and tumor necrosis factor-alpha) biosynthesis in alveolar epithelial cells. J Pharmacol Exp Ther. 2002;300(2):559–66. doi: 10.1124/jpet.300.2.559. [DOI] [PubMed] [Google Scholar]
- 21.Rao KM, Currie MS, McCachren SS, Cohen HJ. Pentoxifylline and other methyl xanthines inhibit interleukin-2 receptor expression in human lymphocytes. Cell Immunol. 1991;135(2):314–25. doi: 10.1016/0008-8749(91)90276-h. [DOI] [PubMed] [Google Scholar]
- 22.Strieter RM, Remick DG, Ward PA, et al. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155(3):1230–6. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 23.Tong Z, Dai H, Chen B, Abdoh Z, Guzman J, Costabel U. Inhibition of cytokine release from alveolar macrophages in pulmonary sarcoidosis by pentoxifylline: comparison with dexamethasone. Chest. 2003;124(4):1526–32. doi: 10.1378/chest.124.4.1526. [DOI] [PubMed] [Google Scholar]
- 24.Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U. Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages. Am J Respir Crit Care Med. 1999;159(2):508–11. doi: 10.1164/ajrccm.159.2.9804085. [DOI] [PubMed] [Google Scholar]
- 25.Tong Z, Chen B, Dai H, Bauer PC, Guzman J, Costabel U. Extrinsic allergic alveolitis: inhibitory effects of pentoxifylline on cytokine production by alveolar macrophages. Ann Allergy Asthma Immunol. 2004;92(2):234–9. doi: 10.1016/S1081-1206(10)61553-0. [DOI] [PubMed] [Google Scholar]
- 26.Co DO, Hogan LH, Il-Kim S, Sandor M. T cell contributions to the different phases of granuloma formation. Immunol Lett. 2004;92(1-2):135–42. doi: 10.1016/j.imlet.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Zissel G, Prasse A, Muller-Quernheim J. Sarcoidosis - immunopathogenetic concepts. Semin Respir Crit Care Med. 2007;28(1):3–14. doi: 10.1055/s-2007-970329. [DOI] [PubMed] [Google Scholar]
- 28.Zabel P, Entzian P, Dalhoff K, Schlaak M. Pentoxifylline in treatment of sarcoidosis. Am J Respir Crit Care Med. 1997;155(5):1665–9. doi: 10.1164/ajrccm.155.5.9154873. [DOI] [PubMed] [Google Scholar]
- 29.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118(6 Pt 2):1–120. [PubMed] [Google Scholar]
- 30.American Medical Association . Guides to the evaluation of permanent impairment. 4th ed. American Medical Association; Chicago: 1993. pp. 153–7. [DOI] [PubMed] [Google Scholar]
- 31.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–6. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 32.American Thoracic Society Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144(5):1202–18. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 33.Standardization of spirometry - 1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis. 1987;136(5):1285–98. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien PC, Zhang D, Bailey K. Semi-parametric and non-parametric methods for clinical trials with incomplete data. Stat Med. 2005;24:341–58. doi: 10.1002/sim.1963. [DOI] [PubMed] [Google Scholar]
- 35.American Thoracic Society Evaluation of impairment/disability secondary to respiratory disorders. Am Rev Respir Dis. 1986;133(6):1205–9. doi: 10.1164/arrd.1986.133.6.1205. [DOI] [PubMed] [Google Scholar]
- 36.Sackett DL, Haynes R, Guyatt G, Tugwell P. Clinical Epidemiology: A Basic Science for Clinical Medicine. 2nd ed. Little, Brown and Company; Boston: 1991. [Google Scholar]
- 37.Sackett D, Richardson W, Rosenberg W, Haynes R. Evidenced-based Medicine. Churchill Livingstone; New York: 1997. [Google Scholar]
- 38.Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17(1):60–6. [PubMed] [Google Scholar]
- 39.Wyser CP, van Schalkwyk EM, Alheit B, Bardin PG, Joubert JR. Treatment of progressive pulmonary sarcoidosis with cyclosporin A. A randomized controlled trial. Am J Respir Crit Care Med. 1997;156(5):1371–6. doi: 10.1164/ajrccm.156.5.9506031. [DOI] [PubMed] [Google Scholar]
- 40.Wang ML, Petsonk EL. Repeated measures of FEV1 over six to twelve months: what change is abnormal? J Occup Environ Med. 2004;46(6):591–5. doi: 10.1097/01.jom.0000128159.09520.2a. [DOI] [PubMed] [Google Scholar]
- 41.Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 42.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, Rabe KF. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 43.Bateman ED, Izquierdo JL, Harnest U, et al. Efficacy and safety of roflumilast in the treatment of asthma. Ann Allergy Asthma Immunol. 2006;96(5):679–86. doi: 10.1016/S1081-1206(10)61065-4. [DOI] [PubMed] [Google Scholar]


