Abstract
Decellularization of native tissues is a promising technique with numerous applications in tissue engineering and regenerative medicine. However, there are various limitations of currently available decellularization methods, such as alteration of extracellular matrix mechanics and restricted use on certain tissues. This study was conducted to explore the effect of serum on the decellularization of various types of tissues. Fetal bovine serum–containing cell culture medium endothelial growth media-2 removed DNA but not cellular β-actin from human umbilical artery after detergent treatment, without compromising the tissue mechanical strength assessed by burst pressure. In addition, the effect of serum-containing endothelial growth media-2 on DNA removal was replicated in other types of tissues such as tissue-engineered vessels and myocardium. Other types of serum, including human serum, were also shown to remove DNA from detergent-pretreated tissues. In conclusion, we describe a novel utilization of serum that may have broad applications in tissue decellularization.
Introduction
Decellularization of tissues and organs for tissue engineering and regenerative medicine has been widely applied to blood vessels,1–5 heart valves,6–10 hearts,11 livers,12,13 urinary bladders,13–15 small intestines,13,16,17 dermis,18 muscles,19,20 tendons, and ligaments.21,22 Decellularization of naturally derived tissues reduces their immunogenicity,23–27 rendering them more favorable for allogenic or xenogenic uses. The extracellular matrix retained in the decellularized tissues can be used as a bio-scaffold for tissue reconstruction.28–30 For example, decellularized biomaterials can be seeded with various types of cells to generate functional tissues,1–3,11,31,32 and have the potential for repair, growth, and remodeling in vivo.16,32–34
The standard of a good decellularization methodology is a combination of complete removal of cellular and nuclear materials for decreased immunogenicity, while minimizing tissue disruption to retain native extracellular matrix structure, with maximal maintenance of mechanical properties for in vivo functionality. A large number of decellularization methods have been reported, which include the utilization of a variety of physical, chemical, and enzymatic approaches to allow the initial lysis of cell and nuclear membranes, the subsequent solubilization of cytoplasmic and nuclear material, and the final removal of all cellular remnants.35 However, many of these decellularization approaches require chemically harsh conditions such as high salt,4,7,22,36 application of proteolytic enzymes or nucleases,1,4,6,9,10,37 and extreme pH,2,31,36 which affect the mechanical integrity of decellularized tissues4,9,36–38 and their ability for recellularization.21 In addition, remnants of such materials may remain in decellularized tissues, and induce a severe inflammatory response upon implantation into a recipient.35 Further, the efficiency of decellularization depends heavily on the original properties of tissue, making it difficult to guarantee a successful decellularization using a single method if applied to many tissues or types of tissues.
Serum, most commonly fetal bovine serum (FBS), has been used widely in cell and tissue culture. Serum is rich in growth factors and other components that are essential for cell attachment, growth, and proliferation. Many cellular and tissue-based products, such as Carticel (Genzyme, Cambridge, MA), Epicel (Genzyme), Dermagraft (Advanced BioHealing, Westport, CT), and TransCyte (Smith & Nephew, Pinetown, South Africa), incorporate the culture of cells in the presence of FBS. These cellular and tissue-based therapeutic products are used for various clinical applications including skin repair and cartilage reconstruction. On the other hand, although less remarked, serum is known to contain serum nucleases that may play a role in DNA/RNA degradation. Thus, protection of therapeutic DNA from degradation by serum nucleases has become an important factor while designing gene delivery vehicles.39 Previously, we have shown that DNA appeared as a diffuse smear in the human umbilical arteries after their incubation with 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and sodium dodecyl sulfate (SDS) buffers.40 As application of exogenous ribonuclease/deoxyribonuclease has been reported in the decellularization of various tissues,1,4,6,9,10,37 these results suggest that the serum component in endothelial growth media-2 (EGM-2) might play a role in removing DNA from the detergent-treated tissues.
In this study, we describe the unique utilization of serum to modify the detergent-based decellularization approach. The effects of EGM-2 on the decellularization of different types of tissues and on tissue mechanical properties were determined. Studies were also performed to examine the effects of using different concentrations of FBS, FBS in different buffers, and serum from other sources on DNA removal. Our results confirm the hypothesis that serum could be used as a novel method with wide application in tissue decellularization.
Materials and Methods
This study was conducted in accordance with the institutional guideline at Yale University. All animal care complied with the Guide for the Care and Use of Laboratory Animals. Human tissue was obtained using protocols approved by the Yale University Human Investigation Committee.
Isolation of human umbilical arteries and rat hearts
Human umbilical cords (anonymized) were obtained from Yale-New Haven Children's Hospital (New Haven, CT) and transported to the laboratories on ice within 24 h after harvesting. Under sterile conditions, umbilical arteries were dissected from the cords as described previously40 and cut into 5 cm segments. The segments were flushed with phosphate-buffered saline (PBS, pH 7.4; Invitrogen, Carlsbad, CA), and stored in PBS containing 1% (v/v) penicillin/streptomycin (Pen/Strep) (Invitrogen) at 4°C. Rat hearts were isolated from 3-month-old 300 g Fischer F344 rats (Charles River Laboratories, Boston, MA) after flushing with PBS to remove the blood. Isolated hearts were then cut into four pieces along the longitudinal and circumferential directions of the heart and stored in PBS containing 1% Pen/Strep at 4°C.
Engineering of vessels
Tissue-engineered porcine arteries were created as previously described.41 Briefly, five million porcine carotid smooth muscle cells were seeded onto a tubular polyglycolic acid mesh (3 mm in diameter and 8 mm in length; Concordia Medical, Coventry, RI) around a silicone tube and cultured in a bioreactor connected to a peristaltic pump at 5% CO2 and 37°C. The culture medium is the same as described previously.42 Engineered vessels were harvested from bioreactors after 8 weeks of culture and rinsed two to three times with PBS to remove traces of culture medium. Within half an hour of isolation, tissues were either used for immediate analysis or subjected to decellularization.
Decellularization of human umbilical arteries
Umbilical arteries were decellularized by a modified detergent-based method that included incubation in CHAPS and SDS buffers.4,43 Briefly, vessel segments were incubated in CHAPS buffer (8 mM CHAPS, 1 M NaCl, and 25 mM ethylenediaminetetraacetic acid (EDTA), in PBS) for 22 h, washed briefly with PBS, and incubated for another 22 h with SDS buffer (1.8 mM SDS, 1 M NaCl, and 25 mM EDTA in PBS) followed by a 2-day wash with PBS to completely remove the detergent. Finally, umbilical arteries were incubated in EGM-2 for 48 h followed by brief PBS rinses. EGM-2 is an endothelial cell basal medium (EBM; Lonza, Walkersville, MD) containing 12% (v/v) FBS (Hyclone, Logan, UT) and 1% Pen/Strep, and supplemented with the components from EGM-2 Single Quots (Lonza) that include hydrocortisone, growth factors (human fibroblast growth factor-B, vascular endothelial growth factor, R3-insulin-like growth factor-1, and human epidermal growth factor), ascorbic acid, and heparin. All decellularization steps were carried out at 37°C with agitation under sterile conditions. Decellularized vessels were stored in PBS containing 1% Pen/Strep at 4°C for up to 2 weeks. Alternatively, vessels were flash-frozen and stored at −80°C for up to 4 weeks for later DNA and protein analysis.
To determine which component in EGM-2 might play a central role in DNA removal, in some studies, after the treatment with CHAPS and SDS buffers, umbilical artery segments were incubated for 48 h in EGM-2 in the absence of FBS or one of the components from EGM-2 Single Quots. In other studies, to determine whether the effect on DNA removal was FBS dose-dependent and was confined to FBS, CHAPS and SDS buffer-treated vessels were incubated for 48 h in EBM containing various concentrations of FBS or other types of serum. In some other studies, to determine whether DNA removal depends on EBM, vessels were treated with CHAPS and SDS buffers for 22 h, respectively, followed by incubation for 48 h in EBM, Dulbecco's modified Eagle's medium (DMEM), PBS, or saline (0.9% (w/v) NaCl) containing 12% FBS. Finally, in studies to determine whether incubation with SDS buffer can be eliminated from the decellularization procedure, umbilical arteries were incubated in CHAPS and SDS buffers for 14 h, respectively, or 22 h, respectively, or were subjected to CHAPS buffer alone for 14 or 22 h, before further 2-day incubation in EGM-2.
Decellularization of engineered vessels and rat heart tissues
Porcine engineered arteries were incubated in CHAPS and SDS buffers for 1 h, respectively, followed by incubation in EGM-2 for 48 h. Rat heart tissues were treated with CHAPS and SDS buffers for 22 h, respectively, before additional treatment in EGM-2 for 48 h. All decellularization steps were carried out at 37°C with agitation under sterile conditions.
Histological analysis
Fresh and decellularized tissue samples were fixed in 10% (v/v) neutral-buffered formalin for 1 h, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin (H&E) for nuclear material. Images were obtained using a Axiovert 200M microscope (Zeiss, Thornwood, NY) equipped with AxioCam HR with software AxioVision Release 4.5.
Immunohistochemical analysis
Paraffin-embedded sections were immunostained for major histocompatibility complex (MHC) Class I molecules using methods described previously.40 Briefly, after sections were deparaffinized, rehydrated, and blocked, rabbit anti-human MHC Class I antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was applied to sections at 1:50 dilution, followed by biotinylated goat anti-mouse IgG (1:200; Vector Laboratories, Burlingame, CA). Bound antibodies were detected using an avidin-biotin-peroxidase complex system (Vector Laboratories), and the color was viewed after incubation with NovaRed peroxidase substrate (Vector Laboratories). Slides were counterstained with hematoxylin, dehydrated, and mounted.
DNA quantification
DNA contents in tissue samples were determined fluorometrically using the PicoGreen assay, as described previously.40 Samples were digested in a papain solution44 and incubated with Quant-iT™ PicoGreen® dsDNA reagent (Molecular Probes, Eugene, OR). Using a fluorometer, the fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Bacteriophage λ DNA (Invitrogen) was used as a standard.
SDS-PAGE and immunoblotting
Proteins were extracted from homogenized frozen tissue samples in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% [v/v] Triton X-100, 0.5% [w/v] sodium deoxycholate, and 0.1% [w/v] SDS) containing freshly added proteinase inhibitors (Sigma-Aldrich, St. Louis, MO). The protein concentration was determined using the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA). Samples were prepared for SDS-Polyacrylamide gel electrophoresis (PAGE) by boiling 30 μg of proteins in SDS sample buffer containing 2% β-mercaptoethanol (final concentration) for 5 min. SDS-PAGE and immunoblotting was performed similarly as previously described.45 Briefly, proteins were separated by electrophoresis, transferred to polyvinylidene difluoride (PVDF) membrane (Bio-Rad), and immunoblotted with mouse monoclonal antibody to β-actin (1:2500; Sigma-Aldrich), followed by horseradish peroxidase–conjugated goat anti-mouse secondary antibody. Blots were developed using enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL).
Mechanical analysis
The mechanical properties of umbilical arteries were determined by pressurizing a vessel segment (2–3 cm in length) attached to a flow system with PBS until failure, as described previously.4 PBS was injected into the flow system at increments of 50 mm Hg pressure until bursting of the vessel occurred. The outer diameter at each pressure was recorded by a CCD camera (Canon XL1 Digital Video Recorder, Canon, Lake Success, NY).
Statistics
Data are expressed as mean or mean ± standard deviation. Statistical significance was determined by one-way analysis of variance (ANOVA). Difference (p value) <0.05 was considered significant.
Results
Effects of EGM-2 on tissue decellularization and its mechanical properties
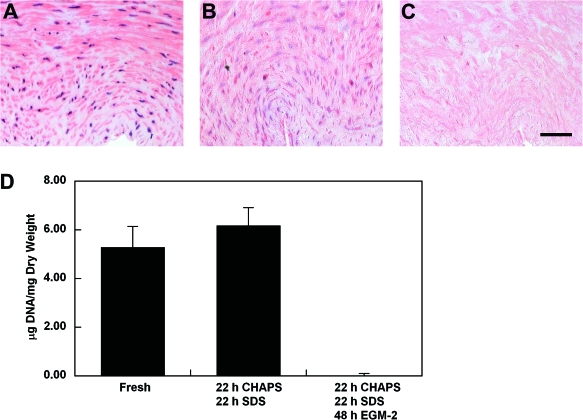
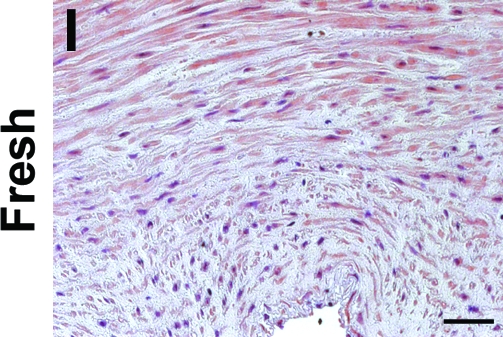
Decellularization efficiency was examined histologically and by DNA quantification, as well as by immunoblotting for β-actin or by immunostaining for MHC antigens. Incubation of freshly isolated human umbilical arteries with CHAPS and SDS buffers caused the nuclear staining to appear as a diffuse smear in the vessel wall (Fig. 1), similar to previous reports.40 A further incubation for 48 h in EGM-2 completely removed the nuclear material as indicated by both H&E staining and by DNA quantification (Fig. 1C, D). These data suggested that EGM-2 or its components might play an important role in achieving an efficient decellularization, as assessed by removal of cellular DNA.
FIG. 1.
(A–C) H&E staining of human umbilical arteries before treatment (A, fresh), after incubation with 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and sodium dodecyl sulfate (SDS) buffers for 22 h, respectively (B, CHAPS/SDS), and after further incubation with endothelial growth media-2 (EGM-2) for 48 h (C, CHAPS/SDS/EGM-2). Scale bar is 50 μm and applies to all panels. (D) Average DNA per dry weight of human umbilical arteries from three independent experiments. Data are presented as mean ± standard deviation (s.d.). Color images available online at www.liebertonline.com/ten.
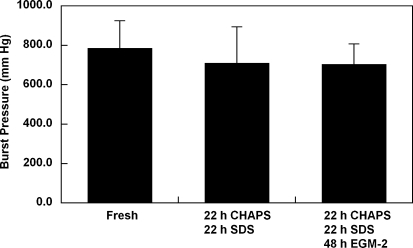
Staining with Masson's trichrome indicates that the extracellular matrix collagen was well preserved in the umbilical arteries that were treated with detergents and EGM-2.40 It has been shown that the decellularization approach utilizing CHAPS and SDS buffers has a minimal effect on vessel burst pressures.4 To verify that an additional incubation in EGM-2 will have no impact on vessel mechanics, the maximum burst pressures of CHAPS and SDS buffer–treated umbilical arteries were compared before and after EGM-2 treatment. No significant decrease in the maximum burst pressure was observed after the treatment of umbilical arteries with CHAPS and SDS buffers (Fig. 2). In addition, umbilical arteries treated with additional EGM-2 incubation had similar maximum burst pressure as compared to those treated with only CHAPS and SDS buffers (Fig. 2). These results show that, while exposure to EGM-2 efficiently removed cellular DNA, it did not impact extracellular matrix mechanics in the decellularized tissue.
FIG. 2.
Average burst strength for human umbilical arteries before treatment (fresh), after incubation with CHAPS and SDS buffers (CHAPS/SDS), and after further incubation with EGM-2 (CHAPS/SDS/EGM-2). Data are presented as mean ± s.d. from three independent experiments. No significant difference was found among three groups.
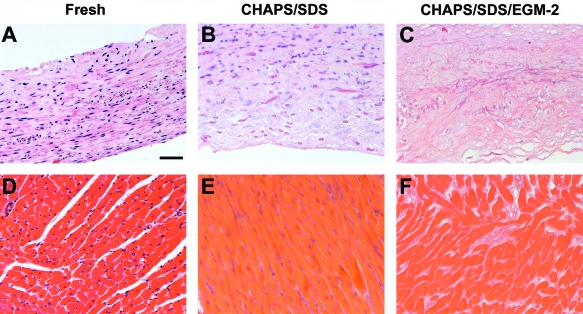
To determine whether the observation of removal of DNA by FBS-containing EGM-2 is limited to human umbilical arteries, we decellularized several other tissues and exposed these tissues to EGM-2 as well. Tissue-engineered porcine arteries41,46 were treated with CHAPS and SDS buffers each for 1 h, followed by EGM-2 incubation for 48 h. The diffuse nuclear material in the vessel wall that was observed after incubation with CHAPS and SDS buffers (Fig. 3B) was completely removed after EGM-2 treatment (Fig. 3C). Similarly, in rat heart tissue, the DNA material was almost absent after EGM-2 treatment (Fig. 3F, and DNA quantification, data not shown). These data suggest that EGM-2, which contains FBS as well as multiple other components, might provide a novel and easy means for quantitative decellularization of various types of tissues.
FIG. 3.
H&E staining of porcine engineered arteries (A–C) and rat heart tissues (D–F) before treatment (A, D), after incubation with CHAPS and SDS buffers (B, E), and after further incubation with EGM-2 (C, F). Scale bar is 50 μm and applies to all panels. Color images available online at www.liebertonline.com/ten.
EGM-2 removes DNA but not cellular β-actin
SDS is an ionic detergent that tends to disrupt native tissue structure35 and has been shown to inhibit recellularization because of its toxicity or damaging effects on extracellular matrix.6,21 Because ionic detergents damage collagenous extracellular matrix, it is desirable to limit the duration of tissue exposure to SDS buffer and other detergents during decellularization.
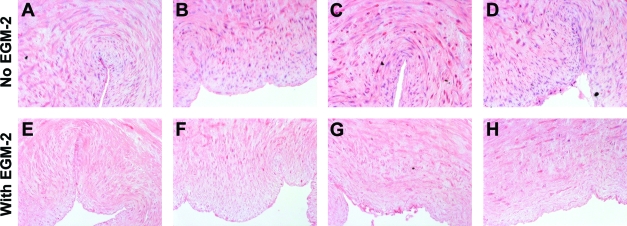
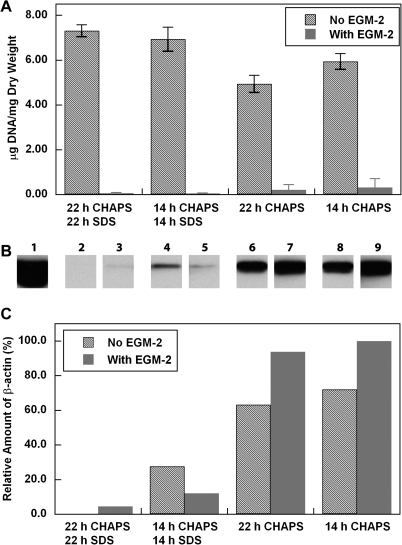
To determine whether incubation with SDS buffer might be eliminated completely from the decellularization procedure by treatment with EGM-2, human umbilical arteries were treated with CHAPS buffer alone, followed by incubation in EGM-2. Vessels treated with both CHAPS and SDS buffers were used as controls. H&E staining indicates that the intact nuclear staining found in the untreated umbilical arteries (Fig. 4I) appeared as a diffuse smear after treatment with CHAPS buffer for either 22 or 14 h (Fig. 4C, D), similar to what was seen in tissues treated with both CHAPS and SDS buffers (Fig. 4A, B). After incubation with EGM-2, the nuclear smear disappeared from all of the tissues (Fig. 4E–H). Quantification of DNA residue in the tissues confirmed the nearly complete removal of DNA from all EGM-2-treated umbilical arteries, and no significant difference was found among these EGM-2 groups (Fig. 5A). Therefore, it appears that the EGM-2 incubation step improved the efficiency of DNA removal regardless of which detergent was employed for initial treatment.
FIG. 4.
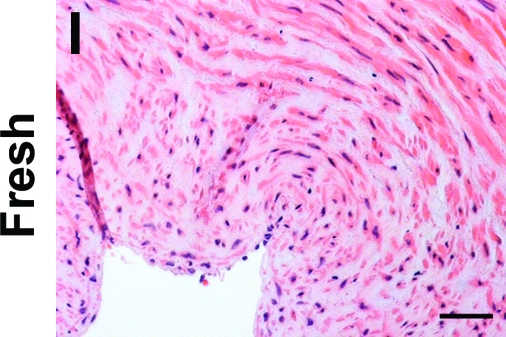
H&E staining of human umbilical arteries treated with CHAPS and SDS buffers for 22 h (A, E) or 14 h (B, F), respectively; or with only CHAPS buffer for 22 h (C, G) or 14 h (D, H), before (A–D) and after (E–H) further EGM-2 incubation. Also shown is freshly isolated umbilical artery (I). Scale bar is 50 μm and applies to all panels. Color images available online at www.liebertonline.com/ten.
FIG. 5.
(A) Quantification of DNA per dry weight of human umbilical arteries treated as described in the legends to Figure 4. Data are presented as mean ± s.d. from three independent experiments. (B) β-Actin staining of human umbilical arteries treated as described in (A). Lanes are labeled as follows: 1, freshly isolated umbilical artery; 2 and 3, CHAPS and SDS buffers for 22 h, respectively; 4 and 5, CHAPS and SDS buffers for 14 h, respectively; 6 and 7, CHAPS buffer for 22 h; 8 and 9, CHAPS buffer for 14 h. Lanes 2, 4, 6, and 7, No EGM-2; lanes 3, 5, 7, and 9, after EGM-2 for 48 h. (C) Quantification of relative β-actin shown in (B). The amount in lane 9 was set as 100%.
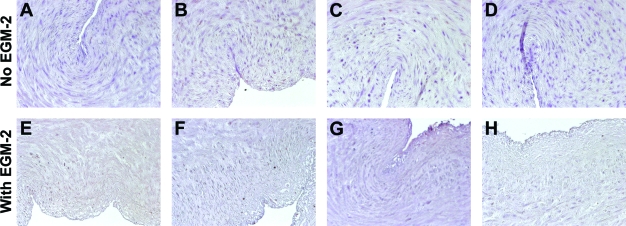
To further determine the effect of EGM-2 on decellularization efficiency, proteins were extracted from tissues and examined with immunoblotting for β-actin. β-Actin was chosen as a sensitive measure of removal of cytoplasmic material, because it is an extremely abundant protein expressed in many types of cells.47 As shown in Figure 5, β-actin was completely absent from tissues that were treated with CHAPS and SDS buffers for 22 h, respectively, with or without the EGM-2 step. The amount of β-actin was also found to be decreased in umbilical arteries treated with CHAPS buffer compared to the fresh, nondecellularized tissue (Fig. 5B, C). However, there was still a significant amount of β-actin remaining in the tissue treated with CHAPS buffer alone, in the absence or presence of additional EGM-2 incubation (Fig. 5). This shows that it is primarily the detergent incubation steps that remove cytoplasmic proteins from these tissues, while exposure to EGM-2 seems to affect primarily nuclear DNA. On the other hand, MHC Class I staining was absent in all the treated tissues (Fig. 6), indicating that staining for this protein is a less sensitive means of quantifying extent of cellular removal than either DNA assay or immunoblotting for β-actin.
FIG. 6.
Staining for MHC Class I molecules in human umbilical arteries treated as described in the legend to Figure 4. Sections were counterstained with hematoxylin. Scale bar is 50 μm and applies to all panels. Color images available online at www.liebertonline.com/ten.
Effect of serum in DNA removal
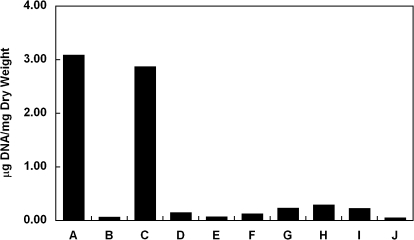
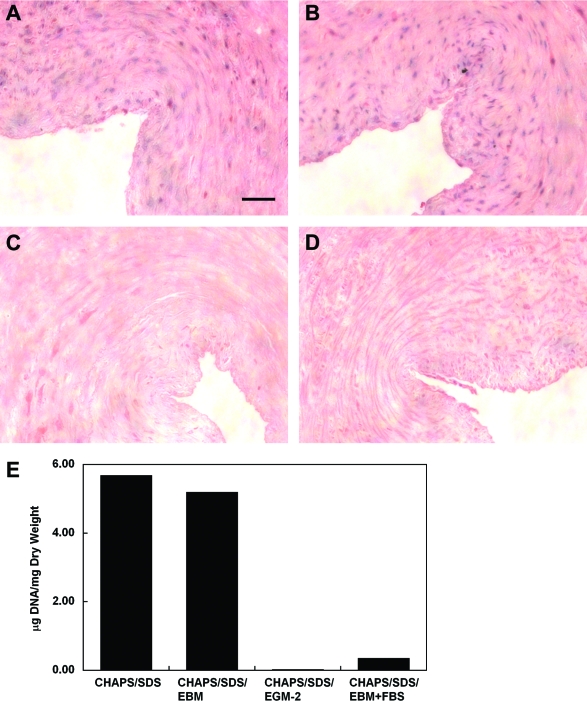
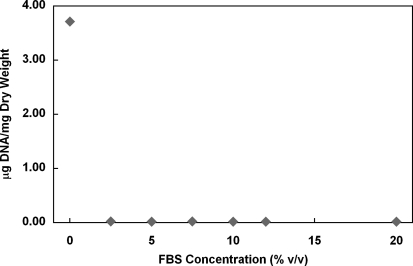
EGM-2 consists of an EBM that is supplemented with 12% FBS and several other components, including growth factors and heparin. To determine whether it is serum or the other components in EGM-2 that plays a central role in DNA removal, detergent-treated umbilical artery segments were incubated with EGM-2 in the absence of either FBS or one of the supplemental components from EGM-2 Single Quots. Interestingly, removing FBS from the EGM-2 formulation completely eliminated its effect on DNA removal (Fig. 7). In contrast, only a very slight change in DNA removal was observed under conditions wherein any of the other components were eliminated from the EGM-2 formulation (Fig. 7). On the other hand, incubation with EBM in the presence of 12% FBS removed DNA from the detergent-treated vessels similarly as EGM-2 did (Fig. 8). The reason FBS has been used at 12% for decellularization is that the EGM-2 used for culturing HUVECs contains 12% FBS.40 However, EBM containing as low as 2.5% FBS was found to be sufficient to remove all DNA from the tissue (Figs. 9 and 11). EBM incubation alone did not change the appearance of nuclear material or the amount of DNA residue in CHAPS and SDS buffer-treated tissue (Fig. 8). These results suggest that FBS plays a major role in DNA removal that is observed with EGM-2 incubation.
FIG. 7.
Average amount of DNA per dry weight of human umbilical arteries treated with CHAPS and SDS buffers (A), followed by EGM-2 (B) or EGM-2 in the absence of fetal bovine serum (FBS) (C), hydrocortisone (D), human fibroblast growth factor-B (E), vascular endothelial growth factor (F), R3-insulin-like growth factor-1 (G), ascorbic acid (H), human epidermal growth factor (I), or heparin (J). Data are presented as mean values from two independent experiments.
FIG. 8.
(A–D) H&E staining of human umbilical arteries treated with CHAPS and SDS buffers for 22 h, respectively (A), followed by further incubation in endothelial cell basal medium (EBM) (B), EGM-2 (C), or EBM containing 12% FBS (D) for 48 h. Scale bar is 50 μm and applies to all panels. (E) Quantification of DNA per dry weight of human umbilical arteries shown in (A–D). Color images available online at www.liebertonline.com/ten.
FIG. 9.
Effects of FBS concentration on DNA removal from human umbilical arteries treated with CHAPS and SDS buffers. Data are presented as mean values from two independent experiments.
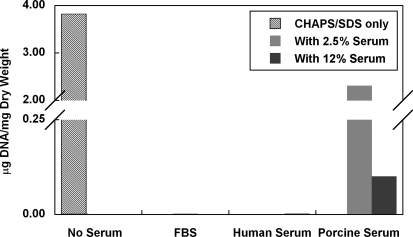
FIG. 11.
Effects of different types of serum on DNA removal from human umbilical arteries treated with CHAPS and SDS buffers. Notice that the amount of DNA per mg dry weight of vessels is less than 0.003 μg for FBS and Human Serum groups. Data are presented as mean values from two independent experiments.
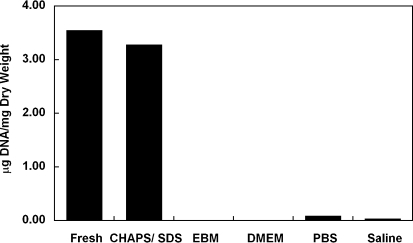
To determine whether the effect of FBS on DNA removal is dependent upon EBM, CHAPS and SDS buffer-treated umbilical arteries were also incubated with 12% FBS in several other solutions, including DMEM, PBS, and saline. DNA was almost completely removed from all groups (Fig. 10), suggesting that DNA removal by FBS is independent of the buffer system.
FIG. 10.
Average amount of DNA per dry weight of human umbilical arteries before treatment (fresh), after incubation with CHAPS and SDS buffers (CHAPS/SDS), and after further treatment in EBM, DMEM, PBS, or saline in the presence of 12% (v/v) FBS. Data are presented as mean values from two independent experiments. PBS, phosphate-buffered saline.
In addition to FBS, human serum as low as 2.5% was also able to completely remove DNA from the detergent-treated vessels (Fig. 11). In comparison, porcine serum was less effective in removing DNA, although an extensive amount of DNA was also removed with 12% porcine serum (Fig. 11). These data suggest that the effect of serum on DNA removal is not limited to FBS but that the efficiency varies among different types of serum.
Discussion
Decellularization of naturally derived tissues is a tissue engineering approach that has gained success in many applications.35,48 Complete decellularization, including the removal of cell membrane antigen epitopes, residual DNA, and damage-associated molecular pattern molecules, is critical to decrease tissue immunogenicity for use in xenogenic or allogenic transplantation.48,49 Herein, we reported a novel use of serum during a detergent-based decellularization procedure. Our studies showed that treating human umbilical arteries with CHAPS and SDS buffers resulted in the diffusion of nuclear material within the vessel wall, which was removed after additional incubation with serum-containing solutions, such as EGM-2. EGM-2 incubation did not affect tissue mechanical properties, and was successful in removing DNA from other types of tissues, such as rat hearts and tissue-engineered porcine vessels, suggesting the broad applicability of these findings.
Although the presence of cell membrane antigens and soluble proteins in the tissue plays a key role in eliciting immune response for allogenic/xenogenic transplantation,48–50 previous studies have indicated that residual DNA could similarly induce adverse immune response,51 suggesting the importance of removing DNA remnants from decellularized tissues as well. In a recent report by Gilbert et al., the authors have shown that various amounts of DNA were retained in several commercially available biomaterials.52 In some cases, retained DNA can be quite extensive, potentially increasing risk of transmission of xenopathogens. In addition, some tissue engineering approaches utilize cells that are genetically engineered.53,54 Quantitative removal of the DNA from tissues engineered from such cells will decrease risk of transmission of transgenes to the host. Human telomerase is an example of one such transgene that enhances engineered tissue formation, but is activated in most cancer cells,55 and hence its removal from engineered tissues would be very important before implantation. Thus, our finding that serum-containing solutions such as EGM-2 quantitatively remove DNA is very useful for developing biomaterials without DNA contamination.
Using various approaches, we have shown that serum might be the primary component in EGM-2 that plays the role in DNA removal from detergent-treated tissues. Serum is an essential component for tissue and cell culture. However, its effect on enhancing tissue decellularization has never been reported, hence the novelty of our findings. In our studies, we found that a simple incubation in serum (FBS and human serum) as low as 2.5% effected the removal of DNA material from tissues after detergent treatment. Compared to other decellularization approaches where exogenous ribonuclease/deoxyribonuclease have been used,1,4,6,9,10,37 utilization of serum not only dramatically cuts the cost of decellularization, but also eliminates the step of removing nuclease remnants from decellularized tissues. The application of nonhuman serum FBS during decellularization to prepare therapeutic products might raise concerns on potential xenogenic rejection responses and possible disease transmission. However, the U.S. Food and Drug Administration (FDA) has approved various cellular and tissue-based products that are developed by culturing cells in FBS-containing culture media. Alternatively, we have shown that efficient DNA removal can be achieved by human serum at as low as 2.5%, allowing the decellularization to be possibly conducted using a patient's own serum. In addition, based upon extensive clinical data obtained from transfusion of blood plasma,56 we anticipate that allogenic human serum could also be used for decellularization without triggering subsequent immune responses in allogenic recipients. However, one limitation of using serum during decellularization is that the remnants of serum, particularly albumin but also immunoglobulins, in the acellular tissues could trigger some mild response in the recipient. Future studies should be performed to determine the amount of serum protein residua in the final decellularized products.
Limiting the duration of decellularization can significantly improve cost efficiency and help retain original tissue mechanics by decreasing total time of exposure to harsh chemical conditions. In our studies to determine whether EGM-2 was also able to remove DNA from tissues with minimal detergent treatment, umbilical arteries were incubated with decreasing time in CHAPS and SDS buffers or with CHAPS buffer alone before a further incubation in EGM-2. Our results showed that EGM-2 incubation allowed an almost complete DNA removal from all detergent-treated tissues regardless of the duration and extent of detergent treatment. However, we observed no additional removal of cellular proteins from detergent-treated tissues by EGM-2 incubation. These results suggest that the role of serum-containing EGM-2 in decellularization is primarily DNA removal and the prerequisite is the lysis of cellular/nuclear membrane. Further studies could be performed to determine whether the serum nuclease activity in EGM-2 plays the key role in DNA removal by inhibiting the nuclease activity such as through heat inactivation.
While many decellularization techniques have been developed and published elsewhere, the information on decellularization efficiency was mainly gained from histological analysis with H&E staining and sometimes with DNA quantification.4,14,25,31,33,57 Detection of remaining soluble proteins, as well as MHC, has also been used for verification of decellularization.8,36 As our data indicated, an absence of β-actin from umbilical arteries treated with CHAPS and SDS buffers for 22 h, respectively, was nonetheless associated with significant amount of DNA materials retained in the tissue before EGM-2 incubation. Similarly, in tissues almost completely free of DNA (CHAPS buffer for 22 h or 14 h, with EGM-2 incubation), there were still abundant protein remnants despite the absence of MHC staining in these tissues. These results suggest that immunoblotting with β-actin provides a more sensitive method for determination of decellularization efficiency. In addition, these studies point out that removal of DNA and cytoplasmic proteins are highly decoupled: it is possible to quantitatively remove either one without removing the other. Hence, it is likely necessary to quantify both DNA and protein remnants using various detection methods to confirm the complete decellularization of tissues.
To summarize, this study describes a modified detergent-based decellularization method with the novel use of serum for DNA removal. This decellularization approach is easy and efficient, and has wide application in the decellularization of various tissues or types of tissues. In addition, we show the importance of using different approaches combined to confirm a complete decellularization.
Acknowledgments
The authors thank Louise Benson for providing the umbilical cords and Thomas Petersen for rat heart tissues. The authors also thank Liping Zhao for technical assistance. This work was supported by NIH R01 HL083895 (to L.E.N.).
Disclosure Statement
L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
References
- 1.Teebken O.E. Bader A. Steinhoff G. Haverich A. Tissue engineering of vascular grafts: human cell seeding of decellularised porcine matrix. Eur J Vasc Endovasc Surg. 2000;19:381. doi: 10.1053/ejvs.1999.1004. [DOI] [PubMed] [Google Scholar]
- 2.Amiel G.E. Komura M. Shapira O. Yoo J.J. Yazdani S. Berry J. Kaushal S. Bischoff J. Atala A. Soker S. Engineering of blood vessels from acellular collagen matrices coated with human endothelial cells. Tissue Eng. 2006;12:2355. doi: 10.1089/ten.2006.12.2355. [DOI] [PubMed] [Google Scholar]
- 3.Kaushal S. Amiel G.E. Guleserian K.J. Shapira O.M. Perry T. Sutherland F.W. Rabkin E. Moran A.M. Schoen F.J. Atala A. Soker S. Bischoff J. Mayer J.E., Jr. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahl S.L. Koh J. Prabhakar V. Niklason L.E. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659. [PubMed] [Google Scholar]
- 5.Abousleiman R.I. Reyes Y. McFetridge P. Sikavitsas V. The human umbilical vein: a novel scaffold for musculoskeletal soft tissue regeneration. Artif Organs. 2008;32:735. doi: 10.1111/j.1525-1594.2008.00598.x. [DOI] [PubMed] [Google Scholar]
- 6.Rieder E. Kasimir M.T. Silberhumer G. Seebacher G. Wolner E. Simon P. Weigel G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg. 2004;127:399. doi: 10.1016/j.jtcvs.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Zeltinger J. Landeen L.K. Alexander H.G. Kidd I.D. Sibanda B. Development and characterization of tissue-engineered aortic valves. Tissue Eng. 2001;7:9. doi: 10.1089/107632701300003250. [DOI] [PubMed] [Google Scholar]
- 8.Ota T. Taketani S. Iwai S. Miyagawa S. Furuta M. Hara M. Uchimura E. Okita Y. Sawa Y. Novel method of decellularization of porcine valves using polyethylene glycol and gamma irradiation. Ann Thorac Surg. 2007;83:1501. doi: 10.1016/j.athoracsur.2006.11.083. [DOI] [PubMed] [Google Scholar]
- 9.Grauss R.W. Hazekamp M.G. van Vliet S. Gittenberger-de Groot A.C. DeRuiter M.C. Decellularization of rat aortic valve allografts reduces leaflet destruction and extracellular matrix remodeling. J Thorac Cardiovasc Surg. 2003;126:2003. doi: 10.1016/s0022-5223(03)00956-5. [DOI] [PubMed] [Google Scholar]
- 10.Bader A. Schilling T. Teebken O.E. Brandes G. Herden T. Steinhoff G. Haverich A. Tissue engineering of heart valves—human endothelial cell seeding of detergent acellularized porcine valves. Eur J Cardiothorac Surg. 1998;14:279. doi: 10.1016/s1010-7940(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 11.Ott H.C. Matthiesen T.S. Goh S.K. Black L.D. Kren S.M. Netoff T.I. Taylor D.A. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 12.Lin P. Chan W.C. Badylak S.F. Bhatia S.N. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 13.Brown B. Lindberg K. Reing J. Stolz D.B. Badylak S.F. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 14.Bolland F. Korossis S. Wilshaw S.P. Ingham E. Fisher J. Kearney J.N. Southgate J. Development and characterisation of a full-thickness acellular porcine bladder matrix for tissue engineering. Biomaterials. 2007;28:1061. doi: 10.1016/j.biomaterials.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert T.W. Nieponice A. Spievack A.R. Holcomb J. Gilbert S. Badylak S.F. Repair of the thoracic wall with an extracellular matrix scaffold in a canine model. J Surg Res. 2008;147:61. doi: 10.1016/j.jss.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Huynh T. Abraham G. Murray J. Brockbank K. Hagen P.O. Sullivan S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat Biotechnol. 1999;17:1083. doi: 10.1038/15062. [DOI] [PubMed] [Google Scholar]
- 17.Badylak S.F. Lantz G.C. Coffey A. Geddes L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47:74. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 18.Chen R.N. Ho H.O. Tsai Y.T. Sheu M.T. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials. 2004;25:2679. doi: 10.1016/j.biomaterials.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 19.Borschel G.H. Dennis R.G. Kuzon W.M., Jr. Contractile skeletal muscle tissue-engineered on an acellular scaffold. Plast Reconstr Surg. 2004;113:595. doi: 10.1097/01.PRS.0000101064.62289.2F. [DOI] [PubMed] [Google Scholar]
- 20.De Coppi P. Bellini S. Conconi M.T. Sabatti M. Simonato E. Gamba P.G. Nussdorfer G.G. Parnigotto P.P. Myoblast-acellular skeletal muscle matrix constructs guarantee a long-term repair of experimental full-thickness abdominal wall defects. Tissue Eng. 2006;12:1929. doi: 10.1089/ten.2006.12.1929. [DOI] [PubMed] [Google Scholar]
- 21.Gratzer P.F. Harrison R.D. Woods T. Matrix alteration and not residual sodium dodecyl sulfate cytotoxicity affects the cellular repopulation of a decellularized matrix. Tissue Eng. 2006;12:2975. doi: 10.1089/ten.2006.12.2975. [DOI] [PubMed] [Google Scholar]
- 22.Woods T. Gratzer P.F. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials. 2005;26:7339. doi: 10.1016/j.biomaterials.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 23.Allaire E. Bruneval P. Mandet C. Becquemin J.P. Michel J.B. The immunogenicity of the extracellular matrix in arterial xenografts. Surgery. 1997;122:73. doi: 10.1016/s0039-6060(97)90267-1. [DOI] [PubMed] [Google Scholar]
- 24.Meyer S.R. Nagendran J. Desai L.S. Rayat G.R. Churchill T.A. Anderson C.C. Rajotte R.V. Lakey J.R. Ross D.B. Decellularization reduces the immune response to aortic valve allografts in the rat. J Thorac Cardiovasc Surg. 2005;130:469. doi: 10.1016/j.jtcvs.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Rieder E. Nigisch A. Dekan B. Kasimir M.T. Muhlbacher F. Wolner E. Simon P. Weigel G. Granulocyte-based immune response against decellularized or glutaraldehyde cross-linked vascular tissue. Biomaterials. 2006;27:5634. doi: 10.1016/j.biomaterials.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Wells W. Malas M. Baker C.J. Quardt S.M. Barr M.L. Depopulated vena caval homograft: a new venous conduit. J Thorac Cardiovasc Surg. 2003;126:498. doi: 10.1016/s0022-5223(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 27.Elkins R.C. Lane M.M. Capps S.B. McCue C. Dawson P.E. Humoral immune response to allograft valve tissue pretreated with an antigen reduction process. Semin Thorac Cardiovasc Surg. 2001;13:82. [PubMed] [Google Scholar]
- 28.Badylak S.F. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 29.Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Badylak S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Cho S.W. Lim S.H. Kim I.K. Hong Y.S. Kim S.S. Yoo K.J. Park H.Y. Jang Y. Chang B.C. Choi C.Y. Hwang K.C. Kim B.S. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borschel G.H. Huang Y.C. Calve S. Arruda E.M. Lynch J.B. Dow D.E. Kuzon W.M. Dennis R.G. Brown D.L. Tissue engineering of recellularized small-diameter vascular grafts. Tissue Eng. 2005;11:778. doi: 10.1089/ten.2005.11.778. [DOI] [PubMed] [Google Scholar]
- 33.Ketchedjian A. Jones A.L. Krueger P. Robinson E. Crouch K. Wolfinbarger L., Jr. Hopkins R. Recellularization of decellularized allograft scaffolds in ovine great vessel reconstructions. Ann Thorac Surg. 2005;79:888. doi: 10.1016/j.athoracsur.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 34.Martin N.D. Schaner P.J. Tulenko T.N. Shapiro I.M. Dimatteo C.A. Williams T.K. Hager E.S. DiMuzio P.J. In vivo behavior of decellularized vein allograft. J Surg Res. 2005;129:17. doi: 10.1016/j.jss.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert T.W. Sellaro T.L. Badylak S.F. Decellularization of tissues and organs. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Meyer S.R. Chiu B. Churchill T.A. Zhu L. Lakey J.R. Ross D.B. Comparison of aortic valve allograft decellularization techniques in the rat. J Biomed Mater Res A. 2006;79:254. doi: 10.1002/jbm.a.30777. [DOI] [PubMed] [Google Scholar]
- 37.Liao J. Joyce E.M. Sacks M.S. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29:1065. doi: 10.1016/j.biomaterials.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grauss R.W. Hazekamp M.G. Oppenhuizen F. van Munsteren C.J. Gittenberger-de Groot A.C. DeRuiter M.C. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg. 2005;27:566. doi: 10.1016/j.ejcts.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 39.Dash P.R. Seymour L.W. Pharmaceutical aspects of gene therapy. In: Chiellini E., editor. Biomedical Polymers and Polymer Therapeutics. New York: Kluwer Academic/Plenum Publishers; 2001. p. 341. [Google Scholar]
- 40.Gui L. Muto A. Chan S. Breuer C. Niklason L. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng Part A. 2009 Feb 10; doi: 10.1089/ten.tea.2008.0526. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niklason L.E. Gao J. Abbott W.M. Hirschi K.K. Houser S. Marini R. Langer R. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 42.Dahl S.L. Rhim C. Song Y.C. Niklason L.E. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng. 2007;35:348. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livesey S.A. Del Campo A.A. Nag A. Nichols K.B. Coleman C. Method for Processing and Preserving Collagen-Based Tissues for Transplantation. The Woodlands, TX: LifeCell Corporation; 1994. United States Patent 5336616. [Google Scholar]
- 44.Kim Y.J. Sah R.L. Doong J.Y. Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 45.Gui L. Wojciechowski K. Gildner C.D. Nedelkovska H. Hocking D.C. Identification of the heparin-binding determinants within fibronectin repeat III1: role in cell spreading and growth. J Biol Chem. 2006;281:34816. doi: 10.1074/jbc.M608611200. [DOI] [PubMed] [Google Scholar]
- 46.Niklason L.E. Abbott W. Gao J. Klagges B. Hirschi K.K. Ulubayram K. Conroy N. Jones R. Vasanawala A. Sanzgiri S. Langer R. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33:628. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 47.Lodish H. Berk A. Zipursky L.S. Matsudaira P. Baltimore D. Darnell J. Molecular Cell Biology. New York: W.H. Freeman; 2000. [Google Scholar]
- 48.Schmidt C.E. Baier J.M. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 49.Badylak S.F. Gilbert T.W. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siemionow M. Klimczak A. Basics of immune responses in transplantation in preparation for application of composite tissue allografts in plastic and reconstructive surgery: part I. Plast Reconstr Surg. 2008;121:4e. doi: 10.1097/01.prs.0000299470.95855.ce. [DOI] [PubMed] [Google Scholar]
- 51.Zheng M.H. Chen J. Kirilak Y. Willers C. Xu J. Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 52.Gilbert T.W. Freundb S.J. Badylak S.F. Quantification of DNA in Biologic Scaffold Materials. J Surg Res. 2009;152:135. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poh M. Boyer M. Solan A. Dahl S.L. Pedrotty D. Banik S.S. McKee J.A. Klinger R.Y. Counter C.M. Niklason L.E. Blood vessels engineered from human cells. Lancet. 2005;365:2122. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 54.Klinger R.Y. Blum J.L. Hearn B. Lebow B. Niklason L.E. Relevance and safety of telomerase for human tissue engineering. Proc Natl Acad Sci USA. 2006;103:2500. doi: 10.1073/pnas.0508184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shay J.W. Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 56.American Cancer Society. Blood component transfusion—what it involves (January 2009) http://www.cancer.org/docroot/ETO/content/ETO_1_4X_Blood_Transfusion.asp. [Apr 11;2009 ]. http://www.cancer.org/docroot/ETO/content/ETO_1_4X_Blood_Transfusion.asp
- 57.Wilshaw S.P. Kearney J.N. Fisher J. Ingham E. Production of an acellular amniotic membrane matrix for use in tissue engineering. Tissue Eng. 2006;12:2117. doi: 10.1089/ten.2006.12.2117. [DOI] [PubMed] [Google Scholar]