Abstract
We have previously reported the results of a 1-y double-blind, placebo-controlled study of embryonic dopamine cell implantation for Parkinson’s disease. At the end of the blinded phase, we found a significant increase in putamen uptake on 18F-fluorodopa (18F-FDOPA) PET reflecting the viability of the grafts. Nonetheless, clinical improvement was significant only in younger (age ≤ 60 y) transplant recipients, as indicated by a reduction in Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores.
Methods
We now report long-term clinical and PET outcomes from 33 of the original trial participants who were followed for 2 y after transplantation and 15 of these subjects who were followed for 2 additional years. Longitudinal changes in UPDRS motor ratings and caudate and putamen 18F-FDOPA uptake were assessed with repeated-measures ANOVA. Relationships between these changes over time were evaluated by the analysis of within-subject correlations.
Results
We found that UPDRS motor ratings declined over time after transplantation (P < 0.001). Clinical improvement at 1 y was relatively better for the younger transplant recipients and for men, but these age and sex differences were not evident at longer-term follow-up. Significant increases in putamen 18F-FDOPA uptake were evident at all posttransplantation time points (P < 0.001) and were not influenced by either age or sex. Posttransplantation changes in putamen PET signal and clinical outcome were significantly intercorrelated (P < 0.02) over the course of the study. Image analysis at the voxel level revealed significant bilateral increases in 18F-FDOPA uptake at 1 y (P < 0.001) in the posterior putamen engraftment sites. PET signal in this region increased further at 2 and 4 y after engraftment. Concurrently, this analysis disclosed progressive declines in radiotracer uptake in the nonengrafted caudate and ventrorostral putamen. Clinical improvement after transplantation correlated with the retention of PET signal in this region at the preoperative baseline.
Conclusion
These results suggest that clinical benefit and graft viability are sustained up to 4 y after transplantation. Moreover, the dependence of clinical (but not imaging) outcomes on subject age and sex at 1 y may not persist over the long term. Last, the imaging changes reliably correlate with clinical outcome over the entire posttransplantation time course.
Keywords: 18F-FDOPA, PET, Parkinson’s disease, transplantation, long-term outcome
Two multidisciplinary teams of investigators have reported results from double-blind, placebo-controlled trials of human embryonic dopaminergic tissue transplantation for advanced Parkinson’s disease (PD). In the first study, 39 PD patients were randomized to either bilateral tissue implantation in the putamen or sham surgery without immunosuppressive therapy and followed for 1 y with blinded clinical and 18F-fluorodopa (18F-FDOPA) PET evaluations (1). We found that transplantation significantly improved standardized motor ratings, although substantial clinical benefit was limited to younger (age ≤ 60 y) subjects. Nonetheless, putamen uptake of 18F-FDOPA, an index of graft viability, increased significantly in the transplanted cohort, regardless of age (2). In the second double-blind study, 34 PD patients underwent bilateral fetal dopaminergic cell transplantation in the putamen, with 1–4 donors per side, or placebo surgery along with pre- and postoperative immunosuppressive therapy over 6 mo (3). That study showed clinical benefit in less severely affected subjects and significant increases in putamen 18F-FDOPA uptake in all the transplant recipients at the 2-y follow-up. The results of these blinded studies agreed with reports from small open-label cohorts of PD patients showing persisting increases in 18F-FDOPA uptake at the site of implantation (4–6), histopathologic evidence of engraftment (7,8), and associations between 18F-FDOPA PET indices of graft viability and clinical outcome (9,10).
To date, reports of the long-term outcome of fetal cell transplantation for advanced PD have been limited to small groups of subjects (4,6). Both double-blind clinical trials also reported the development of graft-induced dyskinesia (GID) in some of the transplant recipients when these recipients were assessed after dopaminergic medications had been stopped (1,3). It remains to be determined whether the clinical response to transplantation is offset by the troubling side effect of GID.
In the present study, we report long-term clinical and 18F-FDOPA PET findings from our blinded transplantation trial (1,2). After the 1-y blinded phase of that study, 14 of the 20 placebo-operated patients went on to receive bilateral putamen grafts, resulting in a total of 33 transplanted patients. We assessed postoperative changes in motor ratings and striatal 18F-FDOPA uptake in these subjects at 1 and 2 y after implantation. Of these subjects, 15 were followed for an additional 2 y. In addition to correlating the clinical and PET changes over time, we determined whether these indices were influenced by subject age or sex. We also assessed the impact on the data of the 5 transplant recipients in whom GID developed (11). Last, we correlated clinical outcome with preoperative 18F-FDOPA PET to identify predictive imaging features (12) that might be used for patient selection in future trials of cell-based interventions for PD and related disorders.
MATERIALS AND METHODS
Patients
We studied 33 transplant patients with advanced PD (mean age ± SD, 57.2 ± 9.9 y; range, 35–76 y) who had participated in the original double-blind placebo-controlled transplantation trial (1). All patients had symptoms of PD for at least 7 y (13.8 ± 5.3 y; range, 7–32 y) and were responsive to levodopa (≥33% improvement in total Unified Parkinson’s Disease Rating Scale [UPDRS] after a first morning dose) at the time of study enrollment. Exclusion criteria included the presence of significant cognitive impairment, depression, or MRI evidence of cerebrovascular disease or another brain lesion. Informed consent was obtained from all patients through a protocol approved by the ethics committees of the participating institutions and the National Institutes of Health.
The subjects received fetal dopamine transplants approximately 8 mo after baseline clinical assessment and 18F-FDOPA PET. All 33 patients underwent clinical and imaging follow-up at 1 y (1.12 ± 0.21 y) after surgery; 29 completed additional postoperative assessments at 2 y (2.47 ± 0.68 y). Of these, 15 patients were reassessed both clinically and with PET 4 y (4.43 ± 0.61 y) after surgery.
As in the blinded phase of the study (1), the participants were stratified by age to assess its effect on clinical outcome and PET measures of graft viability. The young (age ≤ 60 y; n = 18) and older (age > 60 y; n = 15) subgroups had mean ages at baseline of 50.1 ± 7.1 y and 65.8 ± 4.2 y, respectively. The age composition of the sample was similar at 2 y, with 15 young and 14 older subjects. The sample at 4 y consisted of 10 young and 5 older subjects. Fewer participants in the latter group returned for 4-y follow-up because of advanced age and increased disability, which made long-distance travel difficult. To assess the effects of sex on the clinical and imaging changes, the cohort was also divided into male (n = 15) and female (n = 18) subgroups. The sex composition of the sample was 13 men and 16 women at 2 y and 8 men and 7 women at 4 y. At baseline, clinical severity, symptom duration, and striatal 18F-FDOPA uptake were similar for the 2 age and sex subgroups (Tables 1 and 2).
TABLE 1.
Baseline and Postoperative UPDRS Motor Ratings and Operative Changes
| Subject group | Pre | Post 1 y | Post 2 y | Post 4 y | Δ1 (%) | Δ2 (%) | Δ4 (%) |
|---|---|---|---|---|---|---|---|
| All | 38.3 ± 2.0* | 32.6 ± 2.6 | 26.5 ± 2.8 | 28.1 ± 2.7 | −15.1 ± 2.0†‡ | −30.8 ± 4.8§ | −24.9 ± 4.7‡ |
| Young (≤60 y) | 38.5 ± 2.6 | 29.6 ± 3.6 | 21.4 ± 2.9 | 29.5 ± 4.0 | −23.1 ± 4.4 | −44.6 ± 9.2 | −23.8 ± 5.6 |
| Older (>60 y) | 38.1 ± 3.2 | 36.0 ± 3.5 | 31.7 ± 4.3 | 25.4 ± 2.3 | −5.3 ± 1.0 | −16.8 ± 3.7 | −27.4 ± 7.8 |
| Men | 38.4 ± 3.1 | 28.5 ± 3.9 | 25.9 ± 4.3 | 28.1 ± 4.3 | −25.8 ± 5.6 | −32.6 ± 8.0 | −21.5 ± 5.8 |
| Women | 38.2 ± 2.7 | 35.9 ± 3.3 | 27.0 ± 3.7 | 28.2 ± 3.5 | −6.0 ± 1.0 | −29.3 ± 6.1 | −28.5 ± 7.7 |
Off-state UPDRS motor ratings (mean ± SE) at baseline (pre) and 1 (post 1 y), 2 (post 2 y), and 4 (post 4 y) years after transplantation.
Δ = (postoperative − baseline)/baseline × 100%. More negative values reflect greater improvement in parkinsonian signs.
P < 0.05, compared with baseline (1-way RMANOVA from 33 transplant recipients over 2 y [Δ1 and Δ2] and 15 of these subjects over 4 y [Δ4]).
P < 0.0001, compared with baseline (1-way RMANOVA from 33 transplant recipients over 2 y [Δ1 and Δ2] and 15 of these subjects over 4 y [Δ4]).
TABLE 2.
Baseline and Postoperative Putamen 18F-FDOPA Uptake and Operative Changes
| Subject group | Pre | Post 1 y | Post 2 y | Post 4 y | Δ1 (%) | Δ2 (%) | Δ4 (%) |
|---|---|---|---|---|---|---|---|
| All | 0.50 ± 0.02* | 0.65 ± 0.03 | 0.69 ± 0.04 | 0.74 ± 0.05 | 28.7 ± 2.5†‡ | 38.0 ± 3.5‡ | 45.7 ± 6.1‡ |
| Young (≤60 y) | 0.50 ± 0.03 | 0.63 ± 0.04 | 0.66 ± 0.05 | 0.74 ± 0.07 | 26.8 ± 3.0 | 31.9 ± 4.2 | 48.4 ± 8.2 |
| Older (>60 y) | 0.50 ± 0.03 | 0.66 ± 0.05 | 0.73 ± 0.05 | 0.74 ± 0.11 | 30.8 ± 4.2 | 44.9 ± 5.8 | 40.7 ± 9.3 |
| Men | 0.51 ± 0.03 | 0.66 ± 0.04 | 0.75 ± 0.05 | 0.77 ± 0.07 | 30.7 ± 4.0 | 47.8 ± 6.5 | 46.8 ± 7.9 |
| Women | 0.50 ± 0.02 | 0.63 ± 0.04 | 0.65 ± 0.05 | 0.70 ± 0.09 | 26.8 ± 3.1 | 30.6 ± 3.7 | 44.4 ± 9.8 |
18F-FDOPA uptake values (mean ± SE) in predefined anatomic VOI for putamen at baseline (pre) and 1 (post 1 y), 2 (post 2 y), and 4 (post 4 y) years after transplantation. Striatal uptake value is defined as (region − occipital)/occipital at 95 min after tracer injection.
Δ = (postoperative − baseline)/baseline × 100%. More positive values reflect greater improvement in dopaminergic function.
P < 0.0001, compared with baseline (1-way RMANOVA from 33 transplant recipients over 2 y [Δ1 and Δ2] and 15 of these subjects over 4 y [Δ4]).
At each imaging time point, UPDRS motor ratings were obtained in a practically defined off-state (>12 h medication washout) by clinical observers who were unaware of the results of PET procedures (2). Composite UPDRS motor ratings (items 19–31) (13) were obtained on 2 consecutive days before 18F-FDOPA PET. The 2 daily measures were averaged for analysis and correlation with the imaging data.
PET
Scanning was performed on the Advance PET camera (GE Healthcare) at North Shore University Hospital. Baseline and postoperative 18F-FDOPA PET and image processing were performed as described in detail elsewhere (2,11). The patients fasted overnight and stopped taking antiparkinsonian medications at least 12 h before PET.
Volume-of-Interest (VOI) Analysis
VOI data were analyzed interactively as described previously (2,11). Dynamic PET scans between 60 and 100 min after injection were realigned to create a mean count image. In the baseline scans, anatomically-based VOIs for the caudate, putamen, and occipital cortex were placed manually on the mean image summed over central slices encompassing the striatal structures; these VOIs were confirmed for each subject with reference to the coregistered baseline MR images. The postoperative scans were realigned to the baseline scan such that identical brain volumes were compared across the 4 scanning sessions.
In each 18F-FDOPA PET scan, we calculated the ratio of specific to nonspecific activity in the caudate and putamen VOIs during the final 10-min frame (90–100 min after injection) by subtracting occipital from striatal activity and dividing by occipital activity. We have previously shown that this index is comparable with influx constant estimated from graphic analysis of dynamic data in quantifying striatal 18F-FDOPA losses and clinical correlation in PD (14). Right and left values for each structure were averaged before statistical analysis if no asymmetry was found in the uptake values. At each time point, these values were also compared with those from 15 healthy control subjects (age, 45.5 ± 22.1 y) as described by us previously (2).
Longitudinal changes in UPDRS motor ratings and caudate and putamen 18F-FDOPA uptake over time were separately analyzed with 1-way repeated-measures ANOVA (RMANOVA). Because of a large drop in the number of subjects at the fourth time point, this procedure was performed in the 33 transplant patients who were scanned over the first 3 time points, with only minimal loss of data. The analysis was done using an iterative method to fit all the data to a mixed model (15). This approach allowed for the inclusion of subjects with missing data, to provide a more precise and reliable estimation of the data at each time point, thereby increasing the power of the whole model (16,17). Post hoc comparisons were conducted between individual time points (e.g., 2–1, 3–2) using Bonferroni adjustments. The relationships between posttransplantation changes over time in UPDRS motor ratings and caudate and putamen 18F-FDOPA uptake were assessed across the whole cohort by computing Bland–Altman within-subject correlation coefficients (18,19).
We also performed a separate 3-way RMANOVA to assess the effects of age and sex on changes in UPDRS motor ratings and striatal 18F-FDOPA uptake at 1 and 2 y after transplantation. Variables for age (young, older) and sex (men, women) were entered into the model as covariates to evaluate their main and interaction effects over time. We conducted 2 separate analyses on the time course data across the first 2 and 3 time points, respectively.
To assess the effects of transplantation on changes in UPDRS motor ratings and striatal 18F-FDOPA uptake over the 4 y, we repeated the 1-way RMANOVA and correlation analyses in the 15 subjects who were scanned at all 4 time points with no loss of data. The 3-way RMANOVA was not performed because of the smaller sample in the 2 age and sex subgroups. The results for the whole group or the subgroup were compared with and without the inclusion of the individuals with GID. All analyses were considered significant for a P value less than 0.05, corrected for multiple comparisons. All statistical computations were conducted with SAS software, version 9.1 (SAS Institute Inc.).
Voxel-Based Analysis
We also analyzed the posttransplantation imaging changes at the voxel level using statistical parametric mapping (SPM) (2,11). Nonspecific 18F-FDOPA uptake values were computed by averaging regional data from the occipital cortex. Volumetric ratio images of 18F-FDOPA uptake (voxel/occipital − 1) were computed between 90 and 100 min after injection. The ratio images were then spatially normalized onto a 18F-FDOPA brain template in stereotactic space and smoothed with an 8-mm 3-dimensional gaussian filter. For comparability with our earlier published findings, voxelwise changes in the spatial distribution of 18F-FDOPA uptake after surgery were assessed using SPM99 (Well-come Institute of Neurology). These voxelwise changes were assessed with an absolute threshold of 0.2 and without performing global scaling because images were already ratio-normalized by occipital counts.
RMANOVA was performed on the scans from the entire cohort in SPM, using a general linear model with a mixed design. The differences between individual time points were evaluated post hoc by appropriate pairwise comparisons. In RMANOVA, contrasts for increased and decreased 18F-FDOPA uptake over time were defined by [−1 1/3 1/3 1/3] and [1 −1/3 −1/3 −1/3]. The contrasts for increased and decreased 18F-FDOPA uptake between any 2 time points were [−1 1] and [1 −1]. Voxel-based RMANOVA was also performed on the scans from 15 subjects who had complete data at all 4 time points.
SPM contrasts were displayed at an uncorrected voxel-level threshold of P < 0.001 and were considered significant at P < 0.05, cluster-corrected for familywise error rates. Changes at the less stringent uncorrected threshold of P < 0.01 were reported as hypothesis-testing if they occurred within a prespecified striatal mask. We also performed a voxel-based correlation analysis in SPM to assess the relationship between baseline 18F-FDOPA uptake and postoperative changes in UPDRS motor ratings. This exploratory analysis was conducted within the striatal mask and reported at P < 0.05 (uncorrected) to confirm the earlier correlational findings reported by Piccini et al. (12).
In all the voxel-based analyses, coordinates were reported in the standard anatomic space developed at the Montreal Neurologic Institute. The localization of each reported cluster was determined using the Talairach atlas available online (http://www.ihb.spb.ru/~pet_lab/TSU/TSUMain.html). The results of the voxelwise searches were confirmed post hoc by analyzing mean uptake values computed over spheric VOIs (4-mm radius) that were centered on the peak voxel of each significant SPM cluster.
RESULTS
Clinical Outcome
Baseline and posttransplantation clinical data from the transplant recipients with long-term follow-up are presented in Table 1. A significant effect of time on the UPDRS motor ratings (F2,59 = 12.7, P < 0.0001; 1-way RMANOVA; Fig. 1A) was present during the first 2 y of follow-up. Relative to the preoperative baseline, UPDRS motor ratings declined by 15.1% ± 2.0% (mean ± SE; P < 0.02; post hoc test) at 1 y and by 30.8% ± 4.8% (P < 0.0001) at 2 y after engraftment.
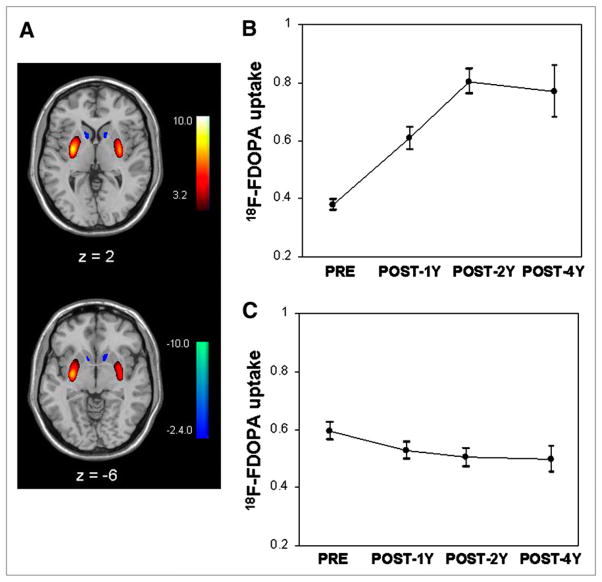
FIGURE 1.
Off-state UPDRS motor ratings and striatal 18F-FDOPA uptake (mean ± SE) at baseline (pre) and at 1 (post 1 y), 2 (post 2 y), and 4 (post 4 y) years after bilateral implantation of fetal dopaminergic cells into putamen of PD patients. Significant treatment effect was noted over 2 y (A) (P < 0.0001; RMANOVA) in whole group and over 4 y (B) (P < 0.001) in 15 participants who were evaluated at all 4 time points. 18F-FDOPA uptake in putamen increased significantly (P < 0.0001) over 2 y in whole group (C) and over 4 y in 15 transplant recipients scanned at all 4 time points (D). These graphs indicated progressive postoperative clinical improvement and increases in 18F-FDOPA uptake relative to baseline. By contrast, no significant changes were evident (P > 0.4) in nongrafted caudate after implantation in whole group (E) and subgroup (F) analyses. 18F-FDOPA uptake was measured in anatomic VOIs and plotted as percentages of normal mean. Dotted lines represent lower limit of striatal 18F-FDOPA uptake (mean − 2 SDs) in 15 healthy control subjects. Asterisks represent P values with respect to baseline. *P < 0.05; †P < 0.005; ‡P < 0.001.
Three-way RMANOVAs (Supplemental Table; supplemental materials are available online only at http://jnm.snmjournals.org) were used to evaluate the effects of age and sex on the changes in the UPDRS motor ratings over the same period of time. The main effect of time was significant (P < 0.005) in each analysis. When analysis was restricted to the first 2 time points, there was a significant age × time interaction effect on the UPDRS motor scores (P < 0.04; Supplemental Table), consistent with greater clinical improvement in the young (23.1% ± 4.4%) relative to the older subgroup (5.3% ± 1.0%) at 1 y. There was also a significant sex × time interaction effect (P < 0.05) on the clinical ratings at 1 y, reflecting greater improvement in men (25.8% ± 5.6%) than in women (6.0% ± 1.0%). Neither of these interactions reached significance when the analysis was extended to include the first 3 time points, although a marginal age × time interaction effect was evident at 2 y (P = 0.06; Supplemental Table). Notably, the effects of age and sex on the outcome data at 1 and 2 y were independent of one another.
The analysis of the data restricted to the 15 subjects who were assessed at all 4 time points showed a significant time effect on the UPDRS motor ratings (F3,41 = 6.98, P < 0.001; Fig. 1B), with improvement of 20.9% ± 5.0%, 42.7% ± 9.2%, and 24.9% ± 4.7% at 1, 2, and 4 y, respectively. In all 1-way RMANOVA analyses, changes in motor ratings after transplantation were found to be independent of disease severity and duration at baseline. Moreover, the results remained significant for the whole group over the 2 y (F2,49 = 7.38, P < 0.002) and for the subgroup over the 4 y (F3,29 = 5.69, P < 0.004) after excluding the 5 patients in whom GID developed.
18F-FDOPA PET: VOI Analysis
Baseline and postoperative 18F-FDOPA uptake values for the putamen and caudate anatomic VOIs are presented in Table 2 and Figure 1. There was no hemispheric asymmetry in striatal 18F-FDOPA uptake at baseline (P > 0.2; Student’s paired t test) or at the posttransplantation time points (P > 0.3; 1-way RMANOVA). Hence, we averaged values from the left and right hemispheres in the subsequent analyses. There was a significant effect of time on putamen 18F-FDOPA uptake for the whole group (F2,58 = 19.1, P < 0.0001; Fig. 1C). Relative to the preoperative baseline, putamen 18F-FDOPA uptake increased by 28.7% ± 2.5% and 38.0% ± 3.5% (mean ± SE) at 1 and 2 y after transplantation, respectively (P < 0.0001; post hoc test).
Three-way RMANOVA of putamen 18F-FDOPA uptake confirmed the significant time effect (P < 0.0003). There was no interaction between age, sex, and time at any of the follow-up time points (P > 0.19). Similar increases in putamen 18F-FDOPA uptake were evident in the young and older subgroups at the 2 postoperative time points relative to the preoperative baseline (young, >26.8% ± 3.0%; older, >30.8% ± 4.2%, P < 0.01). Posttransplantation increases in putamen 18F-FDOPA uptake were also similar for male and female transplant recipients (men, >30.7% ± 4.0%; women, >26.8% ± 3.1%, P < 0.01).
Analysis restricted to the 15 patients who underwent PET at all 4 time points revealed significant increases in putamen 18F-FDOPA uptake after transplantation (F3,42 = 10.1, P < 0.0001; Fig. 1D). The magnitudes of increase were 25.7% ± 3.0%, 31.6% ± 4.0%, and 45.7% ± 6.1% at 1, 2, and 4 y after transplantation (P < 0.02), respectively. Additionally, the results remained significant for the whole group over the 2 y (F2,48 = 10.5, P < 0.0002), as well as for the subgroup over the 4 y (F3,30 = 3.22, P < 0.04) after excluding the 5 transplant recipients in whom GID developed. In these secondary analyses, higher putamen 18F-FDOPA uptake was also evident (P < 0.01; post hoc test) at each of the 3 postoperative time points relative to the baseline.
18F-FDOPA uptake in the caudate anatomic VOIs did not change over the 2 y in the whole group (F2,58 = 0.92, P = 0.41; Fig. 1E) and over the 4 y in the subgroup of 15 patients who were scanned at all 4 time points (F3,42 = 0.52, P = 0.67; Fig. 1F). These analyses also revealed no change (P ≥ 0.36) in caudate 18F-FDOPA uptake after excluding the 5 dyskinesia patients. Subject differences in preoperative caudate and putamen 18F-FDOPA uptake values and in the posttransplantation uptake changes did not correlate with baseline disease severity or symptom duration.
In the whole group, we detected a significant correlation between graft-mediated changes in motor ratings and putamen 18F-FDOPA uptake across the first 3 time points (r = −0.30, P < 0.02). A significant correlation was also present in the 15 subjects with complete clinical and PET data across the 4 time points (r = −0.31, P < 0.04) and in the same subgroup across the first 3 time points (r = −0.38, P < 0.04). Clinical correlations with operative changes in caudate 18F-FDOPA uptake over time were not significant (P ≥ 0.23).
18F-FDOPA PET: Voxel-Based Analysis
Displays of mean 18F-FDOPA uptake for the subjects at baseline and at each posttransplantation time point are presented in Figure 2. Voxelwise analysis of the data from the whole group showed that putamen 18F-FDOPA uptake increased in the transplant recipients relative to baseline (P < 0.001, RMANOVA; Fig. 3A, red). At each time point, the increases (P < 0.001, post hoc tests) were localized to the posterior putamen bilaterally (Table 3). Interestingly, there was evidence of further increases in this region after 1 y (Fig. 3B), with significant posttransplantation increases at both 2 and 4 y relative to the 1-y time point at a lower threshold (P < 0.01). These longitudinal changes remained significant in the 15 subjects who were studied at all 4 time points (Supplemental Fig. 1A).
FIGURE 2.

Maps of mean 18F-FDOPA uptake in transplant recipients scanned at baseline (pre) and at 1 (post 1 y), 2 (post 2 y), and 4 (post 4 y) years after surgery. Postoperative scans showed sustained increases in putamen 18F-FDOPA uptake after transplantation. Maps were created on voxel basis from spatially normalized images of 18F-FDOPA uptake ratio, presented as percentage of normal value in 15 healthy subjects and displayed on standard MRI brain template. Color stripe represents normalized values of 18F-FDOPA uptake in striatal regions thresholded at 30%.
FIGURE 3.
Voxelwise comparisons of 18F-FDOPA uptake within striatum at baseline (pre) and at 1 (post 1 y), 2 (post 2 y), and 4 (post 4 y) years after transplantation. (A) Transplantation resulted in significantly increased 18F-FDOPA uptake in bilateral posterior putamen and concurrent declines in nongrafted caudate and ventrorostral putamen (P < 0.001; RMANOVA). SPM was displayed on standard MRI brain template. Color stripe represents T values thresholded at P < 0.001 for increasing regions (color-coded red) and P < 0.01 for decreasing regions (color-coded blue). (B) Post hoc plot revealed sustained increases of 18F-FDOPA uptake in posterior putamen (coordinates, −28 −6 0 mm) at each postoperative time point relative to baseline. (C) Post hoc plot demonstrated progressive decreases of postoperative 18F-FDOPA uptake in ventrorostral putamen (coordinates, 12 18 −8 mm) relative to baseline. Data from spheric VOI (4-mm radius) centered at peak voxel.
TABLE 3.
Striatal Regions with Significant Changes in 18F-FDOPA Uptake After Putamen Dopaminergic Cell Transplantation
| Change | Left |
Right |
||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Zmax | x | y | z | Zmax | |
| Increases, putamen | ||||||||
| RMANOVA | −28 | −6 | 0 | 7.21 | 34 | −8 | 2 | 6.53 |
| Post 1 y > pre | −28 | −6 | 0 | 5.70 | 36 | −8 | 0 | 5.09 |
| Post 2 y > pre | −28 | −6 | 0 | 7.13 | 32 | −6 | 2 | 6.64 |
| Post 4 y > pre | −28 | −4 | 0 | 5.18 | 32 | −8 | 4 | 4.98 |
| Post 2 y > post 1 y | −26 | −8 | 6 | 2.80* | 30 | −6 | 4 | 3.45† |
| Decreases, caudate | ||||||||
| RMANOVA | −10 | 12 | 8 | 4.73 | 12 | 16 | 2 | 3.81 |
| Post 1 y < pre | −10 | 12 | 8 | 3.76 | 12 | 14 | 4 | 2.83* |
| Post 2 y < pre | −8 | 12 | 4 | 3.65 | 10 | 12 | 0 | 2.94* |
| Post 4 y < pre | −8 | 12 | 4 | 4.40 | 16 | 20 | −6 | 4.61 |
| Post 4 y < post 1 y | −12 | 18 | −8 | 4.22 | 16 | 20 | −6 | 4.29 |
| Post 4 y < post 2 y | −12 | 14 | −6 | 2.97* | 16 | 20 | −6 | 3.34† |
Clusters that were detected within striatum mask at lower threshold of P < 0.01 (uncorrected).
Clusters that were detected within striatum mask at lower threshold of P < 0.001 (uncorrected).
x-, y-, and z-Coordinates are according to Montreal Neurologic Institute anatomic space. Data come from SPM comparisons of longitudinal 18F-FDOPA PET images within striatum at baseline (pre) and 1 (post 1 y), 2 (post 2 y), and 4 (post 4 y) years after transplantation. Listed clusters were significant at P < 0.001 and familywise error–corrected for multiple comparisons (P < 0.05).
There was also evidence of bilateral reductions over time in the ungrafted caudate and ventrorostral putamen (P < 0.001, RMANOVA; Fig. 3A, blue). These localized changes were observed at each of the 3 postoperative time points relative to the preoperative baseline (P < 0.001). The decline in PET signal was most prominent at 4 y, with the maximal loss of uptake in the ventrorostral putamen (Fig. 3C). These decreases persisted in the 15 subjects who were scanned at all 4 time points (Supplemental Fig. 1B). There was no evidence of posttransplantation changes in 18F-FDOPA uptake outside the striatum. Moreover, the longitudinal changes in striatal 18F-FDOPA uptake from voxelwise analyses were similar in the group as a whole after excluding the 5 dyskinesia patients.
Predictive Features in Baseline Scans
Voxelwise analysis within the striatal mask revealed a significant correlation (P < 0.05) between baseline 18F-FDOPA uptake in the nonengrafted anterior putamen and transplant-mediated changes in clinical motor ratings at 1 y (Fig. 4). The maxima of these correlations were localized to the ventrorostral putamen (−26, 8, −10 mm; z score at peak coordinate of each significant cluster [Zmax] = 2.0; r = 0.54, P < 0.02, and 14, 18, −4 mm, Zmax = 2.74; r = 0.59, P < 0.01). Baseline PET signal in the latter region was also predictive of clinical outcome at 2 y (r = 0.46, P < 0.03). Thus, higher baseline 18F-FDOPA uptake in these regions was associated with better clinical outcome after transplantation.
FIGURE 4.
Voxelwise correlation of 18F-FDOPA uptake within striatum at baseline with posttransplantation improvement in UPDRS motor ratings 1 y after surgery. (A) Baseline 18F-FDOPA uptake in ventrorostral putamen correlated with clinical benefit in transplant recipients. SPM was displayed on standard MRI brain template. Color stripe represents T values thresholded at P < 0.05. (B) Post hoc analysis disclosed positive correlation (r = 0.59; P < 0.01) between baseline 18F-FDOPA uptake in this region (coordinates, 14 18 −4 mm) and percentage change in off-state motor ratings in subjects with clinical improvement after transplantation. Data from spheric VOI (4-mm radius) centered at peak voxel.
DISCUSSION
Clinical Outcome
The current results expand on the original findings of the blinded phase of the study, which showed that at 1 y, the grafts improved motor function mainly in young transplant recipients (1). We now report continued clinical improvement at 2 y to nearly twice the degree observed at 1 y (Table 1; Fig. 1). Furthermore, we found that improvement was sustained in the subgroup of transplant recipients who were followed for 4 y after surgery. These long-term findings agree with the results of other open-label studies (5,9,10). In this study, we also confirmed the previous finding that age significantly affected clinical outcome at 1 y after transplantation (2). Although the younger patients still showed relatively better clinical improvement than did the older patients at 2 y after transplant surgery, this group difference was not significant, even with most of the subjects still in the study. Thus, the impact of age on clinical outcome may be time-dependent, with a slower time course of clinical benefit after transplantation in older subjects. Nevertheless, the time course of the age effect on clinical outcome could not be assessed fully because of the predominant dropout of the older subjects by the final time point.
The current study included the 5 transplant patients with GID who were no longer taking dopaminergic medications as reported previously (1,11). These subjects all belonged to the young subgroup of transplant recipients and had greater clinical improvement and putamen 18F-FDOPA uptake at 1 and 2 y than did those who did not develop this complication. That said, similar clinical outcomes and changes in PET signal were seen across the entire cohort whether or not these subjects were included in the analysis. Thus, the therapeutic window for symptom relief after engraftment may not be as narrow as had been previously suggested (1). Nonetheless, GID remains a major challenge for neural transplantation in PD. Studies are ongoing to develop new surgical approaches to avoid this troubling side effect.
Imaging Outcomes
We detected a highly significant increase in 18F-FDOPA uptake in the posterior putamen transplant site but not in the nongrafted caudate and anterior putamen. Indeed, transplantation was associated with an increase in mean putamen 18F-FDOPA uptake from 40% of normal at baseline to an average of 55% after surgery (Figs. 1C and 1D). Although the magnitude of these increases in PET signal was relatively small, the findings are consistent with studies showing a threshold for symptom development in PD of approximately 50% for putamen 18F-FDOPA uptake (20). This localized increase in putamen uptake occurred primarily at 1 y after transplantation. Nonetheless, additional increases were evident at 2 and 4 y on voxelwise searches of the entire striatal volume (Fig. 3B), suggesting the possibility of ongoing graft maturation in the years after dopaminergic cell implantation. Importantly, this approach also revealed progressive loss of PET signal in caudate and ventrorostral putamen, compatible with spread of the neurodegenerative process to dorsomedial regions of the substantia nigra pars compacta (2).
Posttransplantation increases in putamen PET signal over the first 2 postoperative years were found to correlate with improvement in clinical ratings. Moreover, this correlation was also significant in the 15 transplant recipients who had complete clinical and PET data over 4 postoperative years. Thus, clinical improvement, increased putamen 18F-FDOPA uptake, and significant clinical–PET correlations proved to be robust despite the substantial attrition of subjects between the third and final time points. It is not known why further clinical and imaging improvement was not evident between 2 and 4 y after implantation (Figs. 1B and 3B). It is possible that the capacity of the grafts to modulate the neural circuitry of the host brain is limited by local inflammatory responses, as suggested in some postmortem studies (1,3,8). It is also conceivable that α-synuclein pathology can develop in the grafts (21,22), even though in our cohort only 5 y had elapsed after transplantation as opposed to more than 10 y in the reported pathologic studies.
Last, we found that superior clinical outcomes after transplant surgery were associated with the degree of 18F-FDOPA uptake that was present preoperatively in the ventrorostral putamen. Indeed, this region coincided with that in which we detected progressive loss of PET signal over the 4 y after transplantation (Figs. 3 and 4). We note that the accuracy of this preoperative indicator of clinical outcome declined over time. This decline is likely related to the ongoing neurodegeneration that was occurring in the predictive area. Thus, the preoperative imaging feature that predicted a good response is subject to change over time and is valid only in the short term. Nonetheless, the findings indicate that a favorable clinical response to transplantation is dependent on the extent of loss of nigrostriatal dopaminergic terminals at baseline. Thus, subjects with terminal loss restricted to the dorsoposterior putamen appear to respond well to tissue engraftment that targets this area. By contrast, individuals with more extensive nigrostriatal attrition are less likely to exhibit a clinically meaningful response to such local engraftment.
Interestingly, these data also raise the possibility of a time window for optimal transplantation outcomes. Although preoperative preservation of dopaminergic projections to the ventral striatum is predictive of a better treatment response, these specific terminals are likely to degenerate over the ensuing 4–5 y, at which point transplantation may be less beneficial. In this regard, preoperative 18F-FDOPA PET can provide valuable information to assist in the selection of patients for future trials of cell-based dopaminergic therapies for PD. Moreover, the imaging data may also be useful in determining the optimal time for these interventions.
CONCLUSION
We report long-term clinical and imaging outcomes after transplantation from 33 of the original participants in our double-blind, placebo-controlled trial of embryonic dopaminergic cell implantation for PD (1). Relative to pre-operative baseline, clinical improvement in motor ratings and increased 18F-FDOPA uptake in the grafted putamen were evident at 1, 2, and 4 y after the transplantation surgery. Moreover, graft-induced changes in clinical and imaging measures were significantly intercorrelated over the course of the study. The significant improvements in clinical and imaging outcome achieved at 1 y after surgery were found to be sustained over the long term and were not driven by individuals in whom GID developed after transplantation.
Supplementary Material
Acknowledgments
We thank Claude Margouleff for technical support and Toni Fitzpatrick for valuable editorial assistance. This work was supported by the National Institutes of Health (NIH), grant R01 NS 32368.
References
- 1.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T, Dhawan V, Chaly T, et al. Blinded positron emission tomography study of dopamine cell implantation for Parkinson’s disease. Ann Neurol. 2001;50:181–187. doi: 10.1002/ana.1075. [DOI] [PubMed] [Google Scholar]
- 3.Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 4.Freed CR, Breeze RE, Rosenberg NL, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N Engl J Med. 1992;327:1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- 5.Wenning GK, Odin P, Morrish P, et al. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson’s disease. Ann Neurol. 1997;42:95–107. doi: 10.1002/ana.410420115. [DOI] [PubMed] [Google Scholar]
- 6.Piccini P, Brooks DJ, Bjorklund A, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat Neurosci. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 7.Kordower JH, Freeman TB, Snow BJ, et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N Engl J Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 8.Mendez I, Sanchez-Pernaute R, Cooper O, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remy P, Samson Y, Hantraye P, et al. Clinical correlates of [18F]fluorodopa uptake in five grafted parkinsonian patients. Ann Neurol. 1995;38:580–588. doi: 10.1002/ana.410380406. [DOI] [PubMed] [Google Scholar]
- 10.Hauser RA, Freeman TB, Snow BJ, et al. Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol. 1999;56:179–187. doi: 10.1001/archneur.56.2.179. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Feigin A, Dhawan V, et al. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol. 2002;52:628–634. doi: 10.1002/ana.10359. [DOI] [PubMed] [Google Scholar]
- 12.Piccini P, Pavese N, Hagell P, et al. Factors affecting the clinical outcome after neural transplantation in Parkinson disease. Brain. 2005;128:2977–2986. doi: 10.1093/brain/awh649. [DOI] [PubMed] [Google Scholar]
- 13.Fahn S, Elton R. the members of the Unified Parkinson’s Disease Rating Scale Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, editors. Recent Developments in Parkinson’s Disease. New York, NY: MacMillan; 1987. pp. 153–163. [Google Scholar]
- 14.Dhawan V, Ma Y, Pillai V, et al. Comparative analysis of striatal FDOPA uptake in Parkinson’s disease: ratio method versus graphical approach. J Nucl Med. 2002;43:1324–1330. [PubMed] [Google Scholar]
- 15.Searle S, Casella G, McCulloch C. Variance Components. New York, NY: John Wiley & Sons, Inc; 1992. [Google Scholar]
- 16.Ellis SP, Underwood MD, Arango V, Mann JJ. Mixed models and multiple comparisons in analysis of human neurochemical maps. Psychiatry Res. 2000;99:111–119. doi: 10.1016/s0925-4927(00)00051-2. [DOI] [PubMed] [Google Scholar]
- 17.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 18.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1—correlation within subjects. BMJ. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson’s disease. Brain. 2007;130:1834–1846. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrish PK, Sawle GV, Brooks DJ. Regional changes in [18F]DOPA metabolism in the striatum in Parkinson’s disease. Brain. 1996;119:2097–2103. doi: 10.1093/brain/119.6.2097. [DOI] [PubMed] [Google Scholar]
- 21.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 22.Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.