Abstract
Traumatic brain injury (TBI) results in an accumulation of edema and loss of brain tissue. Progesterone (PROG) has been reported to reduce edema and cortical tissue loss in a bilateral prefrontal cortex injury. This study tests the hypothesis that PROG is neuroprotective following a unilateral parietal cortical contusion injury (CCI). Adult male Sprague-Dawley rats were subjected to a moderate unilateral TBI using the CCI model. Rats were given 8 mg/kg PROG 15 min post-injury with four subsequent injections (6 h, and days 1, 2, and 3). Edema was determined 3 days post-injury, while cortical tissue sparing was also evaluated at 7 days post-injury. Animals were injured and given one of four treatments: (I) vehicle; (II) low dose: 8 mg/kg PROG; (III) high dose: 16 mg/kg PROG; (IV) tapered: 8 mg/kg PROG. Animals were given an initial injection within 15 min, followed by five injections (6 h, and days 1, 2, 3, and 4). Group IV received two additional injections (4 mg/kg on day 5; 2 mg/kg on day 6). PROG failed to alter both cortical edema and tissue sparing at any dose. Failure to modify two major sequelae associated with TBI brings into question the clinical usefulness of PROG as an effective treatment for all types of brain injury.
Key words: cortical contusion injury, edema, neuroprotection, progesterone, traumatic brain injury
Introduction
In the United States, the Centers for Disease Control and Prevention (CDC) estimate that 1.5 million Americans sustain head injuries each year. Head injuries are the leading cause of death and life-long disabilities in young adults (Thurman et al., 1999), with an annual cost of over $60 billion in medical expenses and lost productivity (Corso et al., 2006; Langlois et al., 2006). These numbers are expected to continually rise, underscoring the necessity for an effective therapy. Traumatic brain injury (TBI) is heterogeneous in severity and location, resulting in a wide range pathophysiolgical development of detrimental secondary cascades (e.g., edema, excitotoxicity, mitochondrial dysfunction). Experimental research is needed that not only defines responses to TBI and is reproducible across several laboratories, but also employs multiple injury paradigms. Adequate research prior to clinical trials is critical to enhance the probability of positive clinical trial outcomes.
TBI can lead to physical debilitations, emotional stress, and cognitive decline. Treatments for TBIs have improved in recent years, but many survivors experience a decreased quality of life due to their injuries. A large number of new pharmacological treatments have been evaluated in experimental TBI models with a translational emphasis to improve patient outcome (McIntosh et al., 1998; Royo et al., 2003; Marklund et al., 2006). A large portion of these studies have been targeted toward reducing edema, decreasing cell loss, and improving behavior outcome. Clinical trials have tested a variety of neuroprotective compounds shown to be effective in animal models, but most have failed to display a robust beneficial application in the clinic (Narayan et al., 2002; Cernak, 2006; Tenovuo, 2006).
Progesterone (PROG), a new agent currently being used in clinical trials for TBI, has been shown to be safe and has produced possible signs of benefit (Wright et al., 2007). PROG has been used in numerous animal paradigms and is reported to have a neuroprotective effect on cortical tissue following experimental TBI. PROG's neuroprotective qualities have been studied predominately using both a medial frontal cortex (MFC) injury paradigm (Stein et al., 2008) and with a middle cerebral artery occlusion to study stroke (Betz and Coester, 1990; Jiang et al., 1996; Chen et al., 1999; Murphy et al., 2000; Murphy et al., 2002; Gibson et al., 2008). Several of these studies have reported PROG's ability to reduce edema (Roof et al., 1994, 1996; Galani et al., 2001; Wright et al., 2001; Grossman et al., 2004; Guo et al., 2006), lesion/infarct volume (Jiang et al., 1996; Chen et al., 1999; Kumon et al., 2000; Shear et al., 2002; Jones et al., 2005; Cutler et al., 2006a), and modulate the inflammatory response (Grossman et al., 2004; He et al., 2004; Cutler et al., 2005; Jones et al., 2005; Pettus et al., 2005). PROG has also been reported to improve neurological recovery after head trauma (Shear et al., 2002; Goss et al., 2003; Djebaili et al., 2004; Jones et al., 2005; Gibson et al., 2008).
There have been studies that have investigated PROG efficacy in several other types of injury paradigms including a unilateral parietal injury (Grossman and Stein, 2000; Robertson et al., 2006). Others have looked at the effects of PROG on diffuse brain injury (O'Connor et al., 2007) as well as spinal cord injury. One group has published several papers showing that PROG offers molecular neuroprotection and aids in remyelination in the spinal cord following injury (Labombarda et al., 2003; Gonzalez et al., 2004, 2005; Labombarda et al., 2006; Guennoun et al., 2007). In one case, PROG was shown to improve functional recovery and increased the total percent of white matter tracks following spinal cord injury, while another group found PROG offered no morphological or functional improvements (Fee et al., 2007).
There have been several instances where PROG has been shown to be neuroprotective, but ineffective in others. Specifically we are interested in the effects of PROG in the unilateral parietal injury. The Grossman and Stein (2000) publication reported that high physiologic levels of endogenous PROG in psuedopregnant females was ineffective at reducing behavior deficits via the foot fault test, but the ability of PROG to spare cortical tissue was not explored. The Robertson et al. 2006 work, on the other hand, used implanted silastic tubing packed with PROG to maintain physiologic low levels of PROG and found it to reduce mitochondrial dysfunction 1 h after injury and reduced cell injury in the hippocampus, but it failed to reduce cortical lesion volume. Neither of these studies looked at the effects of PROG on edema formation after injury. The goal of this study was to test the neuroprotective abilities of exogenously administered PROG in a well-characterized lateral cortical contusion injury (CCI) model on two major sequelae associated with this injury: edema and cortical tissue sparing. We chose edema because of its clinical relevance and cortical tissue sparing so we could quantify a substrate by which recovery could be possible.
Methods
Surgeries
This study used young adult male (weight, 275–400 g) Sprague-Dawley (Harlan Labs, Indianapolis, IN) rats (n = 55). Animals were housed in group cages (two per cage) on a 12-h light/dark cycle with free access to food and water. All experimental protocols involving animals were approved by the University of Kentucky Animal Use and Care Committee.
Animals were subjected to a moderate unilateral cortical contusion as previously described (Scheff and Dhillon, 2004; Shao et al., 2006). Anesthesia was induced with isoflurane (5%) and reduced to 2.0% after subjects were placed in a Kopf stereotaxic frame (Kopf Instruments, Tujunga, CA). All injuries were produced using a pneumatic controlled cortical impact device (TBI 0310, Precision Systems and Instrumentation, Fairfax Station, VA) with a soft stop Bimba cylinder (Bimba Manufacturing, Monee, IL). The head was positioned in the horizontal plane with the nose bar set at −5. Following a midline incision exposing the skull, a 6.0-mm craniotomy was performed with a Michelle trephine (Miltex, Bethpage, NY) placed lateral to the sagittal suture and centered between bregma and lambda. The skull cap at the craniotomy was carefully removed without damaging the underlying dura, and the exposed cortex was injured (5-mm beveled impactor tip diameter, 1.3-mm depth, 400-ms dwell, and 3.5-m/s velocity). After injury, Surgicel (Johnson & Johnson, Arlington, TX) was laid on the dura and the skull disk replaced. A small amount of dental acrylic was applied over the skull disk and allowed to harden. The skin was sutured with wound clips and the animals returned to their cages for post-operative recovery. Animals were maintained at 37°C.
For the first study, animals were randomly assigned to one of three groups:
Vehicle: 22.5% solution of [2-hydroxypropyl]-β-cyclodextrin (H5784, Sigma, St. Louis, MO) mixed with 0.9% sodium chloride (2B1322, Baxter Healthcare Corp., Deerfield, IL). Initial intraperitoneal (i.p.) injection of cyclodextrin solution was administered within 15 min post-injury followed by subcutaneous injections (s.c.) (6 h, and 1, 2, 3, and 4 days post-injury). Injection volume (0.22–0.32 cc) was determined by weight of the animal.
8 mg/kg progesterone (P8783, Sigma): 8 mg/kg i.p. injection within 15 min post-injury followed by s.c. injections (6 h, and 1, 2, 3, and 4 days post-injury). Drug dissolved in 22.5% cylcodextrin. Injection volume (0.22–0.32 cc) was determined by weight of the animal.
16 mg/kg progesterone (P8783): 16 mg/kg i.p. injection within 15 min post-injury followed by s.c. injections (6 h, and 1, 2, 3, and 4 days post-injury). Drug was dissolved in 22.5% cylcodextrin. Injection volume (0.22–0.32 cc) was determined by weight of the animal.
For the second study, animals were randomly assigned to one of two groups:
Vehicle: peanut oil (LouAna, Ventura Foods, Opelousas, LA). An i.p. injection within 15 min post-injury followed by s.c. injections (6 h, and 1, 2, 3, and 4 days post-injury). Injection volume (0.22–0.32 cc) was determined by weight of the animal.
Tapered progesterone 8 mg/kg (P8783): 8 mg/kg i.p. injection within 15 min post-injury followed by s.c. injections (6 h, and 1, 2, 3, and 4 days post-injury). On day 5, the dose was lowered to 4 mg/kg, and on day 6, to 2 mg/kg. Drug was dissolved in peanut oil. Injection volume(0.22–0.32 cc) was determined by weight of the animal.
All drug treatments were mixed in 10-mL amber serum tubing vials (model 223696; Wheaton Science Production, Millville, NJ) with rubber sleeve stopper caps (model S1320A; Plasticoid Corp., Elkton, MD), so that hypodermic needles could be utilized for administration of drug treatments. PROG readily went into solution upon mixing, but to ensure that PROG went into solution it was mixed on a vortex, warmed in a 37°C water bath for 15–20 min, and mixed again on a vortex.
Cortical Tissue Sparing
At 7 days post-injury, animals were transcardially perfused with 0.1 M phosphate buffer (PB) followed by 4% paraformaldehyde in PB. The brains were removed and postfixed in 20% sucrose in 0.1 PB. Coronal sections (50 μm) were cut with a freezing microtome throughout the rostral-caudal extent of the damaged hemisphere, extending from the septal area (interaural level of 10.7) to the most posterior extent of the hippocampus (interaural level of −0.3) (Paxinos and Watson, 1998). Twelve equidistant sections were stained with cresyl violet and subjected to morphological analysis (Scion Image 4.0.2, Frederick, MD). Quantitative determination of the volume of cortical tissue sparing used the Cavalieri method (Michel and Cruz-Orive, 1988; Mouton, 2002). On each coronal section, the entire cortical mantle was measured independently in the hemispheres ipsilateral and contralateral to the injury. Previous studies have shown that the contralateral cortex of the rat is unaffected at any time point post-injury after using a focal pneumatic cortical contusion (Baldwin et al., 1996). The amount (percent) of tissue sparing is calculated by dividing the volume of the cortex ipsilateral to the injury by the cortical volume contralateral to the injury. In this regard, each animal serves as its own control and histological artifacts such as shrinkage or swelling of tissue that might occur during tissue processing are negated (Sullivan et al., 1999). All quantitative results are reported as percent tissue sparing.
Determination of Percent Water Content
Baseline brain water content was determined using sham-operated animals. The other groups were injured with the parameters outlined above and either given the vehicle, or PROG to assess the drug's ability to modulate secondary injury-related edema at 3 days post-trauma as previously reported (Guo et al., 2006; VanLandingham et al., 2006). Unpublished data from our laboratory show that in our injury paradigm peak edema occurs by day 2 and is not significantly different at day 3.
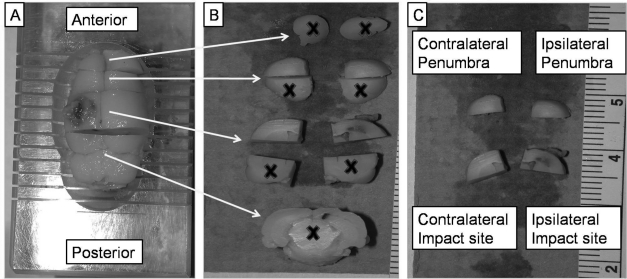
Animals were overdosed with sodium pentobarbital (50 mg/kg), decapitated, and the brain extracted. A complete (6-mm) coronal section encasing the extent of the injury was obtained using a precisely machined brain matrix (model BSR002.1; Zivic-Miller, Pittsburg, PA). Coronal sections (4 mm) immediately anterior were also obtained to determine possible effect of injury to surrounding nervous tissue. The four tissue regions used in these analyses are described in Table 1. Each brain section was bisected at midline and also horizontally through the dorsal thalamus to yield four quartered sections. Only the dorsal quartered sections containing the injured cortex and a portion of the hippocampus were used for analysis (Fig. 1). Samples were placed on pre-weighed antistatic polystyrene weigh boats (model 12577-005; VWR Scientific, St. Paul, MN) and the wet weight of each section determined. The sections were subsequently dried in a vacuum oven for 48 h at 60°C at 0.3 atm (228 mm Hg). Dried sections were reweighed and percent brain water content calculated [(wet weight/dry weight) × 100] (Guo et al., 2006). Mean percent differences were also calculated in all four cortical regions of injured animals and compared to the values of sham animals (Grossman et al., 2004). Using percent brain water content ensures precision regardless of weight differences between quartered sections of brain tissue.
Table 1.
Description of Brain Regions Used for Edema Analyses
| Section | Dorsal portion of a given brain region | Explanation of analyzed sections |
|---|---|---|
| Impact site (6 mm) | Ipsilateral | Site of cortical contusion injury |
| Contralateral | Corresponding contralateral tissue | |
| Penumbra (4 mm) | Ipsilateral | Tissue immediately anterior to injury |
| Contralateral | Corresponding contralateral tissue |
FIG. 1.
The regions of the rat cortex that were used for analysis for percent water content (edema) following injury are shown. Rats given either vehicle (peanut oil) or 8 mg/kg progesterone (PROG) treatments were killed 3 days post-injury and their brains harvested. Brains were cut in a brain matrix (A), resulting in 11 different brain regions (B), and only the dorsal portions containing the injury and penumbral brain tissue were obtained from both hemispheres (C). The X symbol represents portions not used in percent water content analysis. The four regions contained the impact site, corresponding contralateral tissue, and penumbral tissue from the ipsi- and contralateral sides.
Statistical Analysis
All data are reported as group mean ± standard deviation (SD). Cortical tissue sparing data were analyzed using a one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls. Edema data were analyzed using a two-way ANOVA using the same post hoc test. The alpha level was set at 0.05 for significance.
Results
Cortical Tissue Sparing
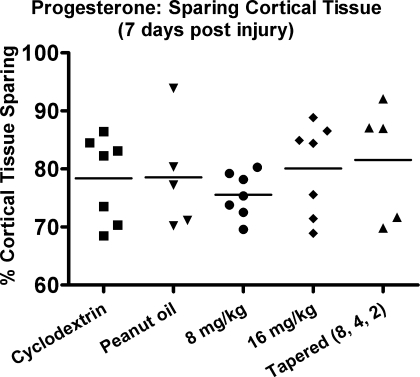
Statistical analysis of cortical tissue sparing failed to reveal a significant difference between group means [F (3, 28) = 0.785, p > 0.1]. Vehicle (cyclodextrin) treated animals had the same level of cortical tissue sparing as animals treated with either 8 or 16 mg/kg PROG (Fig. 2). Animals administered 8 mg/kg PROG demonstrated a trend for less tissue sparing. The animals given 8 mg/kg PROG with a tapered withdrawal, to reduce any negative effects associated with acute PROG withdrawal syndrome (Cutler et al., 2005), showed no significant increase of cortical tissue sparing compared to vehicle treated rats [F (1, 8) = 0.230, p > 0.1].
FIG. 2.
All animals in this study showed obvious cortical damage 7 days post-injury. Statistical analysis found no differences between vehicle-treated animals compared to those given progesterone (PROG). Previous research suggests administering PROG for 5 days or given for 7 days in a tapered withdrawal manner is neuroprotective. Animals given tapered PROG treatment achieved no greater tissue sparing than animals given the vehicle alone. Vehicle-treated (peanut oil) animals demonstrated 78.5 ± 9.6% tissue sparing, while tapered treated rats maintained 81.5 ± 10.1% sparing p > 0.1, suggesting that PROG is not neuroprotective in this injury model. Points indicate individual values. Horizontal line represents the group mean.
Determination of Percent Water Content
The weights for all cortex regions were recorded before and after drying process (Table 2). A thicker section was taken to include the entire injury compared to the penumbral tissue (Fig. 1). The amount of tissue taken in a given brain region was approximately the same across all treatment groups. Ipsi- and contralateral sides taken from the injured tissue section of sham animals were slightly lower in wet weight, but were not significantly different from the same regions of injured rats (p > 0.05). Minor weight differences were probably due to the presence or absence of edema in the area of interest.
Table 2.
Wet and Dry Weights of Brain Sections Used for Edema Analysis
| |
Wet weight in mg (mean ± SD) |
Dry weight in mg (mean ± SD) |
||||
|---|---|---|---|---|---|---|
| Sham | Vehicle | PROG | Sham | Vehicle | PROG | |
| Ipsilateral impact site | 119.3 ± 31.6 | 141.6 ± 27.4 | 141.7 ± 19.6 | 26.1 ± 7.2 | 28.3 ± 5.9 | 27.7 ± 4.0 |
| Contralateral impact site | 113.3 ± 19.7 | 135.5 ± 25.7 | 137.3 ± 25.9 | 24.8 ± 4.6 | 29.7 ± 5.8 | 30.0 ± 5.9 |
| Ipsilateral penumbra | 87.7 ± 7.9 | 88.3 ± 12.6 | 81.5 ± 12.6 | 19.7 ± 1.9 | 19.1 ± 2.7 | 17.9 ± 2.7 |
| Contralateral penumbra | 84.0 ± 12.9 | 85.4 ± 13.3 | 85.3 ± 11.8 | 19.1 ± 3.1 | 18.9 ± 3.1 | 19.0 ± 2.7 |
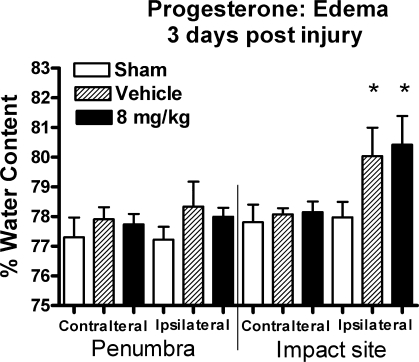
Analyses of the edema data, comparing sham and vehicle-treated injured rats, revealed a significant main effect of injury [F (1, 42) = 18.6, p = 0.001] and for cortical tissue location [F (3, 42) = 29.4, p < 0.0001]. A significant interaction for ipsilateral impact tissue was also observed [F (3, 42) = 10.8, p < 0.0001]. Post hoc analysis revealed that only ipsilateral impact tissue contained significantly higher water content than all other cortical areas (contralateral injured, ipsi- and contralateral penumbra). The injured cortex showed significantly higher mean water content (2.6% increase), while injured penumbra tissue produced only a 1.3% increase, compared to the same area of sham animals (Fig. 3). The analysis failed to reveal a significant main effect for drug treatment when comparing injured animals receiving either PROG or vehicle [F (1, 42) = 0.004, p > 0.95], but did reveal a main effect of cortical tissue location in regard to amount of edema [F (3, 42) = 66.6, p < 0.0001]. There was no significant interaction found in these analyses. PROG failed to reduce cortical edema in tissue containing the injury and had no effect on injured penumbral tissue. Rats given 8 mg/kg PROG had a mean water content of 80.4 ± 1% at the site of injury, while the corresponding uninjured cortex contained 78.1 ± 0.4%. Vehicle-treated animals maintained 80.0 ± 1% water content, with a 77.9 ± 0.4% in the uninjured cortex.
FIG. 3.
The region of cortex that contained injury showed a significant increase in percent water content compared to corresponding contralateral tissue and penumbral tissue. Progesterone (PROG) failed to reduce edema in the area containing the injury and had no effect on any other tissue region. *p < 0.01 compared to all other brain regions and treatment groups.
Discussion
The present study was designed to test whether or not PROG therapy would lead to a more favorable outcome following an experimental lateral cortical contusion injury in the rat. All animals subjected to a unilateral cortical contusion demonstrated a significant loss of cortical tissue immediately below the region of impact. Administering PROG did not appear to be beneficial in terms of total amount of cortical tissue sparing following the injury. Animals subjected to a moderate lateral cortical contusion demonstrated significant increases in brain edema. Subjects treated with PROG failed to demonstrate a significant reduction in tissue edema. These results are in sharp contrast to previously published works demonstrating significant benefit with PROG when treatment is initiated soon after trauma (Roof, 1992, 1996; Galani et al., 2001; Wright et al., 2001; Grossman et al., 2004; Guo et al., 2006; VanLandingham et al., 2006). It is unclear exactly what differences might reconcile the present findings with those of previous studies.
PROG treatment was initiated in the present study relatively quickly (15 min) following trauma, corresponding to previous dosing regiments. These animals were young adult male Sprague-Dawley rats, similar to those used by others, ruling out possible strain and gender effects. The present study evaluated the total amount of cortical tissue sparing following a unilateral injury. PROG failed to enhance tissue sparing compared to vehicle-treated animals. This is in contrast to previous reports that PROG reduces lesion volume (Shear et al., 2002; Cutler et al., 2005, 2006a). Tissue sparing is a more reliable index of neuroprotection than lesion volume since it measures actual tissue present as opposed to the absence of tissue or a cavity, which could be influenced by histological preparations. In the present paradigm, the contralateral side is used as a control and direct comparisons can be made from side to side. Unbiased stereologic techniques were used to calculate the spared tissue volume. Experimental scenarios that use a bilateral frontal contusion injury cannot use each animal as its own control. Other studies have reported that PROG does not reduce lesion volume, which is in agreement with our findings (Roof et al., 1994; Goss et al., 2003; Djebaili et al., 2004, 2005; Robertson, 2004).
Elevated intracranial pressure is an extremely relevant clinical complication that varies with injury type and severity and must be controlled to reduce morbidity and mortality (Stocchetti et al., 2007). Edema following TBI is a primary contributor to elevated intracranial pressure (Barzo et al., 1997). Both cytotoxic and vasogenic edema can cause cerebral swelling. With cytotoxic edema, astrocytes swell or rupture, which leads to neuron damage/loss (Rosenblum, 2007). Vasogenic edema results from compromising the blood-brain barrier, which causes water influx into the extracellular space (Duvdevani et al., 1995). Location and severity of the injury result in varying edema profiles (Unterberg et al., 2004), necessitating different treatment scenarios to reduce edema. Therapeutic strategies have to target specific cells or brain regions with elevated water content without harm to other cell/regions at the same time (Rosenblum, 2007).
The dose of PROG used in our study (8 mg/kg) failed to demonstrate a reduction in edema. The lateral cortical contusion model used in these studies produces a 4% increase in water content at 3 days post-trauma compared to sham-operated controls, which is similar to that reported by others (Unterberg et al., 1997; Fukui et al., 2003). The frontal cortex injury used by groups that report beneficial effects of PROG report a 2–6% increase in water content depending on the day post-trauma, region evaluated, and method used for computing the increase (Roof, 1992; Roof et al., 1996; Galani et al., 2001; Wright et al., 2001; Grossman et al., 2004; Guo et al., 2006; VanLandingham et al., 2006). In the present study, relatively thick sections (6 mm) encompassing the entire injured cortex were taken in addition to penumbral tissue (4 mm) using an unbiased method. Statistical analysis of edema was carried out on both the actual percent water content in the injured area and as the mean percent difference of the injured cortex compared to contralateral adjacent cortex.
Studies reporting reductions in tissue edema with PROG used a substantially different dissection protocol and a different injury model. These edema studies are based on the method of Roof and Stein (1992) in which thinner sections were used and selected small regions of dorsal cortex obtained for analysis in a medial prefrontal contusion (PFC) injury. The statistical analyses in these studies were only performed on the mean percent difference of the peri-contusional region compared to posterior cortex. Studies evaluating the benefits of PROG therapy on edema have been displayed in both 4 mg/kg (Roof, 1992; Roof et al., 1996; Galani et al., 2001; Wright et al., 2001; Grossman et al., 2004) and 16 mg/kg (Guo et al., 2006; VanLandingham et al., 2006), while the present study determined efficacy of 8 mg/kg. A previous study using an extended dosing PROG therapy also failed to demonstrate a significant reduction in cortical edema at 3 days post-trauma (Grossman et al., 2004).
Different amounts of edema are produced depending on the injury paradigms as well as the type of edema: vasogenic or cytotoxic. This fact may help explain the discrepancy between our data and those of others. Most studies that report that PROG reduced edema used a medial PFC that may result in a more vasogenic than cytotoxic edema, since the injury is inflicted over the superior sagittal sinus. The lateral CCI injury model produces a predominantly cytotoxic edema (Unterberg et al., 1997, 2004). PROG may be most effective in reducing vasogenic edema rather than cytotoxic. Differences in amounts of edema could be injury type specific. Failure to observe significant differences in edema at the site of injury in our unilateral parietal injury paradigm does not mean that PROG is not beneficial in some injury paradigms.
It may be possible that the effects of PROG on edema demonstrate a biphasic response such that 8 mg/kg is ineffective, while 4 and 16 mg/kg are therapeutic. This would be somewhat perplexing since 4, 8, and 16 mg/kg PROG have been reported to improve behavioral outcome (Roof et al., 1994; Shear et al., 2002; Goss et al., 2003; Djebaili et al., 2004, 2005; Grossman et al., 2004; Cutler et al., 2005, 2006a) and edema reduction has been linked to behavioral recovery (Roof et al., 1994). Alternatively, reduction of cerebral edema may not be necessary to improve behavioral performance.
The numerous types of PROG available vary in purity, solubility, structure, and source of derivation. Among those is P8783 Sigma, which was used in the current study. This PROG has previously been shown to alter outcome following an experimental frontal cortex injury (Cutler et al., 2006b) and was most likely the type of PROG used in a tapered withdrawal study that promoted behavioral recovery following trauma (Cutler et al., 2006a). Many studies have shown that different PROG preparations are also neuroprotective following TBI, suggesting that several different types of PROG are beneficial (Djebaili et al., 2004, 2005; VanLandingham et al., 2006; Jiang et al., 1996; Chen et al., 1999). Thus, the type of PROG used in the present study cannot account for the observed differences.
The present study evaluated the total amount of cortical tissue sparing following a unilateral injury. PROG failed to spare tissue compared to vehicle-treated animals. This is again in contrast to some previous reports that PROG reduces lesion volume (Shear et al., 2002; Cutler et al., 2005, 2006a). However, other reports have claimed that PROG does not reduce lesion volume (Roof et al., 1994; Goss et al., 2003; Djebaili et al., 2004, 2005; Robertson et al., 2006). If tissue sparing is one mechanism that underlies behavior recovery of function then it would be important to maximize this aspect of the injury response.
Overall PROG failed to modify two of the major sequelae–cortical edema and tissue sparing–that are associated with a lateral cortical contusion type of TBI. PROG was unable to decrease cortical edema 3 days following injury. PROG failed to alter cortical tissue sparing at either a low, high, or tapered doses compared to vehicle treatment. Although PROG has been reported to be neuroprotective, failure to observe similar results in a lateral cortical contusion injury raises concerns of its use as a universal therapy for all types of brain injury. Though we are urging caution, there are many other pathways that neurosteroids may offer some type of neuroprotection–such as increasing myelin production, reduction of inflammatory cytokines, or anti-apoptotic mechanisms. These are just a few of the pathways that PROG have been implicated at being able to modulate following certain insults. Further research appears to be necessary to completely understand PROG's ability to modulate certain aspects of the pathophysiology following brain injury.
Acknowledgments
This study was supported by the Kentucky Spinal Cord and Head Injury Research Trust (grant 5-A) and the National Institutes of Health (grant AG21981).
Author Disclosure Statement
No conflicting financial interests exist.
References
- Baldwin S.A. Fugaccia I. Brown D.R. Brown L.V. Scheff S.W. Blood-brain barrier breach following cortical contusion in the rat. J. Neurosurg. 1996;85:476–481. doi: 10.3171/jns.1996.85.3.0476. [DOI] [PubMed] [Google Scholar]
- Barzo P. Marmarou A. Fatouros P. Hayasaki K. Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J. Neurosurg. 1997;87:900–907. doi: 10.3171/jns.1997.87.6.0900. [DOI] [PubMed] [Google Scholar]
- Betz A.L. Coester H.C. Effect of steroids on edema and sodium uptake of the brain during focal ischemia in rats. Stroke. 1990;21:1199–1204. doi: 10.1161/01.str.21.8.1199. [DOI] [PubMed] [Google Scholar]
- Cernak I. Recent advances in neuroprotection for treating traumatic brain injury. Expert Opin. Invest. Drugs. 2006;15:1371–1381. doi: 10.1517/13543784.15.11.1371. [DOI] [PubMed] [Google Scholar]
- Chen J. Chopp M. Li Y. Neuroprotective effects of progesterone after transient middle cerebral artery occlusion in rat. J. Neurol. Sci. 1999;171:24–30. doi: 10.1016/s0022-510x(99)00247-6. [DOI] [PubMed] [Google Scholar]
- Corso P. Finkelstein E. Miller T. Fiebelkorn I. Zaloshnja E. Incidence and lifetime costs of injuries in the United States. Inj. Prev. 2006;12:212–218. doi: 10.1136/ip.2005.010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S.M. Vanlandingham J.W. Stein D.G. Tapered progesterone withdrawal promotes long-term recovery following brain trauma. Exp. Neurol. 2006a;200:378–385. doi: 10.1016/j.expneurol.2006.02.137. [DOI] [PubMed] [Google Scholar]
- Cutler S.M. Pettus E.H. Hoffman S.W. Stein D.G. Tapered progesterone withdrawal enhances behavioral and molecular recovery after traumatic brain injury. Exp. Neurol. 2005;195:423–429. doi: 10.1016/j.expneurol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Cutler S.M. Vanlandingham J.W. Murphy A.Z. Stein D.G. Slow-release and injected progesterone treatments enhance acute recovery after traumatic brain injury. Pharmacol. Biochem. Behav. 2006b;84:420–428. doi: 10.1016/j.pbb.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Djebaili M. Hoffman S.W. Stein D.G. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Djebaili M. Guo Q. Pettus E.H. Hoffman S.W. Stein D.G. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J. Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Duvdevani R. Roof R.L. Fulop Z. Hoffman S.W. Stein D.G. Blood-brain barrier breakdown and edema formation following frontal cortical contusion: does hormonal status play a role? J. Neurotrauma. 1995;12:65–75. doi: 10.1089/neu.1995.12.65. [DOI] [PubMed] [Google Scholar]
- Fee D.B. Swartz K.R. Joy K.M. Roberts K.N. Scheff N.N. Scheff S.W. Effects of progesterone on experimental spinal cord injury. Brain Res. 2007;1137:146–152. doi: 10.1016/j.brainres.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Fukui S. Signoretti S. Dunbar J.G. Marmarou A. The effect of cyclosporin A on brain edema formation following experimental cortical contusion. Acta Neurochir. Suppl. 2003;86:301–303. doi: 10.1007/978-3-7091-0651-8_65. [DOI] [PubMed] [Google Scholar]
- Galani R. Hoffman S.W. Stein D.G. Effects of the duration of progesterone treatment on the resolution of cerebral edema induced by cortical contusions in rats. Restor. Neurol. Neurosci. 2001;18:161–166. [PubMed] [Google Scholar]
- Gibson C.L. Gray L.J. Bath P.M. Murphy S.P. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131:318–328. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- Gonzalez S.L. Labombarda F. Gonzalez Deniselle M.C. Guennoun R. Schumacher M. De Nicola A.F. Progesterone up-regulates neuronal brain-derived neurotrophic factor expression in the injured spinal cord. Neuroscience. 2004;125:605–614. doi: 10.1016/j.neuroscience.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Gonzalez S.L. Labombarda F. Deniselle M.C. Mougel A. Guennoun R. Schumacher M. De Nicola A.F. Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J. Steroid Biochem. Mol. Biol. 2005;94:143–149. doi: 10.1016/j.jsbmb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Goss C.W. Hoffman S.W. Stein D.G. Behavioral effects and anatomic correlates after brain injury: a progesterone dose-response study. Pharmacol. Biochem. Behav. 2003;76:231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Grossman K.J. Stein D.G. Does endogenous progesterone promote recovery of chronic sensorimotor deficits following contusion to the forelimb representation of the sensorimotor cortex? Behav. Brain Res. 2000;116:141–148. doi: 10.1016/s0166-4328(00)00275-8. [DOI] [PubMed] [Google Scholar]
- Grossman K.J. Goss C.W. Stein D.G. Effects of progesterone on the inflammatory response to brain injury in the rat. Brain Res. 2004;1008:29–39. doi: 10.1016/j.brainres.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Guennoun R. Meffre D. Labombarda F. Gonzalez S.L. Deniselle M.C. Stein D.G. De Nicola A.F. Schumacher M. The membrane-associated progesterone-binding protein 25-Dx: expression, cellular localization and up-regulation after brain and spinal cord injuries. Brain Res. Rev. 2008;57:493–505. doi: 10.1016/j.brainresrev.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Guo Q. Sayeed I. Baronne L.M. Hoffman S.W. Guennoun R. Stein D.G. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp. Neurol. 2006;198:469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- He J. Evans C.O. Hoffman S.W. Oyesiku N.M. Stein D.G. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp. Neurol. 2004;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Jiang N. Chopp M. Stein D. Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735:101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- Jones N.C. Constantin D. Prior M.J. Morris P.G. Marsden C.A. Murphy S. The neuroprotective effect of progesterone after traumatic brain injury in male mice is independent of both the inflammatory response and growth factor expression. Eur. J. Neurosci. 2005;21:1547–1554. doi: 10.1111/j.1460-9568.2005.03995.x. [DOI] [PubMed] [Google Scholar]
- Kumon Y. Kim S.C. Tompkins P. Stevens A. Sakaki S. Loftus C.M. Neuroprotective effect of postischemic administration of progesterone in spontaneously hypertensive rats with focal cerebral ischemia. J. Neurosurg. 2000;92:848–852. doi: 10.3171/jns.2000.92.5.0848. [DOI] [PubMed] [Google Scholar]
- Labombarda F. Gonzalez S.L. Deniselle M.C. Vinson G.P. Schumacher M. De Nicola A.F. Guennoun R. Effects of injury and progesterone treatment on progesterone receptor and progesterone binding protein 25-Dx expression in the rat spinal cord. J. Neurochem. 2003;87:902–913. doi: 10.1046/j.1471-4159.2003.02055.x. [DOI] [PubMed] [Google Scholar]
- Labombarda F. Gonzalez S. Gonzalez Deniselle M.C. Garay L. Guennoun R. Schumacher M. De Nicola A.F. Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J. Neurotrauma. 2006;23:181–192. doi: 10.1089/neu.2006.23.181. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Wald M.M. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Marklund N. Bakshi A. Castelbuono D.J. Conte V. Mcintosh T.K. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr. Pharm. Des. 2006;12:1645–1680. doi: 10.2174/138161206776843340. [DOI] [PubMed] [Google Scholar]
- Mcintosh T.K. Juhler M. Wieloch T. Novel pharmacologic strategies in the treatment of experimental traumatic brain injury: 1998. J. Neurotrauma. 1998;15:731–769. doi: 10.1089/neu.1998.15.731. [DOI] [PubMed] [Google Scholar]
- Michel R.P. Cruz-Orive L.M. Application of the Cavalieri principle and vertical sections method to lung: estimation of volume and pleural surface area. J. Microsc. 1988;150:117–136. doi: 10.1111/j.1365-2818.1988.tb04603.x. [DOI] [PubMed] [Google Scholar]
- Mouton P. Priciples and Practices of Unbiased Stereology: An Introduction for Bioscientists. Johns Hopkins University Press; Baltimore: 2002. [Google Scholar]
- Murphy S.J. Littleton-Kearney M.T. Hurn P.D. Progesterone administration during reperfusion, but not preischemia alone, reduces injury in ovariectomized rats. J. Cereb. Blood Flow Metab. 2002;22:1181–1188. doi: 10.1097/01.WCB.0000037990.07114.07. [DOI] [PubMed] [Google Scholar]
- Murphy S.J. Traystman R.J. Hurn P.D. Duckles S.P. Progesterone exacerbates striatal stroke injury in progesterone-deficient female animals. Stroke. 2000;31:1173–1178. doi: 10.1161/01.str.31.5.1173. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.B. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady S.M. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. Mcintosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G. Temkin N. Tuma R. Wade C. Walker M.D. Weinrich M. Whyte J. Wilberger J. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C.A. Cernak I. Johnson F. Vink R. Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp. Neurol. 2007;205:145–153. doi: 10.1016/j.expneurol.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. 4th. Academic Press; San Diego: 1998. [Google Scholar]
- Pettus E.H. Wright D.W. Stein D.G. Hoffman S.W. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049:112–119. doi: 10.1016/j.brainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Robertson C.L. Mitochondrial dysfunction contributes to cell death following traumatic brain injury in adult and immature animals. J. Bioenerg. Biomembr. 2004;36:363–368. doi: 10.1023/B:JOBB.0000041769.06954.e4. [DOI] [PubMed] [Google Scholar]
- Robertson C.L. Puskar A. Hoffman G.E. Murphy A.Z. Saraswati M. Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp. Neurol. 2006;197:235–243. doi: 10.1016/j.expneurol.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Roof R.L. Duvdevani R. Stein D.G. Progesterone treatment attenuates brain edema following contusion injury in male and female rats. Restor. Neurol. Neurosci. 1992;4:425–427. doi: 10.3233/RNN-1992-4608. [DOI] [PubMed] [Google Scholar]
- Roof R.L. Duvdevani R. Braswell L. Stein D.G. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp. Neurol. 1994;129:64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- Roof R.L. Duvdevani R. Heyburn J.W. Stein D.G. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp. Neurol. 1996;138:246–251. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- Rosenblum W.I. Cytotoxic edema: monitoring its magnitude and contribution to brain swelling. J. Neuropathol. Exp. Neurol. 2007;66:771–778. doi: 10.1097/nen.0b013e3181461965. [DOI] [PubMed] [Google Scholar]
- Royo N.C. Shimizu S. Schouten J.W. Stover J.F. Mcintosh T.K. Pharmacology of traumatic brain injury. Curr. Opin. Pharmacol. 2003;3:27–32. doi: 10.1016/s1471-4892(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Dhillon H.S. Creatine-enhanced diet alters levels of lactate and free fatty acids after experimental brain injury. Neurochem. Res. 2004;29:469–479. doi: 10.1023/b:nere.0000013753.22615.59. [DOI] [PubMed] [Google Scholar]
- Shao C. Roberts K.N. Markesbery W.R. Scheff S.W. Lovell M.A. Oxidative stress in head trauma in aging. Free Radic. Biol. Med. 2006;41:77–85. doi: 10.1016/j.freeradbiomed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Shear D.A. Galani R. Hoffman S.W. Stein D.G. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp. Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- Stein D.G. Wright D.W. Kellermann A.L. Does progesterone have neuroprotective properties? Ann. Emerg. Med. 2008;51:164–172. doi: 10.1016/j.annemergmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Stocchetti N. Colombo A. Ortolano F. Videtta W. Marchesi R. Longhi L. Zanier E.R. Time course of intracranial hypertension after traumatic brain injury. J. Neurotrauma. 2007;24:1339–1346. doi: 10.1089/neu.2007.0300. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Bruce-Keller A.J. Rabchevsky A.G. Christakos S. Clair D.K. Mattson M.P. Scheff S.W. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J. Neurosci. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenovuo O. Pharmacological enhancement of cognitive and behavioral deficits after traumatic brain injury. Curr. Opin. Neurol. 2006;19:528–533. doi: 10.1097/WCO.0b013e328010944f. [DOI] [PubMed] [Google Scholar]
- Thurman D.J. Alverson C. Dunn K.A. Guerrero J. Sniezek J.E. Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Unterberg A.W. Stover J. Kress B. Kiening K.L. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Unterberg A.W. Stroop R. Thomale U.W. Kiening K.L. Pauser S. Vollmann W. Characterisation of brain edema following “controlled cortical impact injury” in rats. Acta Neurochir. Suppl. 1997;70:106–108. doi: 10.1007/978-3-7091-6837-0_33. [DOI] [PubMed] [Google Scholar]
- Vanlandingham J.W. Cutler S.M. Virmani S. Hoffman S.W. Covey D.F. Krishnan K. Hammes S.R. Jamnongjit M. Stein D.G. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology. 2006;51:1078–1085. doi: 10.1016/j.neuropharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Wright D.W. Bauer M.E. Hoffman S.W. Stein D.G. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J. Neurotrauma. 2001;18:901–909. doi: 10.1089/089771501750451820. [DOI] [PubMed] [Google Scholar]
- Wright D.W. Kellermann A.L. Hertzberg V.S. Clark P.L. Frankel M. Goldstein F.C. Salomone J.P. Dent L.L. Harris O.A. Ander D.S. Lowery D.W. Patel M.M. Denson D.D. Gordon A.B. Wald M.M. Gupta S. Hoffman S.W. Stein D.G. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann. Emerg. Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]