Abstract
Previous studies have demonstrated the benefits of cell seeding in the construction of tissue-engineered vascular grafts (TEVG). However, seeding methods are diverse and no method is clearly superior in either promoting seeding efficiency or improving long-term graft function. As we head into an era during which a variety of different TEVG are under investigation in clinical trials around the world, it is important to consider the regulatory issues surrounding the translation of these technologies. In this review, we summarize important advances in the field of vascular tissue engineering, with particular attention on cell-seeding techniques for TEVG development and special emphasis placed on regulatory issues concerning the clinical translation of these various methods.
Introduction
Although native vessels remain the gold standard in revascularization procedures, such tissues are not always available secondary to limited availability or concurrent vascular disease. In these cases, synthetic materials such as expanded polytetrafluoroethylene (Gortex®, W.L. Gore & Associates, Newark, DE) and polyethylene terephthalate (Dacron®, Dupont, Wilmington, DE) have been used with great success as vascular conduits when the graft diameter exceeds 6 mm. Results have been poor, however, with grafts less than 6 mm in diameter, secondary to the development of thrombi and intimal hyperplasia.1–3 In recent years, the development of tissue-engineered vascular grafts (TEVG) has emerged as a promising, clinically applicable alternative to current synthetic grafts.4,5
Tissue engineering has been described as “an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ.”6 A TEVG may be developed by various methods, including construction from cell sheets, or seeding cells onto either biodegradable or decellularized scaffolds. Additionally, the uses of a biological glue or sealant have proven to be useful adjunct to these techniques.7–12 Although several studies have demonstrated that cell seeding promotes TEVG patency and longevity,13–15 in most cases, the exact mechanism by which these cells promote neovessel formation is not fully understood. To date, no one has demonstrated a correlation between the number of cells seeded and results in long-term graft function. However, cell seeding has proven to be a critical component in the construction of tissue-engineered grafts (regardless of the cell type used) and therefore warrants further investigation to optimize this process.
Several TEVG are already in the stage of clinical trials around the world.4,16 Other TEVG are in advanced stages of preclinical development.8,11,13,17 The translation of these technologies from the bench to the clinical is under intense scrutiny by regulatory agencies, and researchers are working to optimize the safety and efficacy of these products. One area of intense investigation is the seeding methods used to create a TEVG.
A diverse array of seeding techniques has developed over the past three decades, but many of these techniques are lengthy and limit clinical applicability.18,19 For the mass production of small-diameter, off-the-counter vascular grafts, considered to be the “Holy Grail” of vascular tissue engineering,20,21 it is necessary to develop a cost-effective, reliable, and efficient seeding technique.22 This technique can be either using seeding a scaffold before implantation11,13,23 or by using manufactured preseeded scaffolds.24 To date, the most common method used in the construction of TEVG is static seeding, in which a concentrated cell suspension is passively introduced on a scaffold. This technique has several limitations that result in low seeding efficiency and minimal cell penetration of scaffold walls. To overcome some of these disadvantages, alternate seeding techniques have been developed, including dynamic, magnetic, vacuum, electrostatic, and centrifugal seeding. Current TEVG seeding methods must be scrutinized in a historical context to determine which factors alone or in combination will aid the development of a readily available artificial vascular conduit.
Cell-Seeding Techniques
Passive seeding (static seeding and gravitational seeding)
Passive seeding is the simplest and most widely used method of cell delivery, but this is also the least efficient approach.13 This method involves pipetting a cell suspension directly into the lumen of the scaffold or onto the outside of the scaffold (Fig. 1). After application of the cell suspension to the graft, the construct is incubated for several minutes and then placed in a Petri dish with media and incubated to allow for cell attachment. Statically seeded cells are incubated with the scaffold for several hours to several days with the goal of maximizing seeding efficiency. This seeding technique yields seeding efficiencies of approximately 10%–25%.25
FIG. 1.
Static seeding. Cell suspension is pipetted directly into the lumen of the scaffold or onto the outside of the scaffold.
Static seeding, despite being a simple method for cell seeding, presents a number of disadvantages.13 One such limitation is the difficulty in achieving a uniform endothelial cell (EC) layer across the entire surface of the scaffold, unless the scaffold position is shifted regularly or culture for a prolonged period.26 Further, when using other types of cells (smooth muscle cells [SMC] or bone marrow cells), the penetration will depend on the scaffold material and porosity.27–29 Additionally, short seeding incubation times (less than 2 h) prevent seeded ECs from reaching a stable, mature, morphologic state, which can result in cell loss upon implantation.13 Although longer cell seeding incubation times allow the cells to obtain a more mature morphology and thus increase efficient cell attachment, this may lead to higher incidences of contamination or unfavorable cellular changes. Although seeding efficiency can be increased with longer incubation times, this method is still inferior compared to other dynamic techniques.30 More importantly, the high operator dependence of this approach may refrain regulatory agencies from approval.
Static seeding with biological glues
A variation of static seeding involves coating the scaffold with one or several commercially available biological glues such as fibrin or fibronectin. The motivation underlying the use of such glues is either to trap cells on the scaffold or to facilitate cell attachment to the matrix or scaffold. Biological glues can mimic extracellular matrix (ECM) and encourage more cells to stably adhere to the scaffold.31 Fibronectin is the most commonly described agent, but other adhesive coatings have been used as well, including fibrin, collagen, laminin, and plasma.13,32 A more empiric approach involves using ligands specific to particular cell types, such as, biomimetic surfactant polymers derived from one of the heparin-binding domains of fibronectin that promote EC adhesion and growth on vascular biomaterials.31 Another approach seems to be the incorporation of cell–matrix signals into a matrix, or to adhere them onto a scaffold by using the adhesive properties of tropoelastin and fibrillins used to coat the scaffolds to enhance luminal EC attachment, and to regulate SMC attachment and function, emphasizing the importance of matrix biology and its components, crucial for the development of TEVG.12 The various coating methods that have been evaluated range from simply dipping the scaffold into the glue or by applying pulsatile perfusion of the scaffold with the biological glue.13
One of the greatest concerns when using these coatings is the potential for thrombogenicity as the glue and cells might not coat the entire surface of the graft, thus leaving uncovered areas prone to platelet adhesion and clot formation. In studies in which fibronectin glue was used to coat scaffolds, experimental animals were treated with anticoagulants to reduce acute graft complications such as thromboembolic events and subsequent graft failure.33 The use of anticoagulants limits the clinical utility of such grafts, as these agents are often associated with significant morbidity and mortality secondary to the increased risk of hemorrhage and bleeding complications. Although biologic glues do have their associated limitations when used in static seeding, these glues may be applicable in increasing seeding efficiency when seeding is conducted by other means and uniform coverage may be attained. It is important for the tissue engineering research community to also provide evidence regarding the safety of these products for the use in human trials, including the origin and source of these substances and the potential deleterious effects to cells.
Dynamic seeding
This method of cell seeding uses techniques that increase cell seeding efficiency, uniformity, and/or penetration of the scaffold. The two main methods of dynamic seeding include those that induce hydrostatic forces, namely, rotational seeding,17,34 and those that create pressure differentials, namely, vacuum seeding.23,35
Rotational systems
Rotational seeding encompasses a diverse array of systems in which a graft is rotated in a cell/medium suspension or spun along with a cell/medium suspension. Seeding conditions range from 0.2 up to 500 rpm with culture periods as low as 12 h and as high as 72 h. Seeding efficiency under these conditions has ranged from 38% to 90%.25,34 Systems using up to 2500 rpm36 have been reported and are subclassified as centrifugal seeding (Fig. 2). Such high-speed rotational (centrifugal) systems have been shown to increase both seeding efficiency and graft wall penetration. Although centrifugal seeding maintains cell viability, concerns have been raised about the effect on cell morphology. Conversely, low-speed rotational systems have not shown an effect on cell morphology. Unfortunately, low rotational systems often require an increased seeding time (∼24 h) and can result in a reduced seeding efficiency at lower cell concentrations.37 The relatively long seeding time associated with rotational seeding limits its practicality when considering same-day seeding/implanting procedures.
FIG. 2.
Rotational seeding. Scaffolds fixed to needle are placed in a spinner flask with cell suspension. The rotation of the medium within the spinner flask drives cells into the scaffold.
Vacuum systems
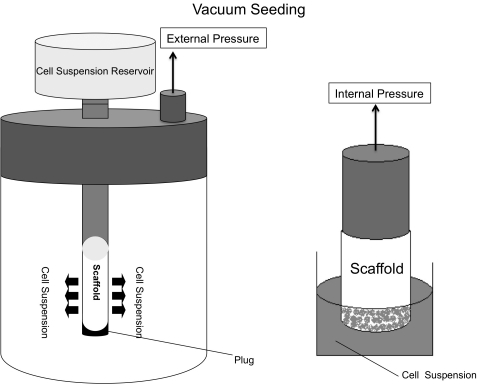
Pressure differential systems have been studied as a seeding method since the mid-1980s.38 They rely on either internal35 or external23,39 vacuum pressure to force a cell suspension through the micropores of a TEVG (Fig. 3). Pressure differential systems are an extremely rapid means of cell seeding with seeding efficiencies ranging from 60% to 90%. Moreover, the simplicity of some vacuum apparatuses allows for their use as an inexpensive disposable seeding device, reducing the risk of contamination. To date, however, there have been no in vivo studies that address concerns regarding adverse effects on cell morphology and viability when using this approach.
FIG. 3.
Vacuum/pressure seeding. A cell suspension is forced through a scaffold by either internal pressure or external vacuum pressure. As cells travel through the scaffold, they become lodged in the pores and thus seed the graft.
Sheet-based cell seeding
Scientists have developed a methodology to engineer tissue grafts that does not rely on foreign material. One technique of creating congruous sheets of cells employs a modified polystyrene tissue culture plate that is coated with a covalently linked temperature responsive polymer, poly(N-isoproplyacrylamide).40 This technique allows the investigator to manipulate and remove an intact sheet of cells without the need to use digestive enzymes to break cellular attachments.41,42
Seeking to develop a fully constructed vascular graft, L'Heureux et al. describe a different methodology of creating cell sheets in which they induce ECM formation using cultured SMCs and fibroblasts. Cells are grown for 30 days in a supplemented medium, and form cells sheets in a standard culture flask. Cell sheets are then wrapped around a cylindrical mandrel to produce concentric sheet layers, which are reported to adhere to one another.7 L'Heureux et al. have tested various production methodologies to construct their vascular grafts, notably using tubularized fibroblast sheets after 8 weeks of maturation as an inner membrane, and subsequently decellularized. After this, the adventitia was formed after adding a second outer fibroblast cell sheet layer for 10 weeks, and finally EC were seeded in the lumen of the living TEBV.43
The main advantages of this seeding methodology come from the creation of completely autologous vascular grafts, eliminating the need for foreign material that acts like an ECM.40,41,43 These autologous grafts can be used in both the venous and arterial system, providing the necessary strength to withstand arterial pressures, which, along with morphological maturation and cell retention of SMC and EC, reduces thrombogenicity and the need for anticoagulation therapy upon implantation. Currently, tubularized cell sheets are under clinical investigation in several countries, with preliminary studies demonstrating clinical utility and effectiveness.16,44 L'Heureux et al. describe a minimum of 3 months for the creation of their vascular grafts, and temperature-sensitive culture plates also require days to weeks for full-confluent cell sheets to develop, although this will be variable depending on the cell type.7 Prolonged culture times would preclude an off-the-shelf use of this technology in addition to carrying a significant risk of contamination or infection.
Electrostatic cell seeding
This method refers to the use and manipulation of the electrostatic properties of vascular scaffolds with the goal of overcoming the two main concerns of EC seeding: morphological maturation before implantation and cell retention after implantation. A temporary positive surface charge is induced on the typically negatively charged expanded polytetrafluoroethylene graft luminal surface to enhance EC adhesion and maturation. Exposure of cells to this system for only 16 min led to seeding efficiencies of up to 90%.13,45
Among the numerous advantages of this technique is the ability to achieve morphological maturation before implantation as demonstrated by scanning electron microscopy.45,46 Additionally, cell retention was confirmed after 1 week of implantation as shown by fluorescent labeling, and a reduced incidence of thrombosis was achieved since the temporary glue disappears when the electrostatic charge is removed. More importantly, this technique of cell seeding had a significant (p < 0.001) impact on acute healing of the specimens, reducing the migration of SMCs and likely decreasing the hyperplastic response.47 On the other hand, there have not been any studies demonstrating the short- and long-term effects of this method (important before clinical use), and the system should be tested on other scaffolds, including biodegradable constructs.
Magnetic cell seeding
This seeding technique involves the use of magnetic forces to increase cell-seeding efficiency. The basic principle of this system is the use of a magnet to attract nanoparticles attached to or inside of seeded cells. Currently, there are two standard approaches to magnetic seeding. The first method involves the use of superparamagnetic, monosized polymer particles (Dynabeads®, DynaBiotec, Oslo, Norway), capable of specifically binding to a desired cell, molecule, or protein. Dynabeads-labeled cells can then be seeded onto a TEVG via a temporary magnetic field. This seeding method can be completed in less than an hour and is followed by 12-h culture. As with the former method, the seeding efficiency using Dynabeads approaches 99%.48,49 The second magnetic seeding method uses cationic liposomes containing superparamagnetic iron oxide particles that can be incorporated into cells. Superparamagnetic iron oxide particle–labeled cells are then attracted and seeded onto a graft containing a removable magnet within its lumen. After a few minutes of magnetic exposure and a 24-h culture period, seeding efficiencies of over 90% were noted.50,51
The advantages of magnetic cell seeding include rapid graft production and reproducible results that are essential for constructing grafts for clinical applications. Additionally, increased cell adhesion with architecture that mimics that of the native vessels is advantageous when constructing such grafts. Although no detrimental short-term effects were found with regard to cell viability when low numbers of particles per cell were used, studies have shown that cell proliferation was reduced when using >100 μm of cationic liposomes per cell50 and >50 beads per cell when using the Dynabeads technology.48 Equally important, the long-term complications of magnetic seeding technologies need to be assessed before clinical application of grafts constructed by these methods. For regulatory purposes a complete panel of tests will be necessary to show cell viability, cell morphology, and also fate of this particles once these cells are replaced (including possible adverse effects in other tissues).
Photopolymerized hydrogels for cell seeding
Photopolymerizable polyethylene glycol derivatives as hydrogel tissue engineering scaffolds have been created to mimic the properties of natural ECM.52 This technology can be used in direct contact with cells and tissues without adverse effects.52,53 Photopolymerization of a cell suspension in an aqueous monomer solution results in a homogeneously seeded hydrogel scaffold.24 This can be accomplished either ex vivo or in situ and constructs can be molded. Advantages of this technique include the manipulation of cell-adhesive characteristics, degradation via hydrolytic or enzymatic processed, and the elution of growth factors to meet the requirements of a given tissue engineering or regeneration application.24,52 From the regulatory stand point, this technique shows promising in vitro results regarding seeding capability, cell viability, and cell proliferation, despite the exposure ultraviolet light during the procedure. A primary concern is whether this hydrogel scaffold possesses the necessary characteristics to withstand the native vascular environment (venous and arterial pressure, thrombogenicity tendencies, etc). Also to be investigated is the scaffold's potential for in vivo ECM formation and cellular infiltration.
Hybrid systems
This method of cell seeding refers to hybrid techniques in which multiple systems of seeding are combined such as rotational systems, pressure differentials system, vacuums and bioreactor systems,54 or combinations of other techniques.55
Rotational vacuum seeding
The rotational vacuum seeding device combines several well-established techniques to produce an efficient, automated, and reproducible seeding methodology.55 A cylindrical extrusion nozzle, fabricated to match the internal diameter of the tubular scaffold, delivers the cell-enriched medium onto, and throughout, the scaffold. The nozzle is attached to an articulating arm that travels the full length of the tubular construct. The scaffold is rotated around the articulating extrusion nozzle, inside an acrylic vacuum chamber.
The advantages of this method are its ability to deliver cells throughout the entire thickness and length of a construct in a reproducible and automated process lending itself well to clinical applications. With the extrusion nozzle approximating very closely with the internal surface of the graft, the cells are better able to penetrate the graft. Further, because the graft is rotating under vacuum there is a pressure gradient to further enhance seeding while the rotation helps to uniformly distribute the cells circumferentially. Seeding efficiency with this methodology is dependent on the pore size of the construct as well as the flow rate of the cell suspension. Soletti et al. reported seeding efficiencies raging from 60% to 90%, depending on the construct used, noting that improved efficiencies were observed with smaller pore sizes and lower flow rates.55
The limitations of this method are its complexity. When successful, it effectively combines several methods of cell seeding resulting in increased efficiencies. However, as a number of methods are incorporated, there is an increased risk of failure for any one method and subsequent compromise of seeding efforts. Also, the inability to adjust for different constructs once the device is fabricated limits its application when constructing grafts of varying lengths and diameters.
Perfusion bioreactor system for cell seeding
A bioreactor system can be applied when constructing a TEVG to mimic the physiologic conditions and biomechanical stresses that vessels and cells are constantly exposed to in vivo (fluid shear stresses, cyclic stretch, and hydrostatic pressures) to promote ECM formation, SMC and EC phenotypic differentiation, and anchoring.8,56,57 A variety of systems have been developed for supporting engineered tissue constructs, including custom-designed mock circulatory systems, pulsatile perfusion bioreactors, and other bioreactor systems that combine cell cultivation with high cell-seeding efficiency of 75%–94%.8,58,59 Despite these advantages, however, many perfusion bioreactor systems require a prolonged period of culture and perfusion, increasing the risk of bacterial and fungal contamination. Also, the use of xenogenic serum as part of the culture medium is a major obstacle for their clinical use.60,61 Moreover, the complexity of such bioreactors is not well suited for more clinical applications and the duration of graft construction limits the use of such constructs. To address the risk of bacterial and fungal contamination with these bioreactor systems, another group54 has created a highly sterile isolated cell culture setting, consisting of a gas supply that fits into a standard humidified incubator. These developments, however, have not addressed the complexity of such bioreactor systems and the prolonged culture times still required when using the systems. This technology has shown promising preclinical animal results regarding safety and reproducibility, but several issues (cell source and use of xenogenic serum) will have to be addressed to meet possible requirements established by regulatory instances.
Quantitative Measures of Seeding Efficiency
Multiple methods for quantifying seeding efficiency are currently available. This creates a problem when trying to compare the different cell-seeding techniques, where a unified method within the field of tissue engineering could create a standardize solution for this important factor. Once a scaffold is seeded by any one of the methods described, seeding efficiency can be determined by quantifying the number of cells that not only have attached to but also have penetrated the construct. This can be done by a variety of methods (Table 1), but can be broken down into two categories: observation and manual counting, or indirect quantification using assays that measure a particular function of metabolically active cells. While the former method provides indisputable evidence of cell attachment, it can be difficult to observe cell attachment deeper than the superficial graft wall. Alternately, indirect assays account for an entire cell population, and although not as accurate as observation, they are generally much faster and can provide the added benefit of distinguishing living cells from dead cells that may have become trapped in the graft matrix.
Table 1.
Quantification Methods Used in Cell Seeding
| Methods | Advantages | Disadvantages |
|---|---|---|
| Hemocytometer | Simple, fast | Not accurate, overestimates cell seeding |
| Histology | Real counting, best overall | Tedious, time consuming, good different cell lines if labeled |
| SEM | Scaffold surface analysis | Only good for scaffold surfaces |
| DNA | Accurate, reliable | Changes when comparing different cell lines or different donor |
| MTS, MTT, WST-1 | Quantitative and qualitative | Changes when comparing different cell lines or different donor |
SEM, scanning electron microscopy; MTS, (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium); MTT, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide); WST-1, (4-(3-4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio)-1,3-benzenedisulfonate).
Hemocytometer
This is a simple approach that involves counting the number of remaining cells in the suspension after seeding, and then calculating number of possible seeded (or at least retained) cells. This approach is simple but quite inaccurate.
Quantitative histology
This method allows the operator to count the number of attached cells per high-power field of seeded scaffold, which has been sectioned. Immunostaining for von Willebrand factor or other cell markers can enable a more precise count of ECs or other specific cell types. This approach provides a more accurate estimate of seeding efficiency but is very tedious and time consuming.
Scanning electron microscopy
This approach enables the operator to obtain magnified images of the graft surface and count the cells present.62 One limitation, however, is that cells that have infiltrated between the fibers and pores of the scaffold are not accounted for by this approach.
Picogreen (DNA) detection assay
This method allows the determination of graft cellularity by measuring DNA content on a sonicated scaffold section (Molecular Probes, Eugene, OR). Once a standard curve is generated by using a known number of the same types of cells and determining the DNA content correlating to this cell number, this curve is then used to determine cell seeding efficiency of the graft by relating the DNA content measured to the cell number obtained during seeding.25 The picogreen DNA detection assay is useful and accurate when the same cell line is used to obtain both the standard curve and to seed the graft.
Cell counting from MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide) and MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) metabolic activity measurements of cell viability
These methods measure cellular metabolic activity by determining enzymatic reduction in the mitochondria.63,64 WST-1 (4-(3-4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio)-1,3-benzenedisulfonate) assays measure the metabolic activity of viable cells, cell proliferation, cell viability, or cytotoxicity and are based on the reduction of WST-1 by viable cells.49 All methods can be used for cellular seeding quantification based on a standard curve.
Assessment of Cell Viability After Seeding
After cell seeding and quantification of seeding efficiency, cell viability must be assessed to ensure that the cells that are attached to the scaffold will have the ability to contribute to the remodeling and growth of the graft.
MTT assay
This assay measures the degree of substrate cleavage by absorbance.62 Since cleavage takes place only in functional mitochondria, this serves as an index of viable cells. MTS and WST-1 assays also can be used to further assess cell viability.
Live-dead assay
This test involves using a Calcein-AM/Ethidium-Homodimer stain (Molecular Probes) to assess for green (viable) cells and red (dead) cells using a confocal microscope.65
Discussion
Investigators in the field of vascular tissue engineering have focused on constructing tissue-engineered vessels that have mechanical and structural properties similar to those of native tissue. The primary goal of this field has been to create a nonthrombogenic, nonimmunogenic implantable graft comprised of a viable endothelium5 that is readily available for clinical application.19 This has been achieved through the use of biodegradable materials and newer cell-seeding techniques. Multiple scaffold materials and techniques have been implemented for the fabrication of such grafts, including a poly glycolic acid sleeve reinforced with a copolymer of ɛ- caprolactone and L-lactide,4 poly glycolic acid, poly-l-lactic acid (PLLA), hydrogels, collagen sheets, decellularized matrices, electrospun fibers, and mandrils of foreign body implanted into the peritoneum of animals.66 Building the scaffolds, however, is just the first step in developing TEVGs for clinical use. Cell seeding remains a more critical step in the construction of these grafts and thus has been a major focus of research.67
A broad spectrum of methods have been developed with varying degrees of complexity in an effort to achieve high levels of seeding efficiency of viable, morphologically undisturbed, functional cells (Table 2). These methods are developed in response to the unavailability of a simpler method that increases seeding efficiency and reduces prolonged periods of culture and its associated risks. Therefore, we believe that a single-step seeding approach would be the ultimate goal for clinical tissue engineering. To date, the seeding method with the highest level of seeding efficiency and most reproducible results is the bioreactor-based seeding,8 cell-sheet-based seeding,16 and the rotational-vacuum seeding.55 Unfortunately, the complexity and high fabrication costs may limit these approaches. Faster and simpler methods, such as magnetic seeding, lead to seeding efficiencies of >90%; however, they require prolonged culture times to promote morphological maturation and cellular retention onto the grafts, thus precluding them from off-the-shelf availability. Currently, all groups have demonstrated improved seeding efficiency with their methods, but no group has demonstrated the in vivo short- or long-term benefits of increased seeding efficiency when compared to static seeding as controls.
Table 2.
Comparison of Different Cell-Seeding Techniques: Advantages and Disadvantages
| Seeding technique | Static | Rotational (low speed) | Centrifugal (high speed) | Vacuum | Cell sheet based | Magnetic | Electrostatic | Hydrogel based | Rotational-vacuum | Bioreactor based |
|---|---|---|---|---|---|---|---|---|---|---|
| Efficiency | 10%–25% | 90% | 38% | 60%–90% | N/A | 90%–99% | 90% | Not shown | 60%–90% | 75%–94% |
| Quantification method | Hemocytometer, DNA assay | Hemocytometer | Picogreen, MTT assay | Picogreen, SEM, histology | Histology | WST-1 assay, hemocytometer | SEM | Picogreen, histology | Hemocytometer | Hemocytometer, DNA assay, MTT assay |
| Cell type | EC, SMC, BM-MNC | EC | BM-MNC | EC, MDSC, | EC, SMC, fibroblast | EC, SMC | EC | SMC, fibroblast | EC, MDSC, BMPC | EC, SMC, EPC, BMSC |
| Scaffold ingrowth | Minimal | None | Good | Gooda | N/A | Good | None | Good | Good | Excellent |
| Phenotype differentiation | None | None | None | Not shown | Yes | Yes | Yes | None | None | Yes |
| Matrix | ↓ Production | ↓ production | ↑ Production | ↓ Production | ↑↑ Production | ↑↑ Production | None | ↑↑ Production | None | ↑↑ Production |
| Reproducibility | Yesb | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes |
| Cost-effectiveness | ↑ | ↑↑↑ | ↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ |
| Clinical use | In vivo (human) | In vitro | In vitro | In vitro | In vivo (human) | In vitro | In vivo (animals) | In vitro | In vitro | In vivo (animals) |
| Advantages | Simple, rapid, well established | High seeding efficiency | High seeding efficiency, increased cell penetration | High seeding efficiency, rapid, simple | High pressure systems, such as fistulas, arteries | High seeding efficiency, simple, cell specific, fast | High seeding efficiency, fast | Scaffold characteristics manipulation | High seeding efficiency, reproducible | Good seeding efficiency, morphological maturation |
| Disadvantages | Variable, operator dependent | Slow (∼24 h), risk of contamination | Adverse effects on cell morphology | Culture for days to weeks, untested in vivo | Complex, prolong culture times | Adverse effects on cell proliferation, long-term adverse effects, prolonged culture time | Only for endothelial cells, long-term adverse effects unknown | UV light exposure, untested in vivo | Complex, costly | Complex, high risk of contamination, prolonged culture times (weeks) |
Graft material dependent.
Operator dependent (regulatory issue).
EC, endothelial cells; SMC, smooth muscle cells; BM-MNC, bone marrow mononuclear cells; MDSC, muscle-derived stem cells; BMPC, bone marrow progenitor cells; EPC, endothelial progenitor cells; BMSC, bone marrow stem cells; UV, ultraviolet.
N/A, non applicable.
The achievement of an optimal cell-seeding method may depend on the cell sources and clinical applications (arterial vs. venous systems). For arterial systems, TEVG construction requires the use of scaffolds with a slow intrinsic degradation property and a seeding technique that provides high seeding efficiency, excellent graft ingrowth, robust growth and organization of SMCs to withstand high pressures, and a confluent EC layer. Moreover, fluid shear stress plays a critical role in blood vessel formation via phenotypic differentiation of ECs. This has a central role in vascular remodeling and more importantly for tissue engineering promotes EC attachment and alignment. Finally, an intact endothelium provides a nonthrombogenic luminal surface.56,57,68 For this model, a more complex technique that requires longer culture periods may be required, as is the case of bioreactors8 and cell-sheet-based technology.7 On the other hand, for the venous system a simpler method that provides a high seeding efficiency and minimal culture time, and uses autologous cell of a neovessel may be used. In this case, a regenerate medicine approach may be appropriate (i.e., seeding with autologous cells that promote cell recruitment).4
Current Challenges
Despite the current research in cell-seeding techniques, many important questions remain unanswered. Foremost, is high efficiency cell seeding even necessary? Is there any clinical relevance in having high versus low seeding efficiencies? This has not been previously demonstrated in vivo and the impact of such a variable remains unknown. Also, it would be important to establish a single quantification method, to provide an accurate comparison when describing seeding efficiencies. Additionally, what is the optimal cell type(s) used in the construction of TEVG and what are the roles of seeded cells subtypes and their effect on TEVG development? Another important issue that has been partially addressed concerns the fate of the seeded cells; several groups have demonstrated that seeded cell density decreases over time, with replacement by host cells.15,69 Importantly, it remains unknown whether seeded cells are lost to cell death or if these cells are flushed out of the remodeling scaffold and embolizing to end organs. The answers to these questions lie in a basic understanding of the process that governs TEVG transformation into a functional blood vessel. Without such understanding cell seeding will continue to operate in the dark. The multitude of seeding methods outlined above is a reflection of the current uncertainty surrounding the fate and function of seeded cells.
We can use current technologies to answer both of these questions and change the way we think about TEVG construction, so that our field could progress further toward the development of a readily available vascular graft for clinical application. It is important to recognize that a cell-seeding technique that satisfies both clinical and regulatory demands is still to be established.
The field of vascular tissue engineering has reached a milestone where a group of experts can reach a consensus on seeding techniques and quantification methods determining the successful implementation of translational research. Organizations such as the National Institute of Standards and Technology could provide a standardized and cost-effective solution. For our group the optimal seeding technique requires a single-step, cost-effective, safe, and reliable method that increases seeding efficiency (with any scaffold size), decreases culture times, maximizes cell attachment (both seeded cells and host cells), avoids undue laboratory processing (xenoserums and risks of culture contamination), and, finally, promotes long-term graft patency.
Disclosure Statement
This work was supported by grants from the U.S. National Institutes of Health (K08HL83980-2) and Doris Duke Charitable Foundation Clinical Scientist Development Award Clinical trial evaluating the safety of the use of autologous TEVG in congenital heart surgery, and by generous gift from the Cytograft and Pall Corporation.
References
- 1.Veith F.J. Gupta S.K. Ascer E. White-Flores S. Samson R.H. Scher L.A. Towne J.B. Bernhard V.M. Bonier P. Flinn W.R. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3:104. doi: 10.1067/mva.1986.avs0030104. [DOI] [PubMed] [Google Scholar]
- 2.Sapsford R.N. Oakley G.D. Talbot S. Early and late patency of expanded polytetrafluoroethylene vascular grafts in aorta-coronary bypass. J Thorac Cardiovasc Surg. 1981;81:860. [PubMed] [Google Scholar]
- 3.Klinkert P. Post P.N. Breslau P.J. van Bockel J.H. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg. 2004;27:357. doi: 10.1016/j.ejvs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Shin'oka T. Matsumura G. Hibino N. Naito Y. Watanabe M. Konuma T. Sakamoto T. Nagatsu M. Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 5.L'Heureux N. Dusserre N. Marini A. Garrido S. de la Fuente L. McAllister T. Technology insight: the evolution of tissue-engineered vascular grafts—from research to clinical practice. Nat Clin Pract Cardiovasc Med. 2007;4:389. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- 6.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 7.L'Heureux N. Paquet S. Labbe R. Germain L. Auger F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 8.Niklason L.E. Gao J. Abbott W.M. Hirschi K.K. Houser S. Marini R. Langer R. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 9.Schaner P.J. Martin N.D. Tulenko T.N. Shapiro I.M. Tarola N.A. Leichter R.F. Carabasi R.A. Dimuzio P.J. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Xu C. Inai R. Kotaki M. Ramakrishna S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004;10:1160. doi: 10.1089/ten.2004.10.1160. [DOI] [PubMed] [Google Scholar]
- 11.Roh J.D. Nelson G.N. Brennan M.P. Mirensky T.L. Yi T. Hazlett T.F. Tellides G. Sinusas A.J. Pober J.S. Saltzman W.M. Kyriakides T.R. Breuer C.K. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials. 2008;29:1454. doi: 10.1016/j.biomaterials.2007.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephan S. Ball S.G. Williamson M. Bax D.V. Lomas A. Shuttleworth C.A. Kielty C.M. Cell-matrix biology in vascular tissue engineering. J Anat. 2006;209:495. doi: 10.1111/j.1469-7580.2006.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawlowski K.J. Rittgers S.E. Schmidt S.P. Bowlin G.L. Endothelial cell seeding of polymeric vascular grafts. Front Biosci. 2004;9:1412. doi: 10.2741/1302. [DOI] [PubMed] [Google Scholar]
- 14.Hashi C.K. Zhu Y. Yang G.Y. Young W.L. Hsiao B.S. Wang K. Chu B. Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A. 2007;104:11915. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho S.W. Lim S.H. Kim I.K. Hong Y.S. Kim S.S. Yoo K.J. Park H.Y. Jang Y. Chang B.C. Choi C.Y. Hwang K.C. Kim B.S. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.L'Heureux N. McAllister T.N. de la Fuente L.M. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357:1451. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- 17.Nasseri B.A. Pomerantseva I. Kaazempur-Mofrad M.R. Sutherland F.W. Perry T. Ochoa E., et al. Dynamic rotational seeding and cell culture system for vascular tube formation. Tissue Eng. 2003;9:291. doi: 10.1089/107632703764664756. [DOI] [PubMed] [Google Scholar]
- 18.Yow K.H. Ingram J. Korossis S.A. Ingham E. Homer-Vanniasinkam S. Tissue engineering of vascular conduits. Br J Surg. 2006;93:652. doi: 10.1002/bjs.5343. [DOI] [PubMed] [Google Scholar]
- 19.Parikh S.A. Edelman E.R. Endothelial cell delivery for cardiovascular therapy. Adv Drug Deliv Rev. 2000;42:139. doi: 10.1016/s0169-409x(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 20.Kakisis J.D. Liapis C.D. Breuer C. Sumpio B.E. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Conte M.S. The ideal small arterial substitute: a search for the Holy Grail? FASEB J. 1998;12:43. doi: 10.1096/fasebj.12.1.43. [DOI] [PubMed] [Google Scholar]
- 22.Ahsan T. Nerem R.M. Bioengineered tissues: the science, the technology, and the industry. Orthod Craniofac Res. 2005;8:134. doi: 10.1111/j.1601-6343.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 23.van Wachem P.B. Stronck J.W. Koers-Zuideveld R. Dijk F. Wildevuur C.R. Vacuum cell seeding: a new method for the fast application of an evenly distributed cell layer on porous vascular grafts. Biomaterials. 1990;11:602. doi: 10.1016/0142-9612(90)90086-6. [DOI] [PubMed] [Google Scholar]
- 24.West J.L. Modification of materials with bioactive peptides. Methods Mol Biol. 2004;238:113. doi: 10.1385/1-59259-428-x:113. [DOI] [PubMed] [Google Scholar]
- 25.Roh J.D. Nelson G.N. Udelsman B.V. Brennan M.P. Lockhart B. Fong P.M. Lopez-Soler R.I. Saltzman W.M. Breuer C.K. Centrifugal seeding increases seeding efficiency and cellular distribution of bone marrow stromal cells in porous biodegradable scaffolds. Tissue Eng. 2007;13:2743. doi: 10.1089/ten.2007.0171. [DOI] [PubMed] [Google Scholar]
- 26.Roh J.D. Brennan M.P. Lopez-Soler R.I. Fong P.M. Goyal A. Dardik A. Breuer C.K. Construction of an autologous tissue-engineered venous conduit from bone marrow-derived vascular cells: optimization of cell harvest and seeding techniques. J Pediatr Surg. 2007;42:198. doi: 10.1016/j.jpedsurg.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 27.Mo X.M. Xu C.Y. Kotaki M. Ramakrishna S. Electrospun P(LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25:1883. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Ravi S. Qu Z. Chaikof E.L. Polymeric materials for tissue engineering of arterial substitutes. Vascular. 2009;17(Suppl 1):S45. doi: 10.2310/6670.2008.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma P.X. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim B.S. Putnam A.J. Kulik T.J. Mooney D.J. Optimizing seeding and culture methods to engineer smooth muscle tissue on biodegradable polymer matrices. Biotechnol Bioeng. 1998;57:46. [PubMed] [Google Scholar]
- 31.Sagnella S. Anderson E. Sanabria N. Marchant R.E. Kottke-Marchant K. Human endothelial cell interaction with biomimetic surfactant polymers containing peptide ligands from the heparin binding domain of fibronectin. Tissue Eng. 2005;11:226. doi: 10.1089/ten.2005.11.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salacinski H.J. Tiwari A. Hamilton G. Seifalian A.M. Cellular engineering of vascular bypass grafts: role of chemical coatings for enhancing endothelial cell attachment. Med Biol Eng Comput. 2001;39:609. doi: 10.1007/BF02345431. [DOI] [PubMed] [Google Scholar]
- 33.Ramalanjaona G. Kempczinski R.F. Rosenman J.E. Douville E.C. Silberstein E.B. The effect of fibronectin coating on endothelial cell kinetics in polytetrafluoroethylene grafts. J Vasc Surg. 1986;3:264. doi: 10.1067/mva.1986.avs0030264. [DOI] [PubMed] [Google Scholar]
- 34.Hsu S.H. Tsai I.J. Lin D.J. Chen D.C. The effect of dynamic culture conditions on endothelial cell seeding and retention on small diameter polyurethane vascular grafts. Med Eng Phys. 2005;27:267. doi: 10.1016/j.medengphy.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Williams C. Wick T.M. Perfusion bioreactor for small diameter tissue-engineered arteries. Tissue Eng. 2004;10:930. doi: 10.1089/1076327041348536. [DOI] [PubMed] [Google Scholar]
- 36.Godbey W.T. Hindy S.B. Sherman M.E. Atala A. A novel use of centrifugal force for cell seeding into porous scaffolds. Biomaterials. 2004;25:2799. doi: 10.1016/j.biomaterials.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 37.Vunjak-Novakovic G. Obradovic B. Martin I. Bursac P.M. Langer R. Freed L.E. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14:193. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- 38.Kempczinski R.F. Rosenman J.E. Pearce W.H. Roedersheimer L.R. Berlatzky Y. Ramalanjaona G. Endothelial cell seeding of a new PTFE vascular prosthesis. J Vasc Surg. 1985;2:424. [PubMed] [Google Scholar]
- 39.Nieponice A. Soletti L. Guan J. Deasy B.M. Huard J. Wagner W.R. Vorp D.A. Development of a tissue-engineered vascular graft combining a biodegradable scaffold, muscle-derived stem cells and a rotational vacuum seeding technique. Biomaterials. 2008;29:825. doi: 10.1016/j.biomaterials.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu T. Yamato M. Kikuchi A. Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24:2309. doi: 10.1016/s0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 41.Yamato M. Okuhara M. Karikusa F. Kikuchi A. Sakurai Y. Okano T. Signal transduction and cytoskeletal reorganization are required for cell detachment from cell culture surfaces grafted with a temperature-responsive polymer. J Biomed Mater Res. 1999;44:44. doi: 10.1002/(sici)1097-4636(199901)44:1<44::aid-jbm5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 42.Okano T. Yamada N. Okuhara M. Sakai H. Sakurai Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials. 1995;16:297. doi: 10.1016/0142-9612(95)93257-e. [DOI] [PubMed] [Google Scholar]
- 43.L'Heureux N. Dusserre N. Konig G. Victor B. Keire P. Wight T.N. Chronos N.A. Kyles A.E. Gregory C.R. Hoyt G. Robbins R.C. McAllister T.M. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAllister T.N. Maruszewski M. Garrido S.A. Wystrychowski W. Dusserre N. Marini A. Zagalski K. Fiorillo A. Avila H. Manglano X. Antonelli J. Kocher A. Zembala M. Cierpka L. de la Fuente L.M. L'Heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 45.Bowlin G.L. Meyer A. Fields C. Cassano A. Makhoul R.G. Allen C. Rittgers S.E. The persistence of electrostatically seeded endothelial cells lining a small diameter expanded polytetrafluoroethylene vascular graft. J Biomater Appl. 2001;16:157. doi: 10.1106/NCQT-JFV9-2EQ1-EBGU. [DOI] [PubMed] [Google Scholar]
- 46.Fields C. Cassano A. Allen C. Meyer A. Pawlowski K.J. Bowlin G.L. Rittgers S.E. Szycher M. Endothelial cell seeding of a 4-mm I.D. polyurethane vascular graft. J Biomater Appl. 2002;17:45. doi: 10.1177/0885328202017001861. [DOI] [PubMed] [Google Scholar]
- 47.Fields C. Cassano A. Makhoul R.G. Allen C. Sims R. Bulgrin J. Meyer A. Bowlin G.L. Rittgers S.E. Evaluation of electrostatically endothelial cell seeded expanded polytetrafluoroethylene grafts in a canine femoral artery model. J Biomater Appl. 2002;17:135. doi: 10.1106/088532802030556. [DOI] [PubMed] [Google Scholar]
- 48.Tiwari A. Punshon G. Kidane A. Hamilton G. Seifalian A.M. Magnetic beads (Dynabead) toxicity to endothelial cells at high bead concentration: implication for tissue engineering of vascular prosthesis. Cell Biol Toxicol. 2003;19:265. doi: 10.1023/b:cbto.0000004929.37511.ed. [DOI] [PubMed] [Google Scholar]
- 49.Perea H. Aigner J. Hopfner U. Wintermantel E. Direct magnetic tubular cell seeding: a novel approach for vascular tissue engineering. Cells Tissues Organs. 2006;183:156. doi: 10.1159/000095989. [DOI] [PubMed] [Google Scholar]
- 50.Ito A. Hayashida M. Honda H. Hata K. Kagami H. Ueda M. Kobayashi T. Construction and harvest of multilayered keratinocyte sheets using magnetite nanoparticles and magnetic force. Tissue Eng. 2004;10:873. doi: 10.1089/1076327041348446. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu K. Ito A. Arinobe M. Murase Y. Iwata Y. Narita Y. Kagami H. Ueda M. Honda H. Effective cell-seeding technique using magnetite nanoparticles and magnetic force onto decellularized blood vessels for vascular tissue engineering. J Biosci Bioeng. 2007;103:472. doi: 10.1263/jbb.103.472. [DOI] [PubMed] [Google Scholar]
- 52.Mann B.K. Gobin A.S. Tsai A.T. Schmedlen R.H. West J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 53.Schmedlen R.H. Elbjeirami W.M. Gobin A.S. West J.L. Tissue engineered small-diameter vascular grafts. Clin Plast Surg. 2003;30:507. doi: 10.1016/s0094-1298(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 54.Sodian R. Lemke T. Fritsche C. Hoerstrup S.P. Fu P. Potapov E.V. Hausmann H. Hetzer R. Tissue-engineering bioreactors: a new combined cell-seeding and perfusion system for vascular tissue engineering. Tissue Eng. 2002;8:863. doi: 10.1089/10763270260424222. [DOI] [PubMed] [Google Scholar]
- 55.Soletti L. Nieponice A. Guan J. Stankus J.J. Wagner W.R. Vorp D.A. A seeding device for tissue engineered tubular structures. Biomaterials. 2006;27:4863. doi: 10.1016/j.biomaterials.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 56.Topper J.N. Gimbrone M.A., Jr. Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today. 1999;5:40. doi: 10.1016/s1357-4310(98)01372-0. [DOI] [PubMed] [Google Scholar]
- 57.Resnick N. Yahav H. Shay-Salit A. Shushy M. Schubert S. Zilberman L.C. Wofovitz E. Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol. 2003;81:177. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 58.Zhao F. Ma T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: dynamic cell seeding and construct development. Biotechnol Bioeng. 2005;91:482. doi: 10.1002/bit.20532. [DOI] [PubMed] [Google Scholar]
- 59.Inoguchi H. Tanaka T. Maehara Y. Matsuda T. The effect of gradually graded shear stress on the morphological integrity of a HUVEC-seeded compliant small-diameter vascular graft. Biomaterials. 2007;28:486. doi: 10.1016/j.biomaterials.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 60.Meuleman N. Tondreau T. Delforge A. Dejeneffe M. Massy M. Libertalis M. Bron D. Lagneaux L. Human marrow mesenchymal stem cell culture: serum-free medium allows better expansion than classical alpha-MEM medium. Eur J Haematol. 2006;76:309. doi: 10.1111/j.1600-0609.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 61.Chevallier N. Anagnostou F. Zilber S. Bodivit G. Maurin S. Barrault A. Bierling P. Hernigou P. Layrolle P. Rouard H. Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials. 2009;31:270. doi: 10.1016/j.biomaterials.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 62.Stitzel J. Liu J. Lee S.J. Komura M. Berry J. Soker S. Lim G. Van Dyke M. Czerw R. Yoo J. Atala A. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27:1088. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 63.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 64.Cory A.H. Owen T.C. Barltrop J.A. Cory J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 65.Berglund J.D. Mohseni M.M. Nerem R.M. Sambanis A. A biological hybrid model for collagen-based tissue engineered vascular constructs. Biomaterials. 2003;24:1241. doi: 10.1016/s0142-9612(02)00506-9. [DOI] [PubMed] [Google Scholar]
- 66.Daly C.D. Campbell G.R. Walker P.J. Campbell J.H. In vivo engineering of blood vessels. Front Biosci. 2004;9:1915. doi: 10.2741/1384. [DOI] [PubMed] [Google Scholar]
- 67.Herring M. Gardner A. Glover J. A single-staged technique for seeding vascular grafts with autogenous endothelium. Surgery. 1978;84:498. [PubMed] [Google Scholar]
- 68.Nesbitt W.S. Westein E. Tovar-Lopez F.J. Tolouei E. Mitchell A. Fu J. Carberry J. Fouras A. Jackson S.P. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 69.Hjortnaes J. Gottlieb D. Figueiredo J.L. Melero-Martin J. Kohler R.H. Bischoff J. Weissleder R. Mayer J. Aikawa E. Intravital molecular imaging of small-diameter tissue-engineered vascular grafts: a feasibility study. Tissue Eng Part C. 2009. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]