Abstract
Although calpain (calcium-activated cysteine protease) inhibition represents a rational therapeutic target for spinal cord injury (SCI), few studies have reported improved functional outcomes with post-injury administration of calpain inhibitors. This reflects the weak potency and limited aqueous solubility of current calpain inhibitors. Previously, we demonstrated that intraspinal microinjection of the calpain inhibitor MDL28170 resulted in greater inhibition of calpain activity as compared to systemic administration of the same compound. In the present study, we evaluated the ability of intraspinal MDL28170 microinjection to spare spinal tissue and locomotor dysfunction following SCI. Contusion SCI was produced in female Long-Evans rats using the Infinite Horizon impactor at the 200-kdyn force setting. Open-field locomotion was evaluated until 6 weeks post-injury. Histological assessment of tissue sparing was performed at 6 weeks after SCI. The results demonstrate that MDL28170, administered with a single post-injury intraspinal microinjection (50 nmoles), significantly improves both locomotor function and pathological outcome measures following SCI.
Key words: locomotion, neurodegeneration, neuroprotection, white matter sparing
Introduction
Following spinal cord injury (SCI), calcium-activated cysteine proteases (calpains) are activated within a few minutes following injury, with maximal activity occurring 1–4 h post-injury (Banik et al., 1984, 1986; Ray et al., 1999; Schumacher et al., 1999; Springer et al., 1997; Zhang et al., 2000). Calpain activation is implicated in the death of motor neurons (Arataki et al., 2005; Das et al., 2005; Momeni and Kanje, 2005), axonal degeneration (O'Hanlon et al., 2003; Schumacher et al., 2000), oligodendrocyte loss, demyelination (James et al., 1998; Ray et al., 2002; Schaecher et al., 2001; Shields et al., 2000), and several aspects of the inflammatory responses (Cuzzocrea et al., 2000; Pianetti et al., 2001; Rock et al., 2000; Schaub et al., 2003; Stewart et al., 1998; Watanabe and Kobayashi, 1994). The putative roles of calpain in the secondary degeneration following SCI has led to investigations examining the effect of calpain inhibition following SCI (Ray et al., 2003).
In vitro studies clearly demonstrate that calpain inhibitors, when provided at a sufficient concentration, protect cultured neurons against a variety of insults relevant to SCI, including excitotoxicity, metabolic impairment, and oxidative stress (Bano et al., 2005; Brorson et al., 1995; Kieran and Greensmith, 2004; Moore et al., 2002). In contrast, pre- and post-injury administration of calpain inhibitors has resulted in modest reductions in tissue damage, cytoskeletal proteolysis, and locomotor impairment in several models of SCI (Arataki et al., 2005; Hung et al., 2005; Ray et al., 2003; Schumacher et al., 2000; Yu and Geddes, 2007). This is thought to reflect the poor selectivity, weak potency, limited aqueous solubility, and rapid metabolism of many current calpain inhibitors (Wang, 1990; Wang and Yuen, 1997; Zhang et al., 2003). Most previous studies have administered a single bolus of a calpain inhibitor via intravenous infusion, either shortly before or following experimental spinal cord injury. With this approach, it is difficult to achieve sufficient levels of the drug in central nervous system (CNS) tissue to effectively inhibit calpain activity (Kupina et al., 2001; Saatman et al., 2000; Zhang et al., 2003).
An alternate route of drug administration is direct tissue injection, which produces high local drug concentrations. Intraspinal injection of the calpain inhibitor CEP-4143 prior to compression injury resulted in significant improvements in locomotor function and tissue preservation (Schumacher et al., 2000). However, post-injury administration was not evaluated, and this inhibitor is no longer available. Previously, we demonstrated that post-injury intraspinal microinjection of calpain inhibitor MDL28170 inhibited calpain activity and reduced the injury-induced proteolysis of α-spectrin and MAP2 to a greater extent than did intravenous administration of the same compound, but did not examine functional outcomes or tissue preservation (Zhang et al., 2003). In the present study, we evaluated the ability of post-injury intraspinal microinjection of MDL28170 to spare spinal tissue, and improve locomotor function following contusive SCI.
Methods
Animals
Adult female Long-Evans rats, 200–250 g, were purchased from Charles River (Indianapolis, IN). Animals were kept under standard housing conditions for at least 1 week following arrival and then randomly assigned to two groups: (1) SCI plus MDL28170 and (2) SCI plus Vehicle. All experimental procedures were approved and carried out in accordance with the Guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Kentucky.
Spinal Cord Contusion Injury
Animals were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.).
SCI was performed using a commercially available Infinite Horizon (IH) SCI device (Precision Systems & Instrumentation, Lexington, KY) as described previously (Scheff et al., 2003; Yu and Geddes, 2007). Briefly, a laminectomy was performed to expose spinal cord segment T10. The exposed vertebral column was stabilized by clamping the rostral T9 and caudal T11 vertebral bodies with two spinal forceps. The SCI was then applied with the IH device using a 200 kilodynes (kdyn) force setting, resulting in severe contusion injury similar to that produced by a 25-mm weight drop using the NYU device (Scheff et al., 2003). Impact analysis, including actual force applied to spinal cord, displacement of spinal cord, and velocity, was recorded (Table 1). The impact tip was automatically retracted, the wound was irrigated with saline, and the muscle and skin openings were closed with sutures. Postoperative care included the manual expression of bladders twice daily until recovery of bladder function, injection with 10 mL of sterile saline s.c. immediately after surgery, 33.3 mg/kg cefazolin i.m. twice a day for 1 week, and 0.02 mg/kg buprenorphine, s.c. for 2 days post-injury.
Table 1.
Injury Parameters
| Groups | Actual force (kdyn) | Displacement (microns) | Velocity (mm/sec) |
|---|---|---|---|
| MDL28170 | 218 ± 10 | 1578 ± 49 | 124 ± 1 |
| Vehicle | 225 ± 9 | 1520 ± 80 | 121 ± 2 |
Values are mean ± SEM. No significant differences in impact force, displacement, and velocity were found between MDL28170 and vehicle-treated groups.
Drug Administration and Experimental Groups
The rats were randomly assigned to the following treatment groups: MDL28170 intraspinal administration (50 nmoles of MDL28170) or vehicle intraspinal administration. The 50-nmole dose is based on a previous study in which this drug concentration was evaluated with respect to calpain activity and proteolysis of calpain substrates including spectrin and MAP2 (Zhang et al., 2003). MDL28170 (Calbiochem, La Jolla, CA) was dissolved in dimethyl sulfoxide (DMSO) as a 1000× stock solution and diluted in sterile saline to a final concentration of 50 mM. Intraspinal microinjection was performed using a beveled glass micropipette (60-μm tip diameter) connected to a nano-injector (Stoelting Co.). At 30 min post-injury, 0.5 μL of 50 mM MDL28170 or vehicle (DMSO diluted in saline) was bilaterally injected into the lesion site at 0.5 mm lateral to the midline and 1.0 mm depth as described previously (Zhang et al., 2003).
Behavioral Assessment
Open-field locomotor function was evaluated using the Basso-Beattie-Breshnahan (BBB) locomotor rating scale (Basso et al., 1996) as described previously (Yu and Geddes, 2007; Yu et al., 2000). Briefly, non-injured rats were exposed daily for 1 week to the behavioral testing environment in order to acclimate them to open field exploration. Two examiners, blinded to experimental treatment, participated in the BBB evaluation. Postoperative open field testing was performed at 3, 7, 14, 21, 28, 35, and 42 days post-injury. The BBB test is widely used to quantify locomotor abilities of rats following experimental SCI (Majczynski et al., 2007). The 6-week survival period allows adequate time for post-injury improvement in locomotor behavior, which can continue until 5 weeks post-injury (Basso et al., 1996).
Spinal Cord Tissue Processing
Following the final assessment of locomotor function 42 days post-injury, the animals were euthanatized with pentobarbital (100 mg/kg) and perfused transcardially with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Spinal cord blocks of 2 cm in length, centered at the lesion epicenter, were immediately dissected, post-fixed in the same fixative solution for 2 h at 4°C, cryoprotected in 30% sucrose/phosphate-buffered saline (PBS), and stored at 4°C. Spinal cords were serially cryosectioned at a thickness of 20 μm. Every fifth section was mounted onto gelatin-coated slides and stored at −20°C.
Eriochrome Cyanine Staining
A modified eriochrome cyanine (EC) staining protocol for myelin that differentiates both white matter and cell bodies was used to visualize spared tissue (Rabchevsky et al., 2001; Yu and Geddes, 2007). Area measurements of gray matter, white matter, spared tissue, and total lesion volume in transverse sections of the injured cords were obtained. Spared tissue was calculated from 15 evenly spaced sections based on the area of necrotic tissue divided by the total cross-sectional area. The total lesion volume was calculated by multiplying the lesion area in each section by the distance between sections.
Statistical Analysis
All data are expressed as the mean ± SEM and analyzed using analysis of variance (ANOVA). Where appropriate, group differences were compared using Fisher's LSD post hoc test. Significance was set at p < 0.05.
Results
Locomotor Function
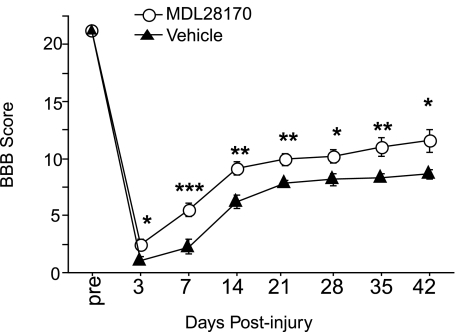
No significant differences in impact force, displacement, and velocity were found between MDL28170 and vehicle-treated groups (Table 1). Immediately following SCI (200 kdyn), all animals exhibited complete bilateral hindlimb paralysis. By 3 days post-injury, intraspinal administration of MDL28170 significantly improved hindlimb locomotor function in comparison to vehicle administration (Fig. 1). The improved locomotor function in the MDL28170-treated animals persisted for 6 weeks post-injury. At 2 weeks post-injury, most MDL28170-treated animals exhibited weight-supported hindlimb movements, whereas vehicle-treated animals exhibited limited joint movements. At the 6-week time period, the MDL28170-treated animals exhibited consistent weight-supported plantar steps with occasional or frequent coordination while vehicle-treated animals exhibited limited hindlimb weight bearing or occasional dorsal stepping.
FIG. 1.
Post-injury intraspinal administration of MDL28170 results in improved locomotor performance as compared to vehicle-treated animals. Contusive spinal cord injury was produced using the Infinite Horizon impactor, 200-kdyn setting, at T10. MDL28170 was administered i.s. (50 nmoles, 30 min post-injury, n = 11, open circles). Vehicle-treated animals (n = 10, filled triangles) received i.s. administration of DMSO/saline without MDL28170. The MDL28170 administration resulted in improved open-field locomotor function, assessed using the Basso, Beattie, and Bresnahan (BBB) test (Basso et al., 1996). Data are represented as mean ± SEM, and were analyzed with analysis of variance (ANOVA) and Fisher's LSD post hoc test, *p < 0.05, **p < 0.01, and ***p < 0.001, for MDL28170 treatment as compared to Vehicle.
Tissue Sparing and Lesion Volume
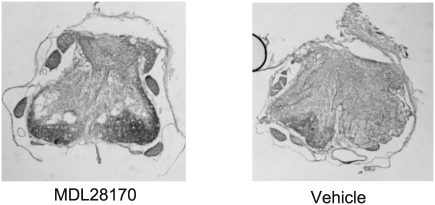
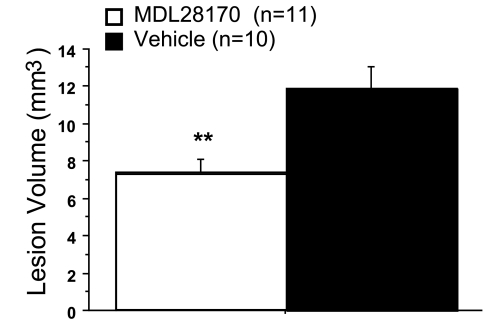
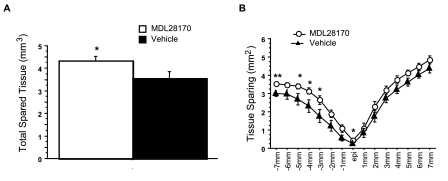
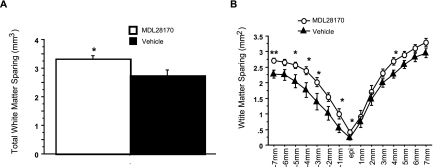
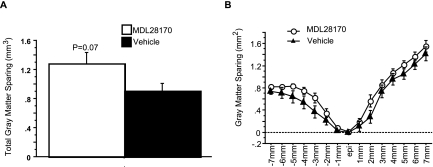
Representative EC-stained transverse spinal cord sections at lesion epicenter after T10 contusion injury and treatment are presented in Figure 2. Damage was most severe at the injury epicenter, and extended several millimeters in both the rostral and caudal directions. Intraspinal MDL28170 treatment significantly reduced lesion volume 6 weeks after SCI, compared to vehicle treatment (Fig. 3). Similar to the lesion volume, an increase in total tissue sparing was significant in the MDL28170 treatment group (Fig. 4). This group exhibited tissue sparing at 3, 4, 5, and 7 mm rostral to the lesion, and also at the lesion epicenter. Similar to the total tissue sparing, intraspinal MDL28170 resulted in improved white matter sparing, primarily rostral to the injury (Fig. 5). The difference in total gray matter sparing between the MDL28170- and vehicle-treated groups did not reach statistical significance (p = 0.07; Fig. 6).
FIG. 2.
Photomicrographs of representative transverse spinal cord sections 42 days following severe contusion spinal cord injury (SCI) at the lesion epicenter. The sections were stained with eriochrome cyanine for myelin. The treatment conditions are as described in Figure 1.
FIG. 3.
Intraspinal administration of MDL28170 resulted in a significant reduction in lesion volume following contusion injury to the spinal cord. Lesion volume was measured at 42 days post-injury, in the same animals in which locomotor performance was evaluated in Figure 1. Data are the mean ± SEM. **p < 0.01.
FIG. 4.
Intraspinal administration of MDL28170 resulted in a significant increase in tissue sparing following contusion injury to the spinal cord. Injury conditions and treatment groups are as described in Figure 1. (A) The total volume of spared tissue is illustrated. (B) The average area of tissue sparing is indicated at 1-mm intervals rostral and caudal to the injury epicenter for animals receiving MDL 28170 i.s. (open circles) or vehicle (filled triangles). *p < 0.05, **p < 0.01.
FIG. 5.
Intraspinal administration of MDL28170 resulted in a significant increase in white matter tissue sparing following contusion injury to the spinal cord. Injury conditions and treatment groups are as described in Figure 1. (A) The total volume of spared white matter is illustrated. (B) The average area of white matter tissue sparing is indicated at 1-mm intervals rostral and caudal to the injury epicenter for animals receiving MDL 28170 i.s. (open circles) or vehicle (filled triangles). *p < 0.05, **p < 0.01.
FIG. 6.
Intraspinal administration of MDL28170 resulted in an insignificant trend for increased total gray matter sparing following contusion injury to the spinal cord. Injury conditions and treatment groups are as described in Figure 1. (A) The total volume of spared gray matter is illustrated. (B) The average area of gray matter tissue sparing is indicated at 1-mm intervals rostral and caudal to the injury epicenter for animals receiving MDL 28170 i.s. (open circles) or vehicle (filled triangles).
Discussion
The results of the present study demonstrate that MDL28170, administered by intraspinal microinjection shortly following injury, results in increased spinal tissue sparing and improved functional recovery following contusive SCI in rats. We previously demonstrated that intraspinal microinjection of MDL28170 was more effective than intravenous infusion in reducing calpain activity and decreasing the injury-induced proteolysis of calpain substrates (Zhang et al., 2003). These findings are similar to those obtained with pre-injury intraspinal administration of calpain inhibitor CEP-4143, which resulted in improved neurological function, increased tissue preservation, and reduced proteolysis of calpain substrates following compression injury (Schumacher et al., 2000). Direct intraspinal administration provides the opportunity to achieve a greater local tissue concentration, and minimizes the rapid hepatic metabolism, of the inhibitor.
Together the above findings support the hypothesis that calpain inhibitors, when delivered to the spinal cord at a sufficient level, attenuate the secondary damage and reduce functional deficits following SCI.
The post-injury administration of MDL28170 was at a single time point, namely 30 min post-injury. Our previous study found that calpain was inhibited up to 24 h following intrastpinal microinjection, although the magnitude of the inhibiton decreased with time (Zhang et al., 2003). Following SCI, the elevation in calpain activity persists for at least 48–72 h (Banik et al., 1997; Springer et al., 1997; Zhang et al., 2000). We recently found that a single i.v. bolus of MDL28170, administered post-injury, combined with daily i.p. injections of MDL28170 resulted in greater tissue sparing than either treatment alone (Yu and Geddes, 2007). Hung et al. (2005) observed improved neurologic function and reduced neuron loss following spinal cord hemisection in animals that received MDL28170 via continuous intrathecal administration infusion for 3 days post-injury. With sustained delivery of the calpain inhibitor, it is therefore possible that tissue sparing and locomotor function may be further improved.
The increase in tissue sparing observed with intraspinal MDL28170 was predominantly in the white matter. Locomotor deficits in the thoracic SCI model used are largely due to loss of descending axon tracts in the white matter (Blight, 1983; Magnuson et al., 1999; Teng and Wrathall, 1997). The improvements in locomotor function are therefore thought to reflect the preservation of descending axons from cortical motor neurons. The lack of significant gray matter sparing (p = 0.07) reflects, at least in part, the greater variability in gray matter volume, as compared to white matter volume, in both vehicle- and MDL28170-treated animals. The drug injection site was designed to be at the border between white and gray matter. However, it is possible that much of the injected drug was distributed in white matter instead of gray matter. Previously, we did not observe improved tissue sparing following a single post-injury i.v. administration of MDL28170 (20 mg/kg), or with i.p. MDL1870 injections, 1 mg/kg, for 7 days post-injury, using the same injury model as in the present study (Yu and Geddes, 2007). However, combined i.v. and i.p. administration of MDL28170 resulted in sparing of both gray and white matter (Yu and Geddes, 2007). There are numerous caveats associated with comparing results obtained with different drug doses, routes of administration, and separate studies. However, the tissue sparing achieved following a single post-injury intraspinal administration of MDL28170 in the present study was greater than observed previously with a single drug bolus administered post-injury via intravenous infusion (Yu and Geddes, 2007).
The tissue sparing achieved with intraspinal MDL28170 was primarily rostral to the injury. In contrast, tissue sparing following MDL28170 administration via combined i.v. and i.p. routes was largely caudal to the injury (Yu and Geddes, 2007). The reason for this discrepancy is uncertain as the drug was injected into the injury epicenter in the present study.
The results support the overall hypothesis that calpain activation contributes to the secondary damage following SCI, and that calpain inhibition represents a viable therapeutic strategy. Intraspinal microinjection results in a greater local tissue concentration of the calpain inhibitor than can be achieved with systemic routes of administration. However, intraspinal microinjection of calpain inhibitors is unlikely to be applied in the clinical setting due to the potential for further injury resulting from the injection and the delay before such surgery could be performed. With improvements in the specificity, cell-permeability, and potency of calpain inhibitors (Cuerrier et al., 2007; Neffe and Abell, 2005; Sanders and Donkor, 2006), it is hoped that a compound will become available which could be administered systemically and provide robust and sustained calpain inhibition.
Acknowledgments
This research was supported by the National Institutes of Health (grant RO1 NS 045726) and the Kentucky Spinal Cord and Head Injury Research Trust.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Arataki S. Tomizawa K. Moriwaki A. Nishida K. Matsushita M. Ozaki T. Kunisada T. Yoshida A. Inoue H. Matsui H. Calpain inhibitors prevent neuronal cell death and ameliorate motor disturbances after compression-induced spinal cord injury in rats. J. Neurotrauma. 2005;22:398–406. doi: 10.1089/neu.2005.22.398. [DOI] [PubMed] [Google Scholar]
- Banik N.L. Hogan E.L. Mcalhaney W.W. A role for calcium and activation of neutral proteinase in myelinolysis? Prog. Clin. Biol. Res. 1984;146:145–152. [PubMed] [Google Scholar]
- Banik N.L. Hogan E.L. Powers J.M. Smith K.P. Proteolytic enzymes in experimental spinal cord injury. J. Neurol. Sci. 1986;73:245–256. doi: 10.1016/0022-510x(86)90149-8. [DOI] [PubMed] [Google Scholar]
- Banik N.L. Matzelle D.C. Gantt-Wilford G. Osborne A. Hogan E.L. Increased calpain content and progressive degradation of neurofilament protein in spinal cord injury. Brain Res. 1997;752:301–306. doi: 10.1016/s0006-8993(96)01488-6. [DOI] [PubMed] [Google Scholar]
- Bano D. Young K.W. Guerin C.J. Lefeuvre R. Rothwell N.J. Naldini L. Rizzuto R. Carafoli E. Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Blight A.R. Cellular morphology of chronic spinal cord injury in the cat: analysis of myelinated axons by line-sampling. Neuroscience. 1983;10:521–543. doi: 10.1016/0306-4522(83)90150-1. [DOI] [PubMed] [Google Scholar]
- Brorson J.R. Marcuccilli C.J. Miller R.J. Delayed antagonism of calpain reduces excitotoxicity in cultured neurons. Stroke. 1995;26:1259–1267. doi: 10.1161/01.str.26.7.1259. [DOI] [PubMed] [Google Scholar]
- Cuerrier D. Moldoveanu T. Campbell R.L. Kelly J. Yoruk B. Verhelst S.H. Greenbaum D. Bogyo M. Davies P.L. Development of calpain-specific inactivators by screening of positional scanning epoxide libraries. J. Biol. Chem. 2007;282:9600–9611. doi: 10.1074/jbc.M610372200. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S. Mcdonald M.C. Mazzon E. Siriwardena D. Serraino I. Dugo L. Britti D. Mazzullo G. Caputi A.P. Thiemermann C. Calpain inhibitor I reduces the development of acute and chronic inflammation. Am. J. Pathol. 2000;157:2065–2079. doi: 10.1016/S0002-9440(10)64845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. Sribnick E.A. Wingrave J.M. Del Re A.M. Woodward J.J. Appel S.H. Banik N.L. Ray S.K. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J. Neurosci. Res. 2005;81:551–562. doi: 10.1002/jnr.20581. [DOI] [PubMed] [Google Scholar]
- Hung K.S. Hwang S.L. Liang C.L. Chen Y.J. Lee T.H. Liu J.K. Howng S.L. Wang C.H. Calpain inhibitor inhibits p35-p25-Cdk5 activation, decreases tau hyperphosphorylation, and improves neurological function after spinal cord hemisection in rats. J. Neuropathol. Exp. Neurol. 2005;64:15–26. doi: 10.1093/jnen/64.1.15. [DOI] [PubMed] [Google Scholar]
- James T. Matzelle D. Bartus R. Hogan E.L. Banik N.L. New inhibitors of calpain prevent degradation of cytoskeletal and myelin proteins in spinal cord in vitro. J. Neurosci. Res. 1998;51:218–222. doi: 10.1002/(SICI)1097-4547(19980115)51:2<218::AID-JNR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Kieran D. Greensmith L. Inhibition of calpains, by treatment with leupeptin, improves motoneuron survival and muscle function in models of motoneuron degeneration. Neuroscience. 2004;125:427–439. doi: 10.1016/j.neuroscience.2004.01.046. [DOI] [PubMed] [Google Scholar]
- Kupina N.C. Nath R. Bernath E.E. Inoue J. Mitsuyoshi A. Yuen P.W. Wang K.K. Hall E.D. The novel calpain inhibitor SJA6017 improves functional outcome after delayed administration in a mouse model of diffuse brain injury. J. Neurotrauma. 2001;18:1229–1240. doi: 10.1089/089771501317095269. [DOI] [PubMed] [Google Scholar]
- Magnuson D.S. Trinder T.C. Zhang Y.P. Burke D. Morassutti D.J. Shields C.B. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp. Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- Majczynski H. Maleszak K. Gorska T. Slawinska U. Comparison of two methods for quantitative assessment of unrestrained locomotion in the rat. J. Neurosci. Methods. 2007;163:197–207. doi: 10.1016/j.jneumeth.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Momeni H.R. Kanje M. The calpain inhibitor VI prevents apoptosis of adult motor neurons. Neuroreport. 2005;16:1065–1068. doi: 10.1097/00001756-200507130-00007. [DOI] [PubMed] [Google Scholar]
- Moore J.D. Rothwell N.J. Gibson R.M. Involvement of caspases and calpains in cerebrocortical neuronal cell death is stimulus-dependent. Br. J. Pharmacol. 2002;135:1069–1077. doi: 10.1038/sj.bjp.0704538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neffe A.T. Abell A.D. Developments in the design and synthesis of calpain inhibitors. Curr. Opin. Drug Discov. Dev. 2005;8:684–700. [PubMed] [Google Scholar]
- O'hanlon G.M. Humphreys P.D. Goldman R.S. Halstead S.K. Bullens R.W. Plomp J.J. Ushkaryov Y. Willison H.J. Calpain inhibitors protect against axonal degeneration in a model of anti-ganglioside antibody-mediated motor nerve terminal injury. Brain. 2003;126:2497–2509. doi: 10.1093/brain/awg254. [DOI] [PubMed] [Google Scholar]
- Pianetti S. Arsura M. Romieu-Mourez R. Coffey R.J. Sonenshein G.E. Her-2/neu overexpression induces NF-kappaB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IkappaB-alpha that can be inhibited by the tumor suppressor PTEN. Oncogene. 2001;20:1287–1299. doi: 10.1038/sj.onc.1204257. [DOI] [PubMed] [Google Scholar]
- Rabchevsky A.G. Fugaccia I. Sullivan P.G. Scheff S.W. Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J. Neurotrauma. 2001;18:513–522. doi: 10.1089/089771501300227314. [DOI] [PubMed] [Google Scholar]
- Ray S.K. Hogan E.L. Banik N.L. Calpain in the pathophysiology of spinal cord injury: neuroprotection with calpain inhibitors. Brain Res. Brain Res. Rev. 2003;42:169–185. doi: 10.1016/s0165-0173(03)00152-8. [DOI] [PubMed] [Google Scholar]
- Ray S.K. Neuberger T.J. Deadwyler G. Wilford G. Devries G.H. Banik N.L. Calpain and calpastatin expression in primary oligodendrocyte culture: preferential localization of membrane calpain in cell processes. J. Neurosci. Res. 2002;70:561–569. doi: 10.1002/jnr.10414. [DOI] [PubMed] [Google Scholar]
- Ray S.K. Shields D.C. Saido T.C. Matzelle D.C. Wilford G.G. Hogan E.L. Banik N.L. Calpain activity and translational expression increased in spinal cord injury. Brain Res. 1999;816:375–380. doi: 10.1016/s0006-8993(98)01128-7. [DOI] [PubMed] [Google Scholar]
- Rock M.T. Dix A.R. Brooks W.H. Roszman T.L. Beta1 integrin-mediated T cell adhesion and cell spreading are regulated by calpain. Exp. Cell Res. 2000;261:260–270. doi: 10.1006/excr.2000.5048. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Zhang C. Bartus R.T. Mcintosh T.K. Behavioral efficacy of posttraumatic calpain inhibition is not accompanied by reduced spectrin proteolysis, cortical lesion, or apoptosis. J. Cereb. Blood Flow Metab. 2000;20:66–73. doi: 10.1097/00004647-200001000-00010. [DOI] [PubMed] [Google Scholar]
- Sanders M.L. Donkor I.O. A novel series of urea-based peptidomimetic calpain inhibitors. Bioorg. Med. Chem. Lett. 2006;16:1965–1968. doi: 10.1016/j.bmcl.2005.12.068. [DOI] [PubMed] [Google Scholar]
- Schaecher K.E. Shields D.C. Banik N.L. Mechanism of myelin breakdown in experimental demyelination: a putative role for calpain. Neurochem. Res. 2001;26:731–737. doi: 10.1023/a:1010903823668. [DOI] [PubMed] [Google Scholar]
- Schaub F.J. Liles W.C. Ferri N. Sayson K. Seifert R.A. Bowen-Pope D.F. Fas and Fas-associated death domain protein regulate monocyte chemoattractant protein-1 expression by human smooth muscle cells through caspase-and calpain-dependent release of interleukin-1 alpha. Circ. Res. 2003;93:515–522. doi: 10.1161/01.RES.0000093205.42313.7C. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Rabchevsky A.G. Fugaccia I. Main J.A. Lumpp J.E., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Schumacher P.A. Eubanks J.H. Fehlings M.G. Increased calpain I-mediated proteolysis, and preferential loss of dephosphorylated NF200, following traumatic spinal cord injury. Neuroscience. 1999;91:733–744. doi: 10.1016/s0306-4522(98)00552-1. [DOI] [PubMed] [Google Scholar]
- Schumacher P.A. Siman R.G. Fehlings M.G. Pretreatment with calpain inhibitor CEP-4143 inhibits calpain I activation and cytoskeletal degradation, improves neurological function, and enhances axonal survival after traumatic spinal cord injury. J. Neurochem. 2000;74:1646–1655. doi: 10.1046/j.1471-4159.2000.0741646.x. [DOI] [PubMed] [Google Scholar]
- Shields D.C. Schaecher K.E. Hogan E.L. Banik N.L. Calpain activity and expression increased in activated glial and inflammatory cells in penumbra of spinal cord injury lesion. J. Neurosci. Res. 2000;61:146–150. doi: 10.1002/1097-4547(20000715)61:2<146::AID-JNR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Springer J.E. Azbill R.D. Kennedy S.E. George J. Geddes J.W. Rapid calpain I activation and cytoskeletal protein degradation following traumatic spinal cord injury: attenuation with riluzole pretreatment. J. Neurochem. 1997;69:1592–1600. doi: 10.1046/j.1471-4159.1997.69041592.x. [DOI] [PubMed] [Google Scholar]
- Stewart M.P. Mcdowall A. Hogg N. LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J. Cell Biol. 1998;140:699–707. doi: 10.1083/jcb.140.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y.D. Wrathall J.R. Local blockade of sodium channels by tetrodotoxin ameliorates tissue loss and long-term functional deficits resulting from experimental spinal cord injury. J. Neurosci. 1997;17:4359–4366. doi: 10.1523/JNEUROSCI.17-11-04359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.K. Developing selective inhibitors of calpain. Trends Pharmacol. Sci. 1990;11:139–142. doi: 10.1016/0165-6147(90)90060-L. [DOI] [PubMed] [Google Scholar]
- Wang K.K. Yuen P.W. Development and therapeutic potential of calpain inhibitors. Adv. Pharmacol. 1997;37:117–152. doi: 10.1016/s1054-3589(08)60949-7. [DOI] [PubMed] [Google Scholar]
- Watanabe N. Kobayashi Y. Selective release of a processed form of interleukin 1 alpha. Cytokine. 1994;6:597–601. doi: 10.1016/1043-4666(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Yu C.G. Geddes J.W. Sustained calpain inhibition improves locomotor function and tissue sparing following contusive spinal cord injury. Neurochem. Res. 2007;32:2046–2053. doi: 10.1007/s11064-007-9347-4. [DOI] [PubMed] [Google Scholar]
- Yu C.G. Marcillo A.E. Fairbanks C.A. Wilcox G.L. Yezierski R.P. Agmatine improves locomotor function and reduces tissue damage following spinal cord injury. Neuroreport. 2000;11:3203–3207. doi: 10.1097/00001756-200009280-00031. [DOI] [PubMed] [Google Scholar]
- Zhang S.X. Bondada V. Geddes J.W. Evaluation of conditions for calpain inhibition in the rat spinal cord: effective postinjury inhibition with intraspinal MDL28170 microinjection. J. Neurotrauma. 2003;20:59–67. doi: 10.1089/08977150360517182. [DOI] [PubMed] [Google Scholar]
- Zhang S.X. Underwood M. Landfield A. Huang F.F. Gison S. Geddes J.W. Cytoskeletal disruption following contusion injury to the rat spinal cord. J. Neuropathol. Exp. Neurol. 2000;59:287–296. doi: 10.1093/jnen/59.4.287. [DOI] [PubMed] [Google Scholar]