Abstract
Background:
Published studies assessing whether asymmetrical facial ultraviolet light exposure leads to underlying differences in skin physiology and morphology report only clinical observations. The aim of this study was to assess the visual impact on the skin of repeated ultraviolet-A (UVA) exposure through a window.
Methods:
Eight women and two men presenting with asymmetrical signs of photoaging due to overexposure of one side of their face to the sun through a window over a long period of time were enrolled in the study. Split-face biometrologic assessments were performed (clinical scoring, hydration with Corneometer®, mechanical properties with Cutometer®, transepidermal water loss with AquaFlux®, skin relief with fringe projection, photography, stripping, and then lipid peroxidation analysis).
Results:
Significant differences were observed in clinical scores for wrinkles, skin roughness assessed by fringe projection on the cheek, and skin heterogeneity assessed with spectrocolorimetry on the cheekbone. Other differences were observed for skin hydration, as well as skin laxity, which tended towards significance.
Discussion:
This study suggests the potential benefit of daily UVA protection during nondeliberate exposure indoors as well as outside.
Keywords: UVA, asymmetry, photodamage, skin aging
Introduction
Terrestrial solar ultraviolet (UV) radiation comprises UVB (280–315 nm) and UVA (315–400 nm) because UV radiation within the UVC waveband (100–280 nm) is absorbed entirely within the atmosphere. During a summer day, approximately 3.5% of the UV radiation reaching the earth’s surface is UVB, whereas approximately 96.5% is UVA.
Whilst UVB radiation is absorbed almost entirely by ordinary glass, generally at least 50% of incident UVA is transmitted.1–3 Photoaging is believed to account for most of the age-related changes in facial skin appearance, with underlying histopathology that reveals dystrophic elastic fibers, reduced collagen, and increased activity of matrix metalloproteinase-1.4,5 Many studies have explored the relative contributions of UVA and UVB to the aging phenotype and whilst UVA photons are on average 1000 times less energetic than UVB photons, they are capable of inducing aging changes even in the dermis, partly due to their greater average depth of skin penetration than UVB photons. Transmission of UV through car windows is more variable. Car windshields are made from laminated glass, which can filter most UVA (gray-tinted laminated glass is able to block about 99% of UVA), whereas nonlaminated side and rear windows can transmit around one-half of incident UVA.6,7 This penetration is highly wavelength-dependent.8 For example, measurements obtained from the excised, nonirradiated epidermis obtained from the lower back of 16 Caucasian subjects showed average transmissions of 10%, 34%, 46%, 54%, and 67% at wavelengths of 290, 300, 320, 350, and 400 nm, respectively.9
Various studies are available that describe the ability of UVA to cause changes in cells in culture and in vivo.10,11,17 Human studies have demonstrated that even relatively low doses of UVA given repetitively can induce changes associated with photoaging, including decreased elastic tissue content and stratum corneum thickening.10,11 These findings have also been replicated in whole animal studies, with hairless mice subjected to repeated UVA exposure developing wrinkles and other signs of photodamage, together with histologic changes in dermal tissues and increased levels of matrix metalloproteinase-1.12 These effects are primarily via reactive oxygen species, generated through endogenous chromophores such as trans-urocanic acid and porphyrins which act as photosensitizers.13,14 The resulting oxidative stress leads to damage to key structural proteins, lipids and DNA, and is further exacerbated by reduction in free radical scavenging enzymes, such as catalase.15 Oxidation in the epidermis could elicit signals that lead to damage in adjacent deeper cells.16
Asymmetry has been observed with regard to photodamage,18 and premalignant19 and malignant20 skin lesions, with incidence higher on the side closest to a car side window. The photodamaging effects of UVA have also been demonstrated21 for a female office worker with extensive Favre-Roucouchot disease on her left cheek, with almost no photodamage observed on the other cheek. This patient’s left side had been near a window for 15 years. In the same way that twin studies are valuable22–24 for assessing the contribution of environment to appearance, those presenting with pronounced asymmetry and who have an unbalanced right-left side UV exposure history, provide an opportunity to assess the possible impact of chronic UV exposure without the interindividual variability brought by the different histories of total UV exposure. However, studies25 reporting only clinical observations are available in the literature or have been presented at scientific meetings,21 and they do not determine whether asymmetric facial UV exposure leads to any underlying differences in skin physiology and morphology.
To assess the worth of a study on asymmetrical skin aging, a radio advertisement was broadcast appealing for men or women with significant differences in the clinical signs of facial aging that they attributed to sun exposure through a nearby window for a great part of their life, due to occupation or activities. The great variety of responders, with occupational histories suggestive of a causal link (drivers, teachers, shopkeepers, saleswomen) demonstrated the worth of performing a pilot study to assess the impact of cumulative UVA exposure through clinical skin measures.
Whilst slight asymmetry is commonplace, if those presenting with strong visible asymmetry have different left-right exposure histories, it does allow for an exploratory assessment of the impact that UV, and in particular UVA, might be having on skin.
Materials and methods
A pilot study was conducted on eight women aged 64.9 ± 5.4 years and two men aged 56–61 years living in the region of Besançon, France. The subjects were likely to have been exposed to a higher proportion of glass-filtered UV radiation on one side of their face.
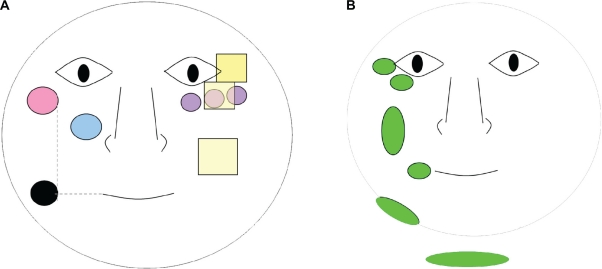
Clinical evaluations were performed on bare skin in controlled environmental conditions (temperature 22.5°C ± 0.7°C, H 51.0% ± 4.5%). Eleven skin characteristics were assessed on the face by a trained dermatologist using photographic scales26 and ordinal scales (Figure 1). Furthermore, a questionnaire dedicated to the subjects’ history in terms of sun exposure and occupation, as well as sun protection was completed. For each side of the face, photographs were taken by VISIA® facial imaging (Canfield Scientific, Fairfield, NJ), skin relief by fringe projection (80 × 60 mm rectangular field; Eotech, France), skin color by spectrocolorimetry (D65 Illuminant, Minolta, France), skin elasticity by Cutometry (SEM 474, suction applied 3 sec/suction off 2 sec, 2 mm probe, 450 mbar pressure, five repeats, Courage and Khazaka, Germany), hydration by Corneometry (CM825, Courage and Khazaka, Cologne, Germany), and transepidermal water loss (TEWL) by AquaFlux® (AF200, Biox, UK).
Figure 1.
Schematic representation of the assessment areas (on the left for biometrologic assessments and stripping, on the right for clinical scoring). Each assessment was performed on both sides of the face A) left; B) right.
Key:  Cutometer®;
Cutometer®;  D-Squame® tape strips; • Corneometer® Aquaflux®;
D-Squame® tape strips; • Corneometer® Aquaflux®;  Spectrophotometer (three measures across the cheekbone);
Spectrophotometer (three measures across the cheekbone);  Fringe projection (roughness + wrinkle volume);
Fringe projection (roughness + wrinkle volume);  Clinical scores.
Clinical scores.
Additionally, D-Squame® tape strippings (CuDerm Corporation, Dallas, TX) were collected (Figure 1) from both sides of the face. Tape strippings were stored at −20°C until analyzed. Phospholipids were extracted27 and detected using LPO–CC (lipid peroxides) kits (Kamiya Biosciences, Seattle, WA). Detection of lipid peroxidation was carried out at 675 nm using a Multiskan RC colorimetric plate reader (Thermo Labsystems, Finland) with results normalized to protein content. Nonparametric tests were performed to compare data from exposed and nonexposed areas (Wilcoxon test) with statistical significance achieved if P < 0.05 (5%).
Results
Ten volunteers with occupational and UV exposure histories suggestive of a link to the asymmetry were recruited (Table 1). Supporting the hypothesis of increased damage to and barrier impairment of photo exposed skin, statistically significant (P < 0.05) differences were observed when comparing window-exposed sides of the face to the nonwindow-exposed sides for:
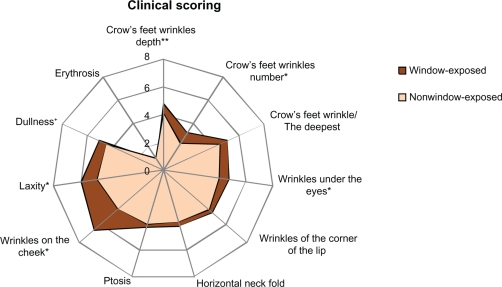
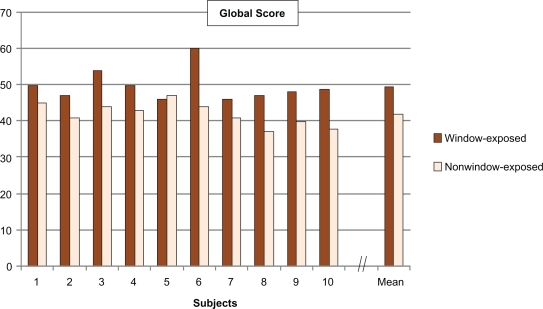
Clinical scores (Figure 2) for wrinkles on the cheek, wrinkles under the eyes, crow’s feet number and laxity; global clinical scores (Figure 3) which were higher on the window-exposed side than on the nonwindow-exposed side, indicating a worsening of each characteristic;
Skin color, with eight subjects showing slightly less heterogeneity, ie, lower ΔE (Table 2), on the window-exposed side than the nonwindow-exposed side;
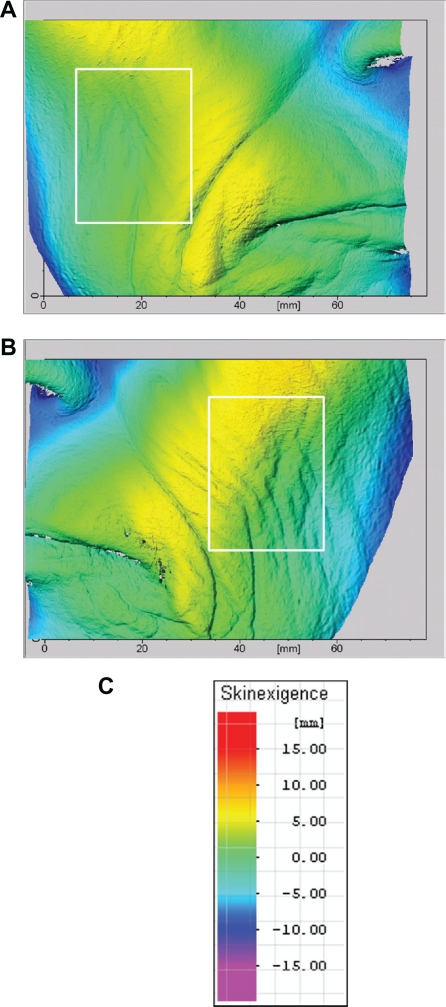
Wrinkle volume and roughness on the cheek (Table 3 and Figure 4), with nine subjects showing deeper wrinkles and less roughness on the window-exposed side than on the nonwindow-exposed side.
Table 1.
Characteristics of the subjects
| Subject (Number) | Age | Gender | Phototype | Profession/activity accounting for asymmetry | Years of asymmetrical ultraviolet exposure/years of life (%) | Overexposed side |
|---|---|---|---|---|---|---|
| 1 | 62 | F | III | Medical saleswoman | 53.2 | Left |
| 2 | 66 | F | II | Childminder | 49.2 | Left |
| 3 | 56 | M | IV | Lorry driver | 32.1 | Left |
| 4 | 59 | F | III | Medical saleswoman | 59.3 | Left |
| 5 | 75 | F | II | Teacher | 49.3 | Right |
| 6 | 64 | F | III | Bus driver | 39.1 | Left |
| 7 | 70 | F | II | Shopkeeper/(shop window) | 48.6 | Right |
| 8 | 63 | F | II | (Sales)/two hours driving every day | 47.6 | Left |
| 9 | 61 | M | II | Salesperson + lorry driver | 24.6 | Left |
| 10 | 60 | F | II | Medical saleswoman | 50.0 | Left |
Figure 2.
Mean clinical scores assessed on each side of the face. Most of the clinical items studied were higher on the window-exposed side (worsening of skin characteristics).
Notes: **Significant differences (P < 0.05) *Tendency (0.05 < P <0.1).
Figure 3.
Global score calculated from the sum of the 11 clinical scores for each subject (significant difference between window-exposed and nonwindow-exposed sides, P = 0.0039).
Table 2.
Results from biometrologic assessments performed on the cheekbone. (ΔEmean = the mean colorimetric distance computed from the three measures (CIELAB 1976), ΔEmax = the maximal distance between the three measures)
| Ue | Ur | Uf | Ur/Ue | ΔEmean | ΔEmax | |
|---|---|---|---|---|---|---|
| WE | 0.263 ± 0.114+ | 0.100 ± 0.022+ | 0.339 ± 0.126+ | 0.449 ± 0.204 | 2.7 ± 1.4* | 3.9 ± 2.2* |
| NWE | 0.178 ± 0.068 | 0.091 ± 0.022 | 0.249 ± 0.076 | 0.567 ± 0.212 | 3.9 ± 1.4 | 5.6 ± 2.0 |
Notes: Data are expressed as mean ± standard deviation.
Differences tend to be significant (0.05 < P < 0.1).
Significant differences (P < 0.05).
Abbreviations: WE, window-exposed side; NWE, nonwindow-exposed side.
Table 3.
Results from fringe projection assessments
| Volume (mm3) | Sa (μm) | ||
|---|---|---|---|
| Cheek | WE | 24.8 ± 9.7* | 82.9 ± 32.6* |
| NWE | 15.8 ± 5.0 | 52.9 ± 16.8 | |
| Wrinkles under eyes | WE | 10.0 ± 3.8 | 88.2 ± 33.8 |
| NWE | 8.9 ± 2.5 | 78.6 ± 22.2 |
Notes: Data are expressed as mean ± standard deviation. Differences tend to be significant (0.05 < P < 0.1).
Significant differences (P < 0.05).
Abbreviations: WE, window-exposed side; NWE, nonwindow-exposed side.
Figure 4.
Images from three-dimensional topographies of subject number 1 (female) who had spent more than half of her life working as a medical saleswoman, driving a car 2–5 hours per day. The regions of interest selected for relief analyses (roughness and volume) are represented in the white frames. A) Nonwindow-exposed side (right side). B) Window-exposed side (left side), where the wrinkles are more numerous and deeper. C) Color scale.
Differences tending towards statistical significance (P < 0.1) were observed for:
Clinical scores for crow’s feet depth and dullness of skin (Figure 2), which were higher on the window-exposed side than on the nonwindow-exposed side, indicating a worsening of each characteristic;
Hydration of cheek skin, with eight subjects showing more dryness (Table 4) on the window-exposed side than on the nonwindow-exposed side;
Laxity of skin on the cheekbone, with seven subjects showing more lax skin, ie, an increase of Uf (Table 2), on the window-exposed side than on the nonwindow-exposed side.
Table 4.
Results from biometrologic assessments performed on lower cheek
| HI | TEWL | LPO | |
|---|---|---|---|
| WE | 34.8 ± 11.1+ | 21.9 ± 6.6 | 0.33 ± 0.14 |
| NWE | 39.0 ± 9.8 | 19.6 ± 4.6 | 0.44 ± 0.18 |
Notes: Data are expressed as mean ± standard deviation.
Differences tend to be significant (0.05 < P < 0.1).
Significant differences (P < 0.05).
Abbreviations: WE, window-exposed side; NWE, nonwindow-exposed side; LPO, lipid peroxidation.
Other results (P > 0.1) worthy of note were found for:
EWL on the cheek (Table 4), with six subjects showing a higher TEWL on the window-exposed side than on the nonwindow-exposed side;
Elasticity, ie, Ur/Ue (Table 2), on the cheekbone, with seven subjects showing lower skin elasticity on the window-exposed side than on the nonwindow-exposed side;
Wrinkle volume measured under the eyes (Table 3 and Figure 5), with seven subjects showing deeper wrinkles on the window-exposed side than on the nonwindow-exposed side (difference of 26% on average between both sides [7%–50%]);
The amount of lipid peroxidation (Table 4) was lower in seven subjects on the window-exposed side than on the nonwindow-exposed side (decrease of 42% [7%–67%]).
Figure 5.
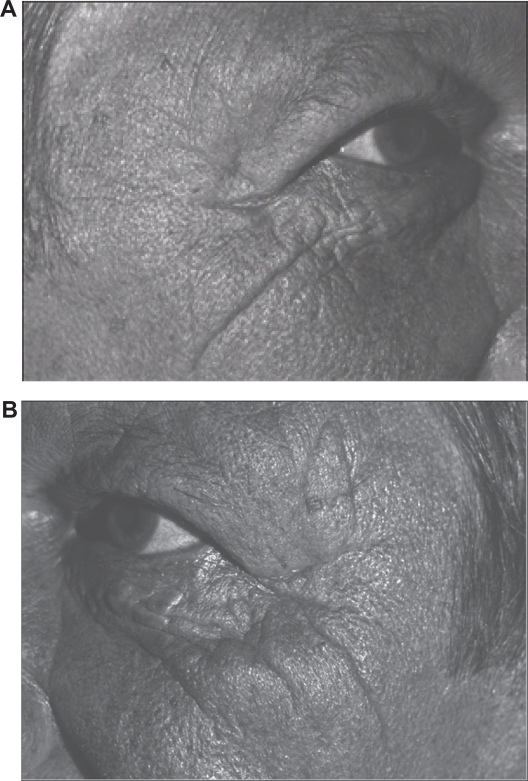
Photographs for subject number 3 (male) who had driven a lorry for a third of his life for 9–10 hours per day. A) Nonwindow-exposed side (his right side). B) Window-exposed side (his left side) where the wrinkles of the crow’s foot are more numerous and more marked.
Discussion
This study indicates the impact of cumulative UVA exposure on skin. All subjects had histories suggestive of unilateral UVA exposure and, although the primary clinical sign was not the same for all subjects (some had more erythrosis [data not shown], others had more wrinkles on the cheeks, some had more wrinkles on the eye contour), significant differences were systematically observed for most of the assessed parameters.
Lowe et al have shown that even suberythemal doses of repetitive UVA are capable of producing photodamage.10 Cumulative UVA induces thickening of the viable epidermis and deposition of lysozyme in elastic fibers.17 Our results demonstrate possible consequences of these previous histologic findings by showing a significant decrease of skin laxity and elasticity on window-exposed skin, as well as a decrease in skin color heterogeneity. Chromophores may be less visible due to increased epidermal thickness. No significant differences were observed for TEWL, lipid peroxidation levels, or wrinkles under the eyes by fringe projection, although this may be a function of low sample size. It would be worth undertaking further work with a larger panel, because differences appear in more than 60% of the enrolled volunteers.
However, larger panel size may demonstrate a significant impairment of skin barrier properties on the window-exposed side. Lower lipid peroxidation levels could indicate depleted lipid and/or sebum production, leading to reduced TEWL and poorer hydration. This would be consistent with reduced lipid content of the stratum corneum in photoaged skin.28
Whilst in this study we focused on the role of cumulative UVA exposure on skin aging, in recent years there has been increasing interest in its ability to promote carcinogenesis following published research using fish models of sunlight-induced malignant melanomas.29 It is now recognized that UVA is able to induce oxidative DNA damage, generating 8-oxoguanine30,31 and cyclobutane pyrimidine dimer photoproducts32 which, in turn, can lead to mutagenesis. Whilst the relative contribution of UVA exposure to carcinogenesis in human skin is unclear, there is growing evidence of a strong association, particularly amongst people subjected to higher UVA levels. For example, a higher incidence of both nonmalignant and malignant skin lesions has been found on the window side of the face of drivers.19,20 Additionally, frequent use of sun beds (which emit predominantly UVA radiation) is known to increase the risk of melanoma.33 Sun-screens, whilst offering good levels of protection against predominately UVB-induced skin burning, still transmit significant levels of UVA.34 This may lead to accumulation of high doses of deeper skin-penetrating UVA and contribute to melanoma risk.35
The great variability of occupations affected by asymmetrical facial aging (Table 1) suggests that more attention should be given to this type of indirect irradiation. The effect of UV radiation is now well recognized, and whilst people are aware of the usefulness of sun protection during peak periods, people are not yet familiar with indirect sun exposure. Moreover, whereas tinted windows in vehicles are now widely provided by car designers, contemporary architectural design incorporates ever larger window areas. This pilot study provides some evidence for the contribution of UVA to photoaging. We cannot exclude the influence of UVB exposure when car windows are opened, for example, nor each subject’s own intrinsic asymmetry. However, this study does suggest that daily protection against nondeliberate UVA exposure indoors, as well as outside, may be an important function of any daily sunscreen.36
Figure 6.
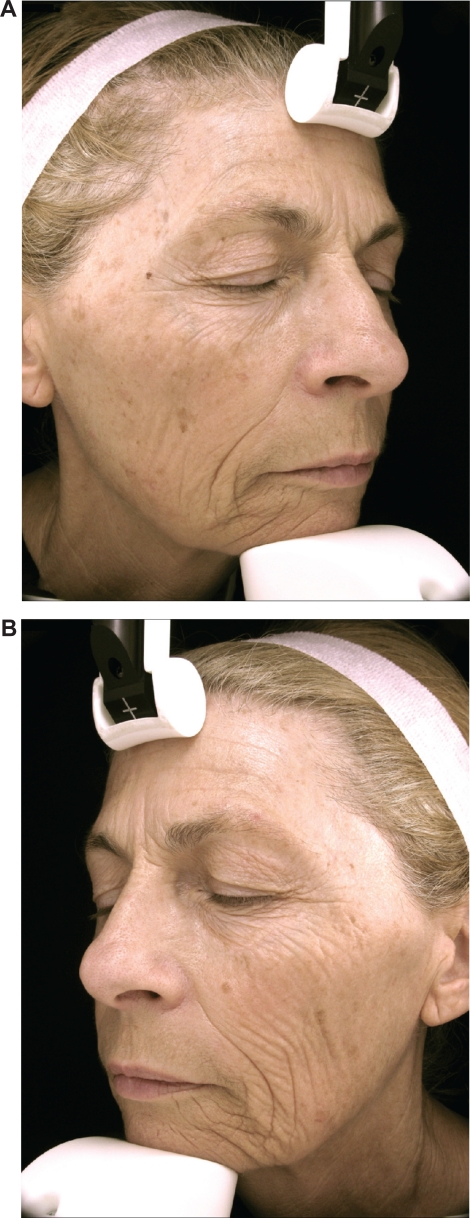
Photographs of the subject number 1 (female) who had spent more than half of her life working as a medical saleswoman and driving a car 2–5 hours per day. A) Nonwindow-exposed side (her right side). B) Window-exposed side (her left side): wrinkles of cheek, crow’s feet and wrinkles under the eyes are more numerous and deeper.
Figure 7.
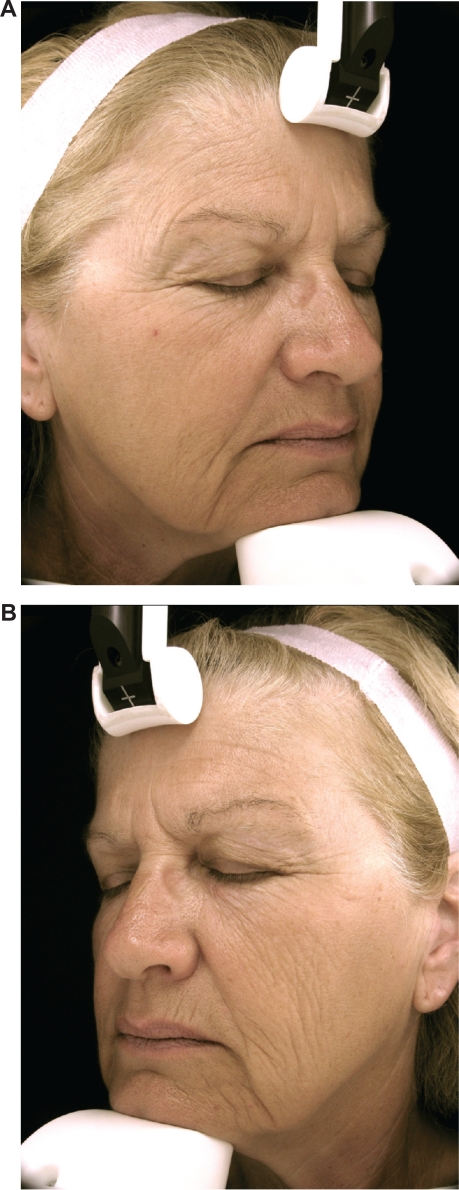
Photographs of subject number 8 (female) who had spent nearly half her life driving a car two hours per day to go to and from work. A) Nonwindow-exposed side (her right side). B) Window-exposed side (her left side) with wrinkles of cheek, crow’s feet, and wrinkles under the eyes being more numerous and deeper.
Acknowledgments
The authors thank Dr Raphaëlle Guerre-Schmidt, Céline Thiébaut, Cécile Tarrit, Perrine Mermet, and Elisabeth Homassel for their contribution to this study. The volunteers who participated gave their written consent to allow their photos to be published, even though they are identifiable.
Footnotes
Disclosure
This research was funded by the Boots Company.
References
- 1.Parisi AV, Wong JCF. The erythemal ultraviolet exposure for humans in greenhouses. Phys Med Biol. 1997;42:2331–2339. doi: 10.1088/0031-9155/42/12/002. [DOI] [PubMed] [Google Scholar]
- 2.Parisi AV, Turnbull DJ, Kimlin MG. Dosimetric and spectroradiometric investigations of glass filtered solar UV. Photochem Photobiol. 2007;83:777–781. doi: 10.1562/2006-08-20-RA-1007. [DOI] [PubMed] [Google Scholar]
- 3.Kimlin MG, Parisi AV. Ultraviolet radiation penetrating vehicle glass: A field based comparative study. Phys Med Biol. 1999;44:917–926. doi: 10.1088/0031-9155/44/4/008. [DOI] [PubMed] [Google Scholar]
- 4.Fligiel SE, Varani J, Datta SC, et al. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins G. Molecular mechanisms of skin ageing. Mech Ageing Dev. 2006;123:801–810. doi: 10.1016/s0047-6374(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 6.Tuchinda C, Srivannaboon S, Lim H. Photo-protection by window glass, automobile glass and sunglasses. J Am Acad Dermatol. 2006;54:845–854. doi: 10.1016/j.jaad.2005.11.1082. [DOI] [PubMed] [Google Scholar]
- 7.Hampton PJ, Farr PM, Diffey BL, Lloyd JJ. Implication for photosensitive patients of ultraviolet A exposure in vehicles. Br J Dermatol. 2004;151:873–876. doi: 10.1111/j.1365-2133.2004.06098.x. [DOI] [PubMed] [Google Scholar]
- 8.Bruls WA. Transmission of human epidermis and stratum corneum as a function of thickness in the ultraviolet and visible wavelengths. Photochem Photobiol. 1984;40:485–494. doi: 10.1111/j.1751-1097.1984.tb04622.x. [DOI] [PubMed] [Google Scholar]
- 9.Hennessy A, Oh C, Rees J, Diffey B. The photoadaptive response to ultraviolet exposure in human skin using ultraviolet spectrophotometry. Photodermatol Photoimmunol Photomed. 2005;21:229–233. doi: 10.1111/j.1600-0781.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 10.Lowe NJ, Meyers DP, Wieder JM, et al. Low doses of repetitive ultraviolet A induce morphologic changes in human skin. J Invest Dermatol. 1995;105:739–743. doi: 10.1111/1523-1747.ep12325517. [DOI] [PubMed] [Google Scholar]
- 11.Lavker RM, Gerberick F, Veres D, et al. Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J Am Acad Dermatol. 1995;32:53–62. doi: 10.1016/0190-9622(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 12.Bissett DL, Hannon DP. Wavelength dependence of histological, physical and visual changes in chronically UV irradiated hairless mouse skin. Photochem Photobiol. 1989;50:763–769. doi: 10.1111/j.1751-1097.1989.tb02907.x. [DOI] [PubMed] [Google Scholar]
- 13.Thiele HH, Dreher F, Packer L. Antioxidant defense systems in skin. In: Elsner P, Maibach HI, editors. Cosmeceuticals – Drugs vs Cosmetics. New York, NY: Dekker; 2000. [Google Scholar]
- 14.Wondrak GT, Jacobson MK, Jacobson EL. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for photoprotection. Photochem Photobiol Sci. 2006;5:215–237. doi: 10.1039/b504573h. [DOI] [PubMed] [Google Scholar]
- 15.Hellemans L, Corstjens H, Neven A, et al. Antioxidant enzyme activity in human stratum corneum shows seasonal variation with an age-dependent recovery. J Invest Dermatol. 2003;120:434–439. doi: 10.1046/j.1523-1747.2003.12056.x. [DOI] [PubMed] [Google Scholar]
- 16.Ridley AJ, Whiteside JR, McMillan TJ, Allinson SL. Cellular and sub-cellular responses to UVA in relation to carcinogenesis. Int J Radiat Biol. 2009;85:177–195. doi: 10.1080/09553000902740150. [DOI] [PubMed] [Google Scholar]
- 17.Lavker R, Kaidbey K. The spectral dependence for UVA induced cumulative damage in skin. J Invest Dermatol. 1997;108:17–21. doi: 10.1111/1523-1747.ep12285613. [DOI] [PubMed] [Google Scholar]
- 18.Singer R, Hamilton TA, Voorhes JJ, et al. Association of assymetrical facial damage to automobile driving. Arch Dermatol. 1994;130:121–123. doi: 10.1001/archderm.1994.01690010127031. [DOI] [PubMed] [Google Scholar]
- 19.Foley P, Lanzer D, Marks R. Are solar keratoses more common on the driver’s side? Br Med J. 1986;293:18. doi: 10.1136/bmj.293.6538.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler ST, Fosko SW. Increased prevalence of left-sided skin cancers. J Am Acad Dermatol. 2010 Mar 10; doi: 10.1016/j.jaad.2009.11.032. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Moulin G, Thomas L, Vigneau M, Fiere A. Un Cas unilateral d’élastose avec kystes et comédons de Favre et Racouchot. Ann Dermatol Venereol. 1994;121:721–732. [PubMed] [Google Scholar]
- 22.Shekar SN, Luciano M, Duffy DL, Martin NG. Genetic and environmental influences on skin pattern deterioration. J Invest Dermatol. 2005;125:1119–1129. doi: 10.1111/j.0022-202X.2005.23961.x. [DOI] [PubMed] [Google Scholar]
- 23.Doshi DN, Hanneman KK, Cooper KD. Smoking and skin aging in identical twins. Arch Dermatol. 2007;143:1543–1546. doi: 10.1001/archderm.143.12.1543. [DOI] [PubMed] [Google Scholar]
- 24.Bachelor MA, Bowden GT. UVA mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin Cancer Biol. 2004;14:131–138. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Rexbye H, Petersen I, Johansen M, et al. Influence of environmental factors on facial aging. Age Aging. 2006;35:110–115. doi: 10.1093/ageing/afj031. [DOI] [PubMed] [Google Scholar]
- 26.Bazin R, Doublet E. Atlas du Vieillissement Cutané. Vol. 1. Paris: Ed Med Com; 2007. [Google Scholar]
- 27.Tateishi T, Yoshimine N, Kazuya F. Serum lipid peroxide assayed by a new colorimetric method. Exp Gerontol. 1987;22:103–111. doi: 10.1016/0531-5565(87)90045-3. [DOI] [PubMed] [Google Scholar]
- 28.Rogers J, Harding C, Mayo A, Banks J, Rawlings A. Stratum corneum lipids: The effects of aging and the seasons. Arch Dermatol Res. 1996;288:765–770. doi: 10.1007/BF02505294. [DOI] [PubMed] [Google Scholar]
- 29.Setlow RB, Woodhead AD, Grist E. Animal model for ultraviolet radiation-induced melanoma: Platyfish-swordtail hybrid. Proc Natl Acad Sci U S A. 1989;86:8922–8926. doi: 10.1073/pnas.86.22.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kvam E, Tyrrell RM. Induction of oxidative DNA base damage in human skin cells by UV and near visible radiation. Carcinogenesis. 1997;18:2379–2384. doi: 10.1093/carcin/18.12.2379. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Rosenstein BS, Wang Y, et al. Induction of 8-oxo-7,8-dihydro-2’deoxyguanosine by ultraviolet radiation in calf thymus DNA and HeLa cells. Photochem Photobiol. 1997;65:119–124. doi: 10.1111/j.1751-1097.1997.tb01886.x. [DOI] [PubMed] [Google Scholar]
- 32.Runger TM, Kappes UP. Mechanisms of mutation formation with long-wave ultraviolet light (UVA) Photodermatol Photoimmunol Photomed. 2008;24:2–10. doi: 10.1111/j.1600-0781.2008.00319.x. [DOI] [PubMed] [Google Scholar]
- 33.Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: A case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557–1568. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haywood R, Wardman P, Sanders R, Linge C. Sunscreens protect against ultraviolet-A induced free radicals in skin: Implications for skin ageing and melanoma? J Invest Dermatol. 2003;121:862–868. doi: 10.1046/j.1523-1747.2003.12498.x. [DOI] [PubMed] [Google Scholar]
- 35.Gorham ED, Mohr SB, Garland CF, et al. Do sunscreens increase risk of melanoma in populations residing at higher latitudes. Ann Epidemiol. 2007;17:956–963. doi: 10.1016/j.annepidem.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Gasparro FP. Sunscreens, skin photobiology and skin cancer: The need for UVA protection and evaluation of efficacy. Environ Health Perspect. 2000;108(Suppl 1):71–78. doi: 10.1289/ehp.00108s171. [DOI] [PMC free article] [PubMed] [Google Scholar]