Abstract
Cervical spinal cord injury (SCI) can severely impair reaching and grasping ability, and several descending systems, including the rubrospinal tract and corticospinal tract, have been implicated in the control of reach-to-grasp movements. The primary aim of this study was to characterize further the forelimb deficits associated with a cervical dorsolateral funiculotomy, which ablates the rubrospinal tract but spares the dorsal and ventral corticospinal tract in the rat. Adult female rats that preferred to use their right forelimb to reach for single pellets received a lesion to the right cervical dorsolateral funiculus between the C3-4 dorsal roots. Gross forelimb motor function was assessed by measuring spontaneous forelimb usage during exploration in a cylinder, and fine motor function was assessed using staircase and single pellet reaching tests. Single pellet reaching was further evaluated by qualitative and quantitative kinematic scoring of the movement components. Histological analysis included the quantification of spared white matter. Cervical dorsolateral funiculotomy produced marked deficits in reaching performance on both the single pellet and staircase reaching tests, with transient deficits in gross forelimb usage in the cylinder. Quantitative kinematics also revealed a reduction in digit abduction during the reach, which persisted throughout the 8-week post-SCI period. Tests of reach-to-grasp function, therefore, were more sensitive than a test of gross forelimb usage after cervical dorsolateral funiculotomy and did not show recovery over the 8-week survival period. We suggest that the staircase test is a useful screening tool for intervention studies because of its ease of implementation, and that the single pellet test is valuable for examining reaching accuracy and detailed kinematics.
Key words: kinematics, reaching, recovery of function, rubrospinal tract, spinal cord injury
Introduction
Cervical spinal cord injuries (SCIs) typically result in tetraplegia, of which the most common neurological category is incomplete tetraplegia (NSCISC, 2006). People with tetraplegia have rated the deficit in hand function as one of their most important losses (Hanson and Franklin, 1976; Anderson, 2004). Loss of hand function translates into increased dependence on attendant care for activities of daily living and impacts a person's occupational status (NSCISC, 2006).
Although the corticospinal tract is often thought to be the primary descending system involved in the control of skilled limb movements (Iwaniuk and Whishaw, 2000), focal ablations of this tract do not prevent many skilled forelimb reaching behaviors such as directed aiming, transport of the forelimb, and grasping in rats (Whishaw et al., 1998; Schrimsher and Reier, 1993), and monkeys (Lawrence et al., 1968; Houk et al., 1988). Other descending systems that contribute to skilled reaching in animals are the rubrospinal tract in the dorsolateral funiculus (Whishaw et al., 1992, 1998; Whishaw and Gorny, 1996) and the tectospinal and reticulospinal tracts in the ventrolateral funiculus and ventral funiculus, respectively (Pettersson, 1990; Pettersson et al., 1997).
Schrimsher and Reier (1993) compared the effects of lesions to the dorsal columns, dorsolateral funiculus, and ventrolateral funiculus on reach-to-grasp behavior in rats over a 1-month period. Only lesions to the dorsolateral funiculus (involving the rubrospinal tract) produced a persistent deficit in reaching success by impairing the ability to flex the digits during the reach-to-grasp task. Similarly, Anderson et al. (2007) showed that lateral lesions to the cervical spinal cord impaired single pellet reaching more than medial lesions in female Sprague-Dawley rats. Consistent with this effect on grasp, Jarratt and Hyland (1999) demonstrated that neuronal activity in the rat red nucleus during a reach-to-grasp task correlated with the distal movements associated with the grasp. The correlation of red nucleus neuronal activity and distal wrist and finger movements has also been shown in monkeys (Miller and Sinkjaer, 1998; Houck et al., 1988). Thus, further investigation of the role of the rubrospinal tract in skilled reaching behavior is warranted.
High-speed video and motion analysis systems allow more detailed quantification of deficits (Whishaw et al., 1998) and alternative reach-to-grasp tests have been developed that force the use of the impaired forelimb (Montoya et al., 1991; Titsworth et al., 2007). The primary aim of this study was to characterize forelimb deficits associated with the cervical dorsolateral funiculotomy lesion model. This study extends the work of Schrimsher and Reier (1993) by examining both gross and fine forelimb motor function and by applying both qualitative and quantitative kinematic analyses. Given the variety of assessments available for forelimb function (Schallert and Woodlee, 2005; Shumsky et al., 2003; Montoya et al., 1991; Whishaw et al., 1992), it is important to determine which are most useful and efficient for the cervical dorsolateral funiculotomy lesion by examining the relationships among the outcomes of the various behavioral tests. The results of this study will allow the creation of a standardized set of assessments for future intervention studies using this lesion model.
Methods
Subjects
Ten out of 20 adult female Sprague-Dawley rats (225–250 g; Taconic, Germantown, NY) demonstrated a consistent right forelimb preference when reaching for single pellets. The 10 animals with a right forelimb reaching preference received a lesion to the right cervical dorsolateral funiculus. These animals were housed on a 12-h light/dark schedule (lights on at 07:00) in standard Plexiglas cages (2–3 animals/cage) for the duration of the experiment and maintained on a food-restricted diet of 12–15 g of standard rat chow per day per animal. This resulted in animals reaching approximately 90% of their free-feeding body weight. Animals were weighed daily to ensure health and 2 days pre-and post-lesion surgery were allowed free access to food. All surgical and behavioral procedures were approved by the Drexel University College of Medicine's Institutional Animal Care and Use Committee and followed NIH Guidelines for working with animals.
Behavior Training and Testing
To assess spontaneous gross forelimb motor function, animals were placed in a Plexiglas cylinder (17.8 cm diameter × 35.5 cm height) for 3 min at a time. When placed in the cylinder, animals spontaneously rear and contact the walls with their forepaws. A mirror was placed behind the cylinder in order to observe the forelimbs irrespective of the position of the animal. The number of forepaw contacts with the wall of the cylinder was scored from video replay. Contacts of the right (impaired limb), left, and both (simultaneous) forepaws were counted and expressed as a percentage of the total placements. Percentage use of the right plus both forepaws were added to reflect the full usage of the impaired limb (Schwartz et al., 2003; Shumsky et al., 2003). The animals performed this test before the lesion, 2–3 days after the lesion surgery, and at 1, 2, 4, 6, and 8 weeks during the recovery period.
We used the staircase-reaching test to examine reach-to-grasp performance in a context that minimizes shoulder function against gravity (Montoya et al., 1991; Biernaskie and Corbett, 2001). The staircase-reaching test was used for several reasons: (1) The shelf of the single pellet reaching apparatus was 4.0 cm above the floor and therefore required control of shoulder muscles to lift and aim the forelimb against gravity to permit contact with the pellet. The staircase-reaching test was able to test reaching function largely independent of shoulder control (reaches in the same direction as gravity), which may be impaired after dorsolateral funiculotomy if it also disrupts the ventral gray matter at the C3/C4 spinal cord level that contains the motoneuron pool for the shoulder musculature. (2) The single pellet test allows the possibility for the rat to “rake” the pellet through the slot rather than grasping it. The staircase test, instead, requires the animal to make a coordinated grasp and lift the pellet to its mouth with no possibility for “raking.” The animals were trained to reach and grasp up to 21 pellets (45 mg; Bioserv, Frenchtown, NJ) from the seven wells (three chocolate pellets per well) located in the animals' right staircase during 2 weeks prior to the lesion surgery (one 15-min session/day). The week prior to the lesion surgery the animals performed the pre-SCI test (two 15-min sessions/day), and the three sessions with the most pellets retrieved were averaged to obtain the measure of pellet retrieval (Biernaskie and Corbett, 2001). Post-injury testing was performed similarly at weeks 1 and 8.
Prior to surgery, all animals were trained on a single pellet reach-to-grasp task for 3 weeks in a manner similar to Whishaw et al. (1992, 1996, 1998). The same handlers conducted training and testing 5 days per week between 08:30 and 11:00. The Plexiglas reaching chamber was 45 cm deep × 40 cm tall × 12.5 cm wide, with a 1-cm-wide slit cut out and centered on the 12.5-cm-wide front wall. On the outside of the front wall, a 3.2 cm deep × 12.5 cm wide shelf was mounted, so that the top surface was 4 cm above the chamber floor. Two food pellet wells were drilled out and centered to each edge of the slit on the front wall and placed 1.5 cm away from the outside front wall. Following habituation to the reaching chamber, the animals were operantly trained to reach with the right forelimb through the slit in order to grasp and consume a 45-mg chocolate food pellet that was placed in the contralateral food well. Training was performed through successive approximation. First, crushed pellets were placed at the shelf so the animal associated the shelf with food. Then a pellet was dropped into the back of the chamber whenever the animal approached the front of the chamber in order to encourage shuttling from back to front before reaching for the pellet on the shelf. Shuttling after a reach creates a natural separation between reaches and encourages consistency in how the animal orients and initiates itself to perform the next reach (Whishaw et al., 1992). The animal was considered to be successfully trained when it reached with the right forelimb and grasped the food pellet with a success rate of ≥50% from a total of 20 reach attempts (Whishaw et al., 1992; Whishaw and Gorny, 1996).
Quantitative assessment of the single pellet reach-to-grasp task was performed in the reaching chamber described above. The quantitative measures that were assessed during the single pellet reach-to-grasp task were as follows: (1) percent successful reaches; (2) peak wrist velocity; (3) deviation of the paw trajectory (tracked 3rd digit) from a straight line during descent over the pellet (path-length ratio = actual path-length of 3rd digit/straight line path length of 3rd digit from first to last frame); (4) amount of paw pronation excursion during the pronation phase using the angle between a plane made between the 2nd and 5th digits and the horizontal; (5) and the amount of spread or distance between the 2nd and 5th digits during digits open and pronation phases. During a single pellet testing session, two video recording systems were simultaneously employed to capture the different types of data. A Sony camcorder was used to record consecutive reach attempts during the first 3 min of the testing session (approximately 15–20 attempts). From this video data, two raters scored the reach attempts as successful or unsuccessful. A successful reach was defined as a reach during which the rat made contact with the food pellet and grasped it to remove it from the pellet well on the platform. A reach during which the animal “raked” the pellet from the well and did not grasp the pellet with digit flexion was not counted as a successful reach. The percent of successful reaches was calculated as follows:
(No. of successful reaches/no. of total reach attempts) × 100
and this was averaged between the two raters. Video for kinematic analysis was recorded using a synchronized, high-speed (500 frames/sec), two-camera digital recording system (Redlake). The two cameras were positioned to get a lateral view of the right side and a frontal view. The animals' right forelimbs were ink marked over the lateral wrist joint and the tips of the digits for kinematic analysis using WINanalyze tracking software (Mikromak).
Qualitative components of the single pellet reach-to-grasp task were scored using a movement rating scale (Whishaw et al., 1992), which assesses 10 different components of a reach. Each component was given a score of 2 if the movement appeared normal, 1 if the movement appeared somewhat abnormal but recognizable, or 0 if the movement was absent or was compensated for by moving other parts of the body. The ratings from each component of the reach were summed to get a deficit score from each trial (20 = no deficit; 0 = maximum deficit with no movement for any of the reaching phases). The first five consecutive reaches from each animal were scored from video replay by two raters and averaged regardless of their success.
The 10 components of the reach are (1) limb lift: the forelimb is lifted from the floor by the upper arm and the digits are brought to the body's midline; (2) digits close: as the forelimb is lifted, the digits are semi flexed and the paw is supinated so that the palm faces the midline of the body; (3) aim: the elbow is adducted by the upper arm so that the forearm and digits are aligned with the body's midline and the paw is held just under the mouth; (4) limb advance: the head is lifted and the limb is advanced above and beyond the pellet; (5) digits open: the digits are extended and opened as the limb is advanced; (6) pronation: the elbow is abducted by the upper arm and the paw is pronated over the food; (7) grasp: as the palmar pads touch the food, the digits close around it, and closure can occur before or during paw withdraw; (8) supination I: as the paw is withdrawn it is dorsiflexed and supinated 90° by a movement around the wrist and by adduction of the elbow; (9) supination II: as the rat sits back on its haunches, the paw is supinated by a further 90° and is ventroflexed so that the food is brought up to its mouth; and (10) release: the digits are opened and the food is transferred to the mouth.
Both qualitative and quantitative assessments of the reach-to-grasp task were performed once during the pre-lesion phase after the animals were trained on the task. Post injury, qualitative and quantitative reaching assessment were performed at 1 and 8 weeks. During the post injury period, the animals were given one practice session per week (20 attempts) to retrieve single pellets.
Lesion Surgery
The animals were deeply anesthetized with an intraperitoneal injection of acepromazine maleate (0.7 mg/kg; Fermenta Animal Health Co., Kansas City, MO), ketamine (95 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA), and xylazine (10 mg/kg; Bayer Co., Shawnee Mission, KS), and received a partial laminectomy of the C3-4 level to expose one spinal cord segment. After hemostasis is achieved, the spinal cord midline and the dorsal root entry zone were identified and a micro scalpel was used to open the dura mater. Next, a shallow (~1 mm deep) rostral-caudal incision was made at the dorsal root entry zone of C3 and extended to C4 in the right dorsal spinal cord (~2–3 mm in length). A fine-tipped, glass-pulled microaspiration device and microscissors were used to extend the lesion laterally. This lesion completely disrupted the dorsolateral funiculus (containing the rubrospinal tract), partially involves the ipsilateral lateral funiculus and gray matter, but leaves the dorsal columns and ventral funiculus intact. The dura was closed with 9-Ø silk sutures, and approximately 3 μL of liquid collagen was injected to fill the lesion cavity. A fat pad was then placed on top of the laminectomy site and the muscle and skin were closed in layers. After the surgery, the animals were given subcutaneous injections of buprenorphine (0.05 mg/kg) for pain immediately post-surgery and then every 12 h for 2 days, a single subcutaneous injection of the antibiotic ampicillin (100 mg/kg) for prophylaxis, 3 mL of lactated Ringer's subcutaneously, and kept on heating pads for 12 h. The animals were returned to their home cages the next day.
Histology
Animals were deeply anesthetized with an intraperitoneal injection of 1 cc of 150 mg/kg Nembutal at the end of the behavioral studies and transcardially perfused with 150 mL of physiological saline and then 500 mL of ice-cold 4% paraformaldehyde in 0.1 phosphate buffer (PB) solution with a pH of 7.4. Spinal cords were dissected and the lesion region was blocked and then placed in 0.1 M PB containing 30% sucrose for 72 h. Specimens, approximately 5 mm long, were frozen in OCT compound (Tissue Tek, Sakura Inc., Japan), and serially sectioned at 20 μm on a freezing microtome. Sectioned tissue was processed for Nissl/myelin staining to evaluate the lesion size and location (Liu et al., 1999). White matter area was outlined and quantified on the intact (left) and lesioned (right) sides of the spinal cord using ImageJ software. The percent spared white matter was calculated by dividing the lesioned side white matter area by the intact side white matter area and multiplying by 100. The percent of spared white matter was averaged every 500 μm through the lesion site and the 500 μm series with the largest lesion area was used for regressions with staircase reaching performance.
Statistical Analysis
For the anatomical data, simple linear regression was performed between the percent white matter sparing and the number of the pellets retrieved from the staircase-reaching test. One-way analysis of variance (ANOVA), with time taken as the repeated measure, was conducted on each behavioral test. Alpha levels were set to 0.05 for all comparisons. If a main effect was found to be significant, then Bonferroni-corrected post-hoc t-tests were performed to identify were the differences existed.
Results
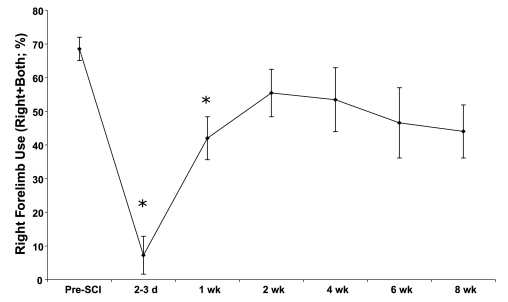
Gross forelimb motor function, as assessed by the number of right forepaw contacts with a cylinder, is depicted in Figure 1. Prior to the cervical SCI, the animals used their right forepaw to contact the cylinder surface (alone or together with the left forepaw) during vertical explorations 68.5% of the time. There was a significant main effect for decreased right forelimb contacts over time following injury (F = 4.66; p < 0.001). Post-hoc t-tests revealed that right forepaw contacts were significantly reduced at 2–3 days (t = 11.17; p < 0.00001) and 1-week (t = 4.27; p < 0.002) post-SCI. Forelimb usage recovered to a slightly lower plateau that was not different from pre-injury baseline at 2 through 8 weeks post-SCI (p > 0.0083; Bonferroni corrected for 6 comparisons).
FIG. 1.
Usage of the affected (right) forelimb in the cylinder showed deficits at 2–3 days and 1 week post-SCI followed by recovery at weeks 2–8. *Significant Bonferroni-corrected post-hoc t-tests, p < 0.0083. Means and standard errors are shown.
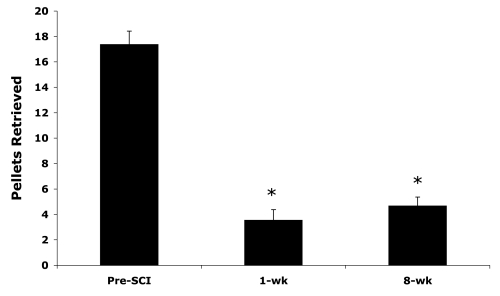
Right forelimb grasp function was assessed using the staircase-reaching test (Fig. 2). Prior to SCI, animals were able to retrieve about 17 of 21 pellets (81%) from the staircase apparatus. There was a significant main effect for a deficit in pellet retrieval performance over time (F = 59.36; p < 0.0001), and post-hoc t-tests revealed that there was no recovery of pellet retrieval (approximately 20% successful) at 1 (t = 10.30; p < 0.0001) and 8 weeks (t = 8.40; p < 0.0001) after SCI.
FIG. 2.
The staircase-reaching test detected large deficits in the ability to grasp and retrieve pellets that did not recover over the 8-week study. *Significant Bonferroni-corrected post-hoc t-tests, p < 0.025. Means and standard errors are shown.
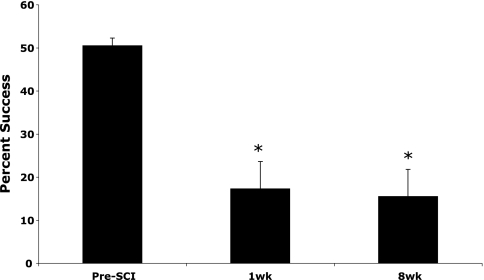
Right forelimb grasp function was also assessed using Whishaw et al. (1992) single-pellet reach-to-grasp test, which requires the animal to accurately aim and retrieve a single pellet from a platform. Prior to SCI, animals were able to retrieve single pellets successfully approximately 50% of the time. After SCI, there was a significant main effect for a deficit in successful single pellet retrievals over time (F = 12.57; p < 0.001). Successful single pellet retrieval did not recover over time with 17% at 1 week (t = 4.86; p < 0.001) and 15% at 8 weeks (t = 4.44; p < 0.01) post-SCI (Fig. 3). Qualitative analysis of the movement components of the single pellet reach-to-grasp test showed a similar pattern with a significant main effect for the presence of abnormal or absent movements over time (F = 17.01; p < 0.0001) that were similar at 1 week (t = 6.02; p < 0.001) and 8 weeks (t = 6.78; p < 0.0001) after SCI, which indicates no recovery (Table 1). When examining the individual movement components of the reach, the movements of the distal forelimb and forepaw (e.g., Digits Open, Grasp, Supination I and II, and Release) appear more affected than movements originating from the shoulder (e.g., Limb Lift, Aim, and Advance; Table 1).
FIG. 3.
The single-pellet reach-to-grasp test detected large deficits in the ability to successfully grasp and retrieve pellets that did not recover over the 8-week study. *Significant Bonferroni-corrected post-hoc t-tests, p < 0.025. Means and standard errors are shown.
Table 1.
Qualitative Reaching Score for the Single Pellet Reach-to-Grasp Test
| Components | Baseline | 1 week | 8 weeks |
|---|---|---|---|
| Limb Lift | 1.89 | 1.63 | 1.53 |
| Digits Closed | 1.90 | 1.59 | 1.54 |
| Aim | 1.98 | 1.47 | 1.36 |
| Advance | 1.93 | 1.13 | 1.05 |
| Digits Open | 1.91 | 0.82 | 0.79 |
| Pronation | 1.87 | 1.00 | 1.01 |
| Grasp | 1.84 | 0.63 | 0.61 |
| Supination I | 1.90 | 0.24 | 0.31 |
| Supination II | 1.60 | 0.18 | 0.18 |
| Release | 1.94 | 0.36 | 0.32 |
| Mean score | 1.87 | 0.90 | 0.87 |
| SD | 0.10 | 0.52 | 0.48 |
| Sum score | 18.74 | 9.03 | 8.68 |
2, Normal movement; 1, abnormal movement; 0, absent movement.
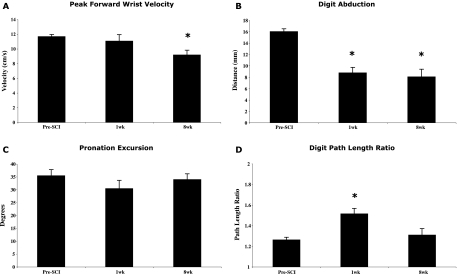
Kinematic analysis from digitized high-speed video was performed on selected components of the single pellet reach-to-grasp test. Forward velocity at the wrist (Fig. 4A) was measured during the Advance phase of the reach and found to decrease over time (F = 3.54; p < 0.05) such that by 8 weeks post-SCI the decrease in forward velocity was significant (t = 4.04; p < 0.01) but modest in magnitude. The maximum distance attained between the second and fifth digits (digit abduction; Fig. 4B) during the Digits Open or Digit Spread phase (Whishaw et al., 1992; McKenna and Whishaw, 1999) was diminished by half at 1 week and did not recover by 8 weeks (F = 55.96; p < 0.0001; 1 week, t = 5.71; p < 0.001; 8 weeks, t = 4.81; p < 0.001). The amount of pronation excursion of the forepaw (Pronation phase; Fig. 4C) showed no deficits over the post-SCI time periods (F = 1.24; p > 0.05). Although during qualitative scoring the raters scored the Pronation phase movement as “abnormal” (Table 1), the individual data from quantitative analysis of pronation excursion shows some animals losing motion, while others gained (individual data not shown). The forepaw path length ratio (Fig. 4D) shows longer path lengths to the food pellet 1 week after SCI (F = 6.60; p < 0.01; t = −4.42; p < 0.01) followed by recovery of path length (shorter) at 8 weeks (t = −0.657; p > 0.05).
FIG. 4.
Kinematic analysis of selected aspects of the single-pellet reach-to-grasp test. (A) Peak forward wrist velocity. (B) Digit abduction. (C) Pronation excursion. (D) Digit path length ratio. *Significant difference from pre-SCI with a Bonferroni-corrected post-hoc t-test, p < 0.025. Means and standard errors are shown.
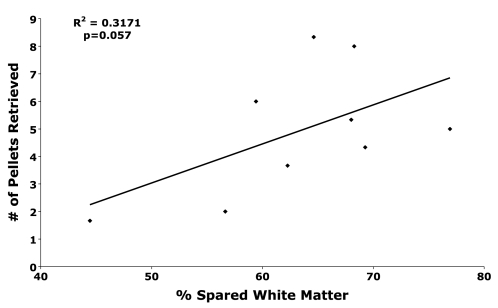
All animals were evaluated histologically and verified to have lesions that affected the dorsolateral funiculus. One animal had damage of the dorsal columns, but was excluded from behavior analysis except for the regression of spared white matter and staircase-reaching performance. Spared white matter ranged from 44.44% to 76.92% (mean = 63.32%; SD = 8.73; Fig. 5). The amount of spared white matter was modestly related to staircase reaching performance at 8 weeks post-SCI (r2 = 0.32; F = 3.25; p = 0.057; Fig. 6). This failed to reach statistical significance because of the limited number of subjects and limited variation in lesion size; however, the regression analysis did reveal that the variation in the percent spared white matter did explain approximately 32% of the variance in staircase reaching performance.
FIG. 5.
Example of the cervical dorsolateral funiculotomy lesion in cross-section.
FIG. 6.
Performance on the staircase-reaching test at 8-weeks post-SCI was modestly related to the percent of spared white matter.
Discussion
In this study, we compared the degree of deficit and recovery from a cervical SCI using several tests of forelimb function. The unilateral cervical dorsolateral funiculotomy lesion produced marked deficits in reaching performance on both the single pellet and staircase tests, but only transient deficits in gross forelimb function as measured by forelimb preference for use during exploration in a cylinder. We also were able to identify both qualitatively and kinematically a previously undescribed deficit in digit abduction during the digits open phase of the reach after a dorsolateral funiculotomy.
The single pellet and staircase reaching tests, and the measure of the loss of digit abduction all revealed robust changes (loss of function) as a result of this lesion and may be useful outcome measures for intervention studies that foster recovery of function after partial cervical SCI. The staircase test of pellet retrieval had results comparable to the single pellet reach test (Figs. 2 and 3). The ease with which animals are trained and the objectivity of the results for the staircase test may make it a preferable behavioral test for studies of partial cervical SCI. Onifer et al. (1997) studied the effects of a C7 contusion on forelimb function, and the staircase-reaching test showed a decrease in performance from approximately 13 to five pellets retrieved, similar to the effect seen in our C3-4 dorsolateral funiculotomy. The staircase test may especially be useful when screening for treatments that focus on improving forelimb grasp function (Garcia-Alias et al., 2007), because, since less time is spent training the animals, several animals can be tested simultaneously by one examiner, video analysis is unnecessary, and the examiner does not have to distinguish a “rake” from a true “grasp” as must be done in the single pellet test. Unless the researcher has specific hypotheses regarding the animal's reaching accuracy or the individual movement components or kinematics of the single pellet reach, we view the staircase test as a good alternative testing procedure for partial cervical hemisection lesions.
Forelimb motor function as assessed by the single pellet reach test revealed persistent deficits that were present in the distal forelimb. Using Whishaw's qualitative reaching score, we identified five phases of the reach that scored between a 0 (movement phase did not occur) and 1 (movement appeared abnormal). These phases were digits open, grasp, supination I, supination II, and release. Our results for single pellet reaching were similar to those of Schrimsher and Reier (1993), who used a bilateral C4 dorsolateral funiculotomy model, and Anderson and colleagues (2005), who used a C5 hemisection injury model, and McKenna and Whishaw (1999) for their C2 dorsolateral lesion. Schrimsher and Reier (1993), Anderson and colleagues (2005), and the present study found a persistent deficit in the grasp phase that consisted of a lack of active digit flexion despite making pellet contact with the palmar surface of the forepaw and often prevented successful pellet retrieval. These studies also observed deficits in the supination and release phases with the animals commonly using both forepaws to control the pellet and to bring it to its mouth, or the pellet was dropped as the limb was withdrawn from the slot. This is despite Onifer and colleagues (2005) finding that dorsolateral funiculotomies did not impair attention to stickers placed on the palmar surface of the injured side forelimb. McKenna and Whishaw (1999) found that reach-to-grasp success in the single pellet test returned to control levels by 1 week post-injury in the dorsolateral injured group; however, this difference might be the result of a strain difference in motor patterns during reaching between Long-Evans and Sprague-Dawley rats as described by Whishaw et al. (2003).
We, unlike Schrimsher and Reier (1993), found that the dorsolateral funiculotomy abolished most of the digit abduction (digits open phase) that takes place during limb advancement through the slot in the apparatus. In addition, our qualitative score for the lack of digit abduction is supported by our quantitative kinematic assessment, which shows an average loss of 7-8 mm of maximum digit abduction (Fig. 4B). The lack of digit abduction is a novel finding after a dorsolateral funiculotomy but has previously been described after dorsal column injury (Schrimsher and Reier, 1993) and complete hemisection (Anderson, 2005), but not after injury to the dorsolateral or ventrolateral funiculi (Schrimsher and Reier, 1993; McKenna and Whishaw, 1999; Muir et al., 2007). Again, the absence of an impairment in the digits open phase observed by McKenna and Whishaw (1999) and Muir et al. (2007) may be due to strain differences, but further study would need to be performed to elucidate this notion.
We also examined the pronation excursion movement kinematically. Qualitatively, we found that the pronation movement was performed abnormally; however, quantitative kinematics did not show a strong pattern of change in the total pronation excursion. This kinematic measure was probably influenced by compensation in the movement pattern from the shoulder and from passive displacement of the paw against the shelf (Whishaw and Gorny, 1996) and should be interpreted with caution. Other kinematic measures that we employed were a measure of the “smoothness” of the paw trajectory as it descended upon the pellet using the path-length ratio and the forward wrist velocity during the advance phase. Both of these measures showed only transient deficits with the path-length being longer at 1 week and recovering toward baseline by 8 weeks, and the forward wrist velocity slowing only at 8 weeks. Whishaw et al. (1998) have noted increased variability in the reaching movement's smoothness in animals that have received pyramidal tract and combined pyramidal tract-red nucleus lesions. Whishaw et al. (1998) also reported a greater segmentation in the velocity profile in these same animals. We, however, did not see differences in our velocity profiles (data not shown) following dorsolateral funiculotomy, but the peak velocity slowed only by a modest amount at the end of the 8-week study.
The dorsolateral funiculotomy produced only minor deficits in gross forelimb function that were only found early after the lesion injury (2–3 days, 1 week), but did not differ statistically from baseline from 2–8 weeks post-injury. The lack of a forelimb use deficit in the cylinder test is in contrast to prior work involving C3-4 partial hemisection model (Liu et al., 1999; Schwartz et al., 2003; Shumsky et al., 2003; Tobias et al., 2005; Xiao et al., 2005, 2007) and is likely due to a smaller lesion that spares gray matter, which was used in the current study (Fig. 5). In fact, Muir et al. (2007) recently reported that bilateral lesions confined to the dorsolateral funiculus did not impair forelimb usage in cylinder exploration. Additionally, Anderson et al. (2007) reported that lateral column lesioned rats recovered grip strength but not pellet retrieval success, whereas rats who had a medial lesion through the dorsal and ventral columns lost grip strength but recovered pellet retrieval. This supports our observation that gross motor function is only modestly disrupted but fine motor function (i.e., pellet retrieval) is severely impaired after damage to the dorsolateral funiculus.
It is difficult to assign the movement deficits that we report after a dorsolateral funiculotomy to a particular ascending or descending spinal system, because this lesion interrupts the rubrospinal tract (Liu et al., 1999), lateral corticospinal fibers (Weidner et al., 2001), and some parts of reticulospinal tract (Houle and Jin, 2001). Ablation studies of the red nucleus have implicated its role in the control of fine forelimb/forepaw movements in the rat (Whishaw et al., 1992, 1998; Whishaw and Gorny, 1996). The movement components of single pellet reaching that have been affected by red nucleus ablations are the aim, advance, pronation, grasp, supination I and II phases (Whishaw et al., 1992; Whishaw and Gorny, 1996), while after dorsolateral funiculotomy we found that the advance, digits open, pronation, grasp, supination I and II, and release phases were affected. In the rat, anatomical data show that some rubrospinal fibers do make direct projections onto the motoneurons of the extensor and flexor digitorum and the flexor and abductor digiti (Kuchler et al., 2002), electrophysiologic data support that the red nucleus is active during the reach (Jarratt and Hyland, 1999; Hermer-Vazquez et al., 2004), and microstimulation to the red nucleus resulted in shorter electromyographic latencies in the distal forepaw musculature than the proximal forelimb/shoulder musculature (Kuchler et al., 2002). Based on our qualitative and quantitative measures and the anatomical data from Kuchler et al. (2002), we suggest that digit abduction is a movement component that may be partially under rubrospinal control during the single pellet reach.
In summary, deficits in forelimb function after cervical dorsolateral funiculotomy are detected using sensitive assessments of reach-to-grasp function, but not gross function tests such as preference for forelimb use. We suggest that the staircase test would be the preferred forelimb functional test as a screening tool for future intervention studies in the cervical dorsolateral funiculotomy because it is easy to implement, analyze and demonstrates persistent deficits over an 8-week recovery period. Although our results could suggest that behavioral recovery in the single pellet or staircase-reaching tests implies restoration of rubrospinal connections, appropriately designed re-lesion experiments would need to be performed to confirm anatomical restoration versus behavioral compensation. The single pellet reach test may also be valuable when more detailed information is needed because it can measure reaching accuracy and provide kinematics of the reaching movement.
Acknowledgments
We would like to thank Jacyln Nicolai, Omar Fischer, Kyle Horn, Bronwyn Kilby, Valerie Miller, Gregory Smith, Catherine LaRocco, Laura Prosser, and Natasha Townsend for their technical assistance. This research was supported in part by the PVA Research Foundation (grant 2378), the Stacy Anne Vitetta ‘82 professorship from Arcadia University, and the NIH (PO1-NS-24707).
Author Disclosure Statement
No conflicting financial interests exist.
References
- Anderson K.D. Targeting recovery: priorities of the spinal cord–injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Anderson K.D. Gunawan A. Steward O. Quantitative assessment of forelimb motor function after cervical spinal cord injury in rats: relationship to the corticospinal tract. Exp. Neurol. 2005;194:161–174. doi: 10.1016/j.expneurol.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Anderson K.D. Gunawan A. Steward O. Spinal pathways involved in the control of forelimb motor function in rats. Exp. Neurol. 2007;206:318–331. doi: 10.1016/j.expneurol.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Biernaskie J. Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J. Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alias G. Lin R. Akrimi S.F. Story D. Bradbury E.J. Fawcett J.W. Therapeutic time window for the application of chondroitinase ABC after spinal cord injury. Exp. Neurol. 2008;210:331–338. doi: 10.1016/j.expneurol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Hermer-Vazquez L. Hermer-Vazquez R. Moxon K.A. Kuo K.H. Viau V. Zhan Y. Chapin J.K. Distinct temporal activity patterns in the rat M1 and red nucleus during skilled versus unskilled limb movement. Behav. Brain Res. 2004;150:93–107. doi: 10.1016/S0166-4328(03)00226-2. [DOI] [PubMed] [Google Scholar]
- Hanson R.W. Franklin M.R. Sexual loss in relation to other functional losses for spinal cord injured males. Arch. Phys. Med. Rehabil. 1976;57:291–293. [PubMed] [Google Scholar]
- Houk J.C. Gibson A.R. Harvey C.F. Kennedy P.R. Van Kan P.L.E. Activity of primate magnocellular red nucleus related to hand and finger movements. Behav. Brain Res. 1988;28:201–206. doi: 10.1016/0166-4328(88)90097-6. [DOI] [PubMed] [Google Scholar]
- Houle J.D. Jin Y. Chronically injured supraspinal neurons exhibit only modest axonal dieback in response to a cervical hemisection lesion. Exp. Neurol. 2001;169:208–217. doi: 10.1006/exnr.2001.7645. [DOI] [PubMed] [Google Scholar]
- Iwaniuk A.N. Whishaw I.Q. On the origin of skilled forelimb movements. Trends Neurosci. 2000;23:372–376. doi: 10.1016/s0166-2236(00)01618-0. [DOI] [PubMed] [Google Scholar]
- Jarratt H. Hyland B. Neuronal activity in rat red nucleus during forelimb reach-to-grasp movements. Neuroscience. 1999;88:629–642. doi: 10.1016/s0306-4522(98)00227-9. [DOI] [PubMed] [Google Scholar]
- Küchler M. Fouad K. Weinmann O. Schwab M.E. Raineteau O. Red nucleus projections to distinct motor neuron pools in the rat spinal cord. J. Comp. Neurol. 2002;448:349–359. doi: 10.1002/cne.10259. [DOI] [PubMed] [Google Scholar]
- Lawrence D.G. Kuypers H.G.J.M. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brainstem pathways. Brain. 1968;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- Liu Y. Kim D. Himes B.T. Chow S.Y. Schallert T. Murray M. Tessler A. Fischer I. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J. Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna J.E. Whishaw I.Q. Complete compensation in skilled reaching success with associated impairments in limb synergies after dorsal column lesion in the rat. J. Neurosci. 1999;19:1885–1894. doi: 10.1523/JNEUROSCI.19-05-01885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.E. Sinkjaer T. Primate red nucleus discharge encodes the dynamics of limb muscle activity. J. Neurophysiol. 1998;80:59–70. doi: 10.1152/jn.1998.80.1.59. [DOI] [PubMed] [Google Scholar]
- Montoya C.P. Campbell-Hope L.J. Pemberton K.D. Dunnett S. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J. Neurosci. Methods. 1991;36:219–228. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Muir G.D. Webb A.A. Kanagal S. Taylor L. Dorsolateral cervical spinal injury differentially affects forelimb and hindlimb action in rats. Eur. J. Neurosci. 2007;25:1501–1510. doi: 10.1111/j.1460-9568.2007.05411.x. [DOI] [PubMed] [Google Scholar]
- NSCISC. National Spinal Cord Injury Statistical Center (NSCISC) Annual Report 2006. 2006. http://images.main.uab.edu/spinalcord/pdffiles/NSCIC%20Annual%2006.pdf. [Jul 6;2008 ]. http://images.main.uab.edu/spinalcord/pdffiles/NSCIC%20Annual%2006.pdf
- Onifer S.M. Rodriguez J.F. Santiago D.I. Benitez J.C. Kim D.T. Brunschwig J.P.R. Pacheco J.T. Perrone J.V. Llorente O. Hesse D.H. Martinez-Arizala A. Cervical spinal cord injury in the adult rat: assessment of forelimb dysfunction. Restor. Neurol. Neurosci. 1997;11:211–223. doi: 10.3233/RNN-1997-11405. [DOI] [PubMed] [Google Scholar]
- Pettersson L.G. Forelimb movements in the cat; kinetic features and neuronal control. Acta Physiol. Scand. 1990;594(Suppl):1–60. [PubMed] [Google Scholar]
- Pettersson L.G. Lundberg A. Alstermark B. Isa T. Tantisira B. Effect of spinal cord lesions on forelimb target-reaching and on visually guided switching of target-reaching in the cat. Neurosci. Res. 1997;29:241–256. doi: 10.1016/s0168-0102(97)00093-x. [DOI] [PubMed] [Google Scholar]
- Schallert T. Woodlee M.T. In: Orienting and placing, in: The Behavior of the Laboratory Rat; A Handbook with Tests. Whishaw I.Q., editor; Kolb B., editor. Oxford University Press; New York: 2005. pp. 132–134. [Google Scholar]
- Schrimsher G.W. Reier P.J. Forelimb motor performance following dorsal column, dorsolateral funiculi, or ventrolateral funiculi lesions of the cervical spinal cord in the rat. Exp. Neurol. 1993;120:264–276. doi: 10.1006/exnr.1993.1060. [DOI] [PubMed] [Google Scholar]
- Schwartz E.D. Shumsky J.S. Wehrli S. Tessler A. Murray M. Hackney D.B. Ex vivo MR determined apparent diffusion coefficients correlate with motor recovery mediated by intraspinal transplants of fibroblasts genetically modified to express BDNF. Exp. Neurol. 2003;182:49–63. doi: 10.1016/s0014-4886(03)00036-0. [DOI] [PubMed] [Google Scholar]
- Shumsky J.S. Tobias C.A. Tumolo M. Long W.D. Giszter S.F. Murray M. Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT-3 into a spinal cord injury site is associated with limited recovery of function. Exp. Neurol. 2003;184:114–130. doi: 10.1016/s0014-4886(03)00398-4. [DOI] [PubMed] [Google Scholar]
- Titsworth W.L. Onifer S.M. Liu N.K. Xu X.M. Focal phospholipases A2 group III injections induce cervical white matter injury and functional deficits with delayed recovery concomitant with Schwann cell remyelination. Exp. Neurol. 2007;207:150–162. doi: 10.1016/j.expneurol.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Tobias C.A. Han S.S.W. Shumsky J.S. Kim D. Tumolo M. Dhoot N.O. Wheatley M.A. Fischer I. Tessler A. Murray M. Alginate encapsulated BDNF-producing fibroblast grafts permit recovery of function after spinal cord injury in the absence of immune suppression. J. Neurotrauma. 2005;22:138–156. doi: 10.1089/neu.2005.22.138. [DOI] [PubMed] [Google Scholar]
- Weidner N. Ner A. Salimi N. Tuszynski M.H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl. Acad. Sci. USA. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw I.Q. Gorny B. Does the red nucleus provide the tonic support against which fractionated movements occur? A study on forepaw movements used in skilled reaching by the rat. Behav. Brain Res. 1996;74:79–90. doi: 10.1016/0166-4328(95)00161-1. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q. Gorny B. Foroud A. Kleim J.A. Long-Evans and Sprague-Dawley rats have similar skilled reaching success and limb representations in motor cortex but different movements: some cautionary insights into the selection of rat strains for neurobiological motor research. Behav. Brain Res. 2003;145:221–232. doi: 10.1016/s0166-4328(03)00143-8. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q. Gorny B. Sarna J. Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behav. Brain Res. 1998;93:167–183. doi: 10.1016/s0166-4328(97)00152-6. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q. Pellis S.M. Pellis V.C. A behavioral study of the contributions of cells and fibers of passage in the red nucleus of the rat to postural righting, skilled movements, and learning. Behav. Brain Res. 1992;52:29–44. doi: 10.1016/s0166-4328(05)80322-5. [DOI] [PubMed] [Google Scholar]
- Xiao M. Klueber K.M. Lu C. Guo Z. Marshall C.T. Wang H. Roisen F.J. Human adult olfactory neural progenitors rescue axotomized rodent rubrospinal neurons and promote functional recovery. Exp. Neurol. 2005;194:12–30. doi: 10.1016/j.expneurol.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Xiao M. Klueber K.M. Zhou J. Guo Z. Lu C. Wang H. Roisen F.J. Human adult olfactory neural progenitors promote axotomized rubrospinal tract axonal reinnervation and locomotor recovery. Neurobiol. Dis. 2007;26:363–374. doi: 10.1016/j.nbd.2007.01.012. [DOI] [PubMed] [Google Scholar]