Abstract
Background: We sought to investigate the characteristics and survival rate of patients with gastrointestinal stromal tumor (GIST) associated with other primary malignancies.
Patients and methods: A total of 783 patients with GIST were identified from 1995 to 2007. Additional primaries included tumors not considered metastasis, invasion, or recurrence of GIST, nor non-melanoma skin cancer. Data on gender, age at diagnosis, follow-up time after diagnosis, and death were collected.
Results: Of the 783 patients with GIST, 153(20%) were identified with at least one additional primary. Patients with additional primaries were more often men (M : F 1.5 versus 1.3) and older (66 versus 53 years). More patients had another cancer diagnosed before (134) than after (52) GIST. Primaries observed before GIST were cancers of the prostate (25), breast (12), esophagus (9), and kidney (7) and melanoma (6). Lung (5) and kidney (5) primaries were the most frequent after GIST. The 5-year survival was 68% for patients with primaries before GIST, 61% for patients with primaries after GIST, 58% for patients with GIST only, and 49% for patients with two or more primaries in addition to GIST (P = 0.002).
Conclusions: Approximately 20% of patients with GIST develop other cancers. Inferior median 5-year survival was observed in patients with GIST with two or more other cancers. The etiology and clinical implications of other malignancies in patients with GIST should be investigated.
Keywords: GIST, imatinib, predisposition, risk, synchronous
Gastrointestinal stromal tumor (GIST) is a rare cancer of the digestive tract that accounts for 0.1%–3.0% of all gastrointestinal neoplasms, 10% of small-bowel tumors, and 10%–15% of all sarcomas [1–4]. There are ∼5000 newly diagnosed cases per year in the USA. Although most of these cases are sporadic, there are reported syndromes of autosomal dominant hereditary GIST that result from inheritance of a germ-line KIT or platelet-derived growth factor receptor (PDGFR) mutation [5]. Additionally, there are predisposition syndromes in which affected individuals may develop GIST as well as other types of cancer. Examples include malignant peripheral nerve sheath tumor in neurofibromatosis [6], paraganglioma and pulmonary chondroma in Carney’s triad [7], and paraganglioma in the Carney–Stratakis syndrome [8]. Recently, Agaimy et al. [9] found that the most common association with GIST is gastric carcinoma (47%), prostate cancer (9%), lymphoma/leukemia (7%), and breast cancer (7%). Au et al. [10] reported that GIST and papillary renal cell carcinoma may occur as familial tumors related to mutations in the proto-oncogenes c-MET and c-KIT. An association has been demonstrated between GIST and acute myeloid leukemia (AML) as well as chronic myelogenous leukemia [11].
The clinical use of imatinib mesylate (imatinib), an oral inhibitor of the Kit receptor tyrosine kinase, has increased the survival of patients with metastatic GIST [12]. Due to the marked success of imatinib in both the adjuvant primary and the metastatic settings, many patients with GIST are treated with imatinib for many years. There are limited data as to the occurrence of additional malignancies after diagnosis of GIST in patients treated with imatinib. Thus, it is critical to investigate the prevalence and type of malignancies that develop before and after diagnosis in the era of increasing use of imatinib for GIST.
In this study, we used the University of Texas M. D. Anderson Cancer Center (MDACC) cancer registry to identify additional primary malignancies among our patients with GIST. Furthermore, we have rigorously determined detailed patient demographics, histological type of all malignancies, and whether these additional cancers occurred before or after the diagnosis of GIST. Finally, we estimated the survival of patients with GIST with none, one, or multiple other primary tumors.
materials and methods
patients
This study included 783 individuals who were confirmed to have the diagnosis of GIST at MDACC from 1995 to 2007. After institutional review board approval, we used medical records and tumor registry information to collect data on these patients. Synchronous cancers were defined as two or more tumors present or detected at the same time as the GIST. Whereas metachronous cancers were defined as tumors detected within a time interval of 1 year of GIST diagnosis and not considered to be metastases, invasion, or recurrence. All intra-abdominal or gastrointestinal tumors were reviewed to confirm that these were not metastatic GIST. Non-melanoma skin cancers such as basal and squamous cell carcinoma were not considered as a primary tumor as these are highly prevalent in this patient population and rarely influence survival.
The patients were divided into four groups as per Duchateau and Stokkel [13]: group I (patients with GIST diagnosed before another malignancy), group II (patients with GIST diagnosed after another malignancy), group III (GIST as the only malignancy), and group IV (patients diagnosed with GIST and more than two other primary tumors).
statistical analysis
Patient characteristics between the groups were compared by chi-square test. Survival curves and rates were calculated by the Kaplan–Meier method [14]. Statistical analysis was carried out with STATA version 10 (STATA Corp., College Station, TX).
results
patient characteristics
Among the 783 diagnosed with GIST between 1995 and 2007, 159 (20.3%) patients were found to have one or more second malignancies diagnosed before or after their GIST. Since the date of diagnosis of the other primary cancer was not known in five patients with GIST having another primary, they were not included in the study. As a result, there were 154 patients with 186 additional malignancies. Table 1 describes the overall characteristics of patients with GIST. The age at diagnosis of GIST ranged from 17 to 91 years, with a mean and a median age at diagnosis of 56 and 57 years, respectively. Approximately 57% of the patients were male, with a male to female ratio of 1 : 3. The majority of the patients were Caucasian. About 67% of the patients received imatinib, whereas 33% did not receive imatinib or any KIT inhibitor since they were not living at the time these therapies became available. The stomach (50%) was the predominant primary site of occurrence of GIST. The tumor size ranged from 0.1 to 35 cm, with a median size of 7.5 cm. Approximately equal number of patients were distributed in the three size categories (<0.5, 5–10, and >10 cm).
Table 1.
Overall patient characteristics
| Characteristics | n (%) |
| Patients | 783 (100) |
| Median time from diagnosis with GIST (months) | 57 |
| Gender | |
| Men | 444 (57) |
| Women | 339 (43) |
| Age at diagnosis of GIST (years) | |
| Mean | 56.7 |
| Range | 17–91 |
| Race | |
| White | 603 (77) |
| Black | 74 (9) |
| Hispanic | 73 (9) |
| Others | 33 (5) |
| Treated with imatinib | |
| Yes | 443 (57) |
| No | 340 (43) |
| Vital status | |
| Alive | 524 (67) |
| Dead | 259 (33) |
| GIST site | |
| Stomach | 386 (49) |
| Small intestine | 273 (35) |
| Retroperitoneal space | 56 (7) |
| Colorectal | 50 (6) |
| Omentum/mesentery | 18 (2) |
| GIST size (cm) | |
| <5 | 200 (26) |
| 5–10 | 206 (26) |
| >10 | 196 (25) |
| No data | 181 (23) |
GIST, gastrointestinal stromal tumor.
additional primary malignancies in patients with GIST
A second primary tumor developed after the diagnosis of GIST in 32 patients (4.1%; group I), 97 patients (12.5%) had a previous malignancy (group II) in their history, and 629 patients (80.3%) had GIST as their only malignancy (group III). In 24 patients (3.1%), two or more additional cancers were found in addition to GIST (group IV); 10 patients had a history of two additional malignancies before GIST, whereas 5 patients were found to have two new cancers after a diagnosis with GIST; 5 patients had one primary before and one primary after diagnosis with GIST; and 1 patient was diagnosed with two primaries before GIST and one other primary developed after a finding of GIST. Surprisingly, one patient had a history of three primaries before a diagnosis of GIST and subsequently developed four other primaries after the diagnosis of GIST.
characteristics of patients with an additional primary malignancy
The patient characteristics of each group are given in Table 2. There were more men than women in each group. Mean age at diagnosis of GIST was almost the same in groups I and II. Patients tended to be younger in group III (54) and older in group IV (67). The large majority of patients were Caucasians in each group. The stomach was the most frequent site of occurrence of GIST in all four groups, followed by small intestine. There was no significant difference in the GIST primary site among the groups. However, significant difference was observed for tumor size categories (<5, 5–10, and >10 cm) among the groups (P = 0.001).
Table 2.
Patients’ characteristics among the groups studied
| Characteristics | Group I | Group II | Group III | Group IV |
| Patients (all), n (%) | 32 (4.1) | 97 (12.4) | 630 (80.5) | 24 (3.1) |
| Average interval from GIST diagnosis (years) | 4.7 | 1.4 | – | 7.0 |
| Gender, n (%) | ||||
| Men | 21 (65.6) | 55 (56.7) | 353 (56.0) | 15 (62.5) |
| Women | 11 (34.40) | 42 (43.3) | 277 (44.0) | 9 (37.5) |
| Age at diagnosis of GIST (years) | ||||
| Mean | 62.5 | 62.2 | 54.1 | 67.4 |
| Range | 39–82 | 17–91 | 25–86 | 48–85 |
| Race, n (%) | ||||
| White | 26 (81.3) | 74 (76.30) | 481 (76.4) | 22 (91.7) |
| Black | 1 (3.1) | 8 (8.3) | 64 (10.2) | 1 (4.2) |
| Hispanic | 3 (9.4) | 12 (12.4) | 57 (9.1) | 1 (4.2) |
| Others | 2 (6.3) | 3 (3.1) | 28 (4.4) | – |
| Treated with imatinib, n (%) | ||||
| Yes | 16 (50.0) | 29 (29.9) | 288 (45.7) | 7 (29.2) |
| No | 16 (50.0) | 68 (70.1) | 342 (54.3) | 17 (70.8) |
| Vital status, n (%) | ||||
| Alive | 23 (71.9) | 64 (66.0) | 423 (67.1) | 14 (58.3) |
| Dead | 9 (28.1) | 33 (34.0) | 207 (32.90 | 10 (41.7) |
| GIST site, n (%) | ||||
| Stomach | 18 (56.3) | 50 (51.6) | 305 (48.4) | 13 (54.2) |
| Small intestine | 10 (31.3) | 31 (32.0) | 224 (35.6) | 8 (33.3) |
| Retroperitoneal space | 1 (3.1) | 11 (11.3) | 42 (6.7) | 2 (8.3) |
| Colorectal | 3 (9.4) | 5 (5.1) | 41 (6.5) | 1 (4.2) |
| Omentum/mesentery | – | – | 18 (2.9) | – |
| GIST size in cm, n (%) | ||||
| <5 | 8 (25.0) | 48 (49.50 | 129 (20.5) | 15 (62.5) |
| 5–10 | 10 (31.3) | 15 (15.5) | 178 (28.3) | 3 (12.5) |
| >10 | 3 (9.4) | 15 (15.5) | 176 (28.0) | 2 (8.3) |
| Unknown | 11 (34.4) | 19 (19.6) | 147 (23.3) | 4 (16.7) |
| No. of other primaries | 32 | 97 | – | 56 |
| Synchronous | 19 | 48 | – | 30 |
| Metachronous | 13 | 49 | – | 26 |
GIST, gastrointestinal stromal tumor.
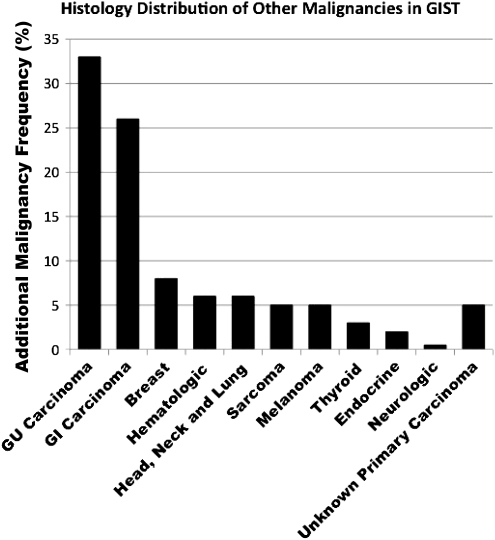
The number and the type of other primaries diagnosed before and after the diagnosis of GIST is shown in Table 3 and Figure 1. It was not too surprising to find that prostate (25) and breast cancer (12) were the most common other primaries noticed before the diagnosis of GIST, but cancers of the esophagus (9) and kidney (7) were also found. Interestingly, cancers of the kidney (5) and lung (5) as well as leukemia (4) were the highest among the other primaries that occurred in our patient population after or at the diagnosis of GIST. Thus, ∼20% of patients with GIST are at risk for additional malignancies. Biopsy of metastatic lesions, particularly if the lesion is atypical in appearance, should be considered as a potential second primary lesion.
Table 3.
Number and type of other primaries before and after GIST
| Cancer type | Before GIST (n) | After GIST (n) | Total, n (% of patients with additional malignancy) | |
| GU carcinoma | 50 | 12 | 62 (33) | |
| Prostate | 25 | 3 | ||
| Kidney | 7 | 5 | ||
| Uterus | 7 | |||
| Bladder | 5 | 1 | ||
| Ovary | 5 | 2 | ||
| Testis | 1 | |||
| Ureter | 1 | |||
| GI carcinoma | 39 | 9 | 48 (26) | |
| Colorectal | 13 | 5 | ||
| Esophagus | 9 | |||
| Pancreas | 5 | |||
| Appendix | 3 | 1 | ||
| Small intestine | 3 | 1 | ||
| Stomach | 3 | 2 | ||
| Liver | 2 | |||
| Gall bladder | 1 | |||
| Breast | Breast | 12 | 3 | 15 (8) |
| Hematologic | 6 | 6 | 12 (6) | |
| Leukemia | 4 | 4 | ||
| Lymphoma | 2 | 2 | ||
| Head, neck, and lung | 5 | 6 | 11 (6) | |
| Lung | 4 | 6 | ||
| Retinoblastoma | 1 | |||
| Sarcoma | 4 | 5 | 9 (5) | |
| Leiomyosarcoma | 2 | |||
| Sarcoma, unclassified | 1 | 3 | ||
| Sarcoma, epithelioid | 1 | |||
| Sarcoma, desmoid | 1 | |||
| Liposarcoma | 1 | |||
| Melanoma | Melanoma | 7 | 2 | 9 (5) |
| Thyroid | Thyroid | 3 | 3 | 6 (3) |
| Endocrine | 1 | 2 | 3 (2) | |
| Parotid salivary gland carcinoma | 1 | 1 | ||
| Adrenal | 1 | |||
| Neurological | – | 1 | 1 | |
| Cerebral hemisphere | 1 | |||
| Unknown primary carcinoma | Unknown | 7 | 3 | 10 (5) |
| Total | 134 | 52 | 186 |
GI, gastrointestinal; GIST, gastrointestinal stromal tumor; GU, genitourinary.
Figure 1.
Histological subtype of additional malignancies in patients with GIST. Percentages represent the proportion of patients with additional malignancies. GI, gastrointestinal; GIST, gastrointestinal stromal tumor; GU, genitourinary.
survival of patients with GIST and an additional primary malignancy
We next sought to gain an understanding of the clinical outcomes of patients with GIST who also had an additional primary cancer. We divided our GIST patients into the four groups proposed by Duchateau and Stokkel [13]: group I (patients with GIST diagnosed before another malignancy), group II (patients with GIST diagnosed after another malignancy), group III (GIST as the only malignancy), and group IV (patients diagnosed with GIST and more than two other primary tumors).
The 1-, 5-, and 10-year survival rates for group I (patients with GIST as the first malignancy and another in the follow-up period) were 0.86 [95% confidence interval (CI) 0.68–0.94], 0.68 (95% CI 0.48–0.82), and 0.68 (95% CI 0.48–0.82). Patients with a first primary and a subsequent GIST (group II) had 1-, 5-, and 10-year survival as 0.85 (95% CI 0.80–0.94), 0.61 (95% CI 0.47–0.71), and 0.23 (95% CI 0.05–0.48). The 1-, 5-, and 10-year survival rates for group III (patients with GIST without any other primary) were 0.94 (95% CI 0.92–0.96), 0.58 (95% CI 0.53–0.63), and 0.45 (95% CI 0.39–0.52). Patients with more than two primaries (group IV) had their 1-, 5-, and 10-year survival as 0.87 (95% CI 0.65–0.96), 0.49 (95% CI 0.20–0.73), and 0.49 (95% CI 0.20–0.73). It was only those patients with GIST and two or more other cancers who had an inferior overall survival compared with those with GIST alone or GIST plus one other cancer (P = 0.007, Figure 2). Additionally, we found no difference in the overall survival whether patients had their GIST diagnosed before or after the other malignancy. Thus, diagnosis of a second malignancy in a patient with GIST does not appear to alter patient survival and should not preclude appropriate therapy for both cancers.
Figure 2.
Survival probability by additional malignancy grouping. Patients with GIST alone appeared to have a trend toward the best overall survival (group III). Patients with GIST and two or more cancers were found to have a statistically significant inferior overall survival (P = 0.007). Patients with GIST as the first malignancy and a subsequent tumor (group I) and patients with another malignancy in their history (group II) had an intermediate survival compared with groups III and IV. GIST, gastrointestinal stromal tumor.
discussion
Although rare, GIST is the most common mesenchymal tumor of the gastrointestinal tract [15]. The recent advent of imatinib has lead to dramatic improvements in long-term survival of patients with GIST [16]. With this increase in survival time, it is not surprising that these patients may have an increased time to develop additional malignant tumors.
In this article, we have used one of the largest single institution-based tumor registries to identify additional cancers in patients with GIST. Surprisingly, ∼20% of the 783 patients with GIST were identified as having at least one additional malignant tumor, a number much higher than that expected in the general population. This figure is higher than the one reported by Wronski et al. [17] (14%) and Kalender et al. [18] (16%), and this is possibly due to the longer follow-up time in our study, referral bias of a tertiary referral center, or simply a different patient population. In the present study, genitourinary (62; 8% of all patients with GIST) and gastrointestinal (48; 6% of all patients with GIST) carcinomas were the most common additional cancers. However, other studies have reported that the most common GIST-associated malignancies were gastrointestinal carcinomas (47% of all patients with GIST), prostate cancer (9%), lymphoma/leukemia (7%), and breast cancer (7%) and kidney (n = 27; 6%), lung (n = 26; 5%), female genital tract (n = 25; 5%), and carcinoid tumors (n = 13; 3%) [9]. Our clinical experience is that it is unlikely that nearly half of all patients with GIST also have a gastrointestinal carcinoma (JCT, personal communication). In a different study, Miettinen et al. [11] demonstrated a nonrandom association between GIST and AML. The reason for the high percentage of other malignancies among patients with GIST is unlikely related to imatinib therapy since secondary tumors are observed before imatinib exposure. It is possible that an underlying genetic instability or mismatch repair may lead to mutation in KIT resulting in GIST but also other oncogenes that result in other cancers. This mechanism of cancer predisposition has not been sufficiently evaluated and is currently under investigation.
The primary objective of our study was to determine the patient demographics and survival of patients with GIST and additional malignancies. Our analysis of patients diagnosed with GIST over a period of 12 years resulted in the identification of a spectrum of second malignancies that occur in patients before and after their diagnosis with GIST. Thus, surveillance for not only recurrent GIST but also of second malignancies is an important component of the management of patients with GIST. Importantly, the occurrence of two or more other cancers in a patient with GIST appears to be a negative prognostic factor. This may be improved with earlier diagnosis and appropriate treatment of these other cancers.
Although our study was not a case–control design, the occurrence of other malignancies in patients with GIST appears to be more common than what would be expected. Longer follow-up, larger studies, and perhaps a case–control design may help distinguish whether there is a common predisposition syndrome between GIST and other malignancies.
funding
Institutional Physician-Scientist award to JCT; National Institutes of Health/National Caner Institute grant (1K23CA109060-05) to JCT; Amschwand Sarcoma Cancer Foundation to JCT.
References
- 1.Chou FF, Eng HL, Sheen-Chen SM. Smooth muscle tumors of the gastrointestinal tract: analysis of prognostic factors. Surgery. 1996;119:171–177. doi: 10.1016/s0039-6060(96)80165-6. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JJ, Brennan MF. Soft tissue sarcomas. Curr Probl Surg. 1996;33:817–872. [PubMed] [Google Scholar]
- 3.Demetri GD. Targeting the molecular pathophysiology of gastrointestinal stromal tumors with imatinib. Mechanisms, successes, and challenges to rational drug development. Hematol Oncol Clin North Am. 2002;16:1115–1124. doi: 10.1016/s0889-8588(02)00052-7. [DOI] [PubMed] [Google Scholar]
- 4.Trent JC, Benjamin RS. New developments in gastrointestinal stromal tumor. Curr Opin Oncol. 2006;18:386–395. doi: 10.1097/01.cco.0000228747.02660.e2. [DOI] [PubMed] [Google Scholar]
- 5.Gupta P, Tewari M, Shukla HS. Gastrointestinal stromal tumor. Surg Oncol. 2008;17:129–138. doi: 10.1016/j.suronc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Fuller CE, Williams GT. Gastrointestinal manifestations of type 1 neurofibromatosis (von Recklinghausen’s disease) Histopathology. 1991;19:1–11. doi: 10.1111/j.1365-2559.1991.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 7.Carney JA. The triad of gastric epithelioid leiomyosarcoma, pulmonary chondroma, and functioning extra-adrenal paraganglioma: a five-year review. Medicine (Baltimore) 1983;62:159–169. doi: 10.1097/00005792-198305000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Carney JA, Sheps SG, Go VL, Gordon H. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med. 1977;296:1517–1518. doi: 10.1056/NEJM197706302962609. [DOI] [PubMed] [Google Scholar]
- 9.Agaimy A, Wunsch PH, Sobin LH, et al. Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol. 2006;23:120–129. doi: 10.1053/j.semdp.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Au WY, Ho KM, Shek TW. Papillary renal cell carcinoma and gastrointestinal stromal tumor: a unique association. Ann Oncol. 2004;15:843–844. doi: 10.1093/annonc/mdh191. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M, Kraszewska E, Sobin LH, Lasota J. A nonrandom association between gastrointestinal stromal tumors and myeloid leukemia. Cancer. 2008;112:645–649. doi: 10.1002/cncr.23216. [DOI] [PubMed] [Google Scholar]
- 12.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 13.Duchateau CS, Stokkel MP. Second primary tumors involving non-small cell lung cancer: prevalence and its influence on survival. Chest. 2005;127:1152–1158. doi: 10.1378/chest.127.4.1152. [DOI] [PubMed] [Google Scholar]
- 14.Meier K. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 16.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 17.Wronski M, Ziarkiewicz-Wroblewska B, Gornicka B, et al. Synchronous occurrence of gastrointestinal stromal tumors and other primary gastrointestinal neoplasms. World J Gastroenterol. 2006;12:5360–5362. doi: 10.3748/wjg.v12.i33.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalender ME, Sevinc A, Kucukdurmaz Z, et al. Gastric and prostate adenocarcinoma in a patient with metastatic gastrointestinal stromal tumor. Onkologie. 2007;30:568–570. doi: 10.1159/000108640. [DOI] [PubMed] [Google Scholar]