Kinases catalyze protein phosphorylation to regulate signal transduction in a cell. Thus, the identification of substrates of a particular kinase is a fundamental step toward understanding the signaling cascades in normal and disease states. With over 500 known kinases and a paucity of tools available, the identification of genuine kinase substrates has been difficult.[1–7] New approaches to detect substrates are needed to fully characterize cell-signaling networks.
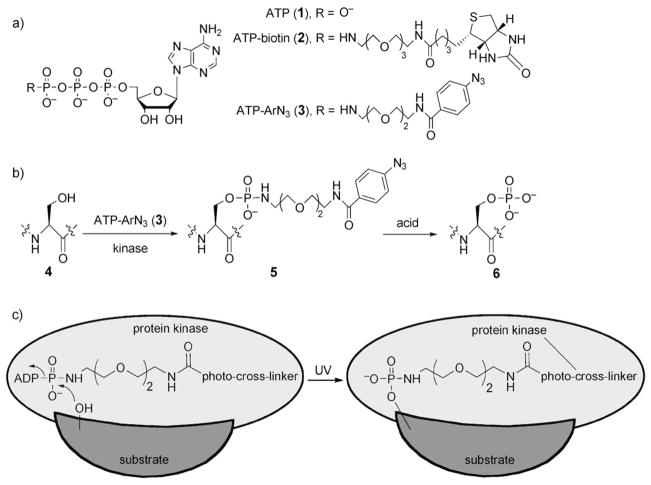
Adenosine 5′-triphosphate (ATP, 1; Scheme 1a) is the universal cosubstrate responsible for protein phosphorylation. During phosphorylation, the γ phosphate group of ATP is transferred to serine, threonine, and tyrosine residues on eukaryotic protein substrates. Recently, we demonstrated that cellular kinases promiscuously accept ATP analogues with γ-phosphate modifications. Specifically, we coupled ATP analogues modified at the γ-phosphate position with either a dansyl[8] or a biotin[9] group (see derivative 2 in Scheme 1a) with cellular kinases to conveniently label peptides and proteins with the dansyl or biotin group for subsequent analysis by mass spectrometry (MS) or gel electrophoresis. Kinase-catalyzed phosphoprotein labeling is a useful enzymatic approach to attach a desired functional probe to a substrate protein.
Scheme 1.
a) Chemical structures of ATP (1) and the γ-phosphate-modified ATP analogues ATP-biotin (2) and ATP-ArN3 (3). b) Kinase-mediated phosphorylation of a hydroxy-containing amino acid (serine is shown) with ATP-ArN3 (3) produces a photo-cross-linking protein product 5. The phosphoramidate bond is cleaved with acid (50% TFA). c) UV irradiation during kinase catalysis promotes covalent cross-linking of the kinase and the substrate through the ATP-ArN3 probe.
To address the challenge of identifying substrates, we envisioned the use of kinase-catalyzed labeling in combination with photo-cross-linking. Enzyme–substrate photo-cross-linking has been applied previously to kinases.[4] We hypothesized that kinases would accept a photoactivatable ATP analogue 3 (ATP-ArN3, Scheme 1a) as a cosubstrate and generate a protein product 5 capable of cross-linking (Scheme 1b). With simultaneous UV irradiation during phosphorylation, the photo-cross-linker would covalently link the substrate to the kinase to produce a high-molecular-weight protein complex (Scheme 1c). The kinase and substrate in the cross-linked product could be identified by using traditional gel electrophoresis and western blotting. A powerful feature of the photo-cross-linking ATP analogue is that it requires phosphorylation for cross-linking, in contrast to the previously reported kinase–substrate photo-cross-linking approach.[4] As a result, all cross-linked complexes necessarily contain a kinase substrate. To our knowledge, the photo-cross-linking analogue ATP-ArN3 (3) would be the first phosphorylation-dependent reagent for kinase–substrate cross-linking.
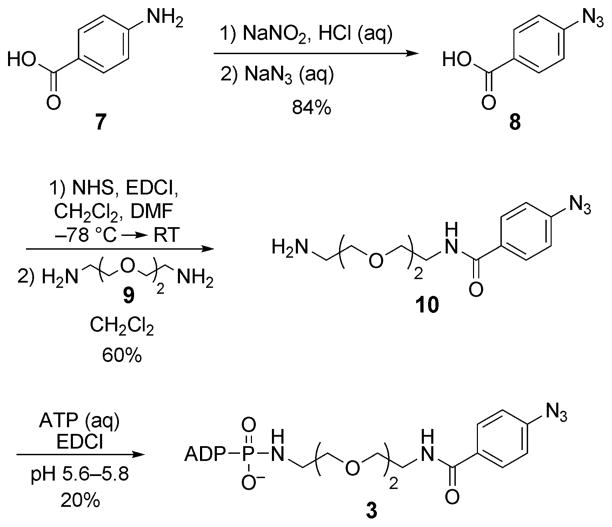
An aryl azide (ArN3 in 3) was selected as the photo-cross-linker since it is small, is stable under standard biological conditions, and has been used previously to photo-cross-link proteins.[10] A hydrophilic linker that mimics that in ATP-biotin (2; Scheme 1a) was also chosen. Thus, the aryl azide 8 was synthesized from commercially available 4-aminobenzoic acid (7; Scheme 2),[11] activated with N-hydroxysuccinimide (NHS), and incubated with 1,8-diamino-3,6-dioxaoctane (9) to give amine 10. The final compound 3 was obtained by coupling amine 10 with ATP in the presence of a water-soluble carbodiimide (EDCI) at pH 5.6–5.8.[4,12]
Scheme 2.
Synthesis of ATP analogue 3. EDCI=1-ethyl-3-(3′-dimethyl-aminopropyl)carbodiimide, DMF = dimethylformamide.
To test the compatibility of ATP-ArN3 (3) with kinase-catalyzed labeling, we incubated peptide substrates 11–13 containing serine, threonine, and tyrosine with 3 along with their respective kinase: PKA, CK2, or Abl (Table 1). We analyzed the products by quantitative matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS to determine the efficiency of the labeling, as described previously.[8,9,13,14] Quantitative MS data (Table 1) indicated that each kinase modified its corresponding peptide substrate with 51–86% conversion (see Schemes S1–S3 in the Supporting Information). The conversion efficiencies observed with ATP-ArN3 (3) were similar to those observed with ATP-biotin (2; 56–80%).[9] These results confirm the ATP-cosubstrate promiscuity of kinases and prove that peptide substrates can be labeled with a photo-cross-linker by using ATP-ArN3 (3).
Table 1.
MALDI-TOF MS analysis of peptides 11–13 after incubation with ATP-ArN3 (3) and PKA, CK2, or Abl kinase.
| Peptide substrate | Kinase | Conversion [%]a |
|---|---|---|
| Ac-LRRTSIIGT (11) | PKA | 86 |
| RRREEETEEE (12) | CK2 | 51 |
| EAIYAAPFAKKK (13) | Abl | 78 |
Conversion was calculated on the basis of quantitative MS by comparison with phosphorylation with ATP (100%).
Having validated that ATP-ArN3 (3) is a cosubstrate, we used the photo-cross-linker to covalently couple kinases with substrates. Cross-linking was tested by incubating CK2 kinase and ATP-ArN3 (3) with the rhodamine-labeled CK2 peptide substrate 14 (Rho-RRREEETEEEE, Rho-14). Simultaneously the reaction mixture was irradiated with UV light (365 nm) to afford a covalent cross-link (Scheme 1c). If kinase–substrate cross-linking occurred, we expected the kinase to become fluorescently labeled. As expected, in-gel fluorescence scanning showed the presence of fluorophore-labeled CK2 after UV-mediated cross-linking with ATP-ArN3 (3) and Rho-14 (Figure 1a, lane 6). No fluorescence labeling was observed without UV light or with natural ATP (Figure 1a, lanes 3–5). These results indicate that the aryl azide and photoactivation are necessary for cross-linking. The addition of competing ATP or the unlabeled CK2 substrate peptide 12 to the reaction mixture prevented fluorophore labeling (Figure 1a, lane 8; see also Figure S4, lane 5 in the Supporting Information). Fluorophore-labeled kinase was absent when the 5(6)-carboxy-X-rhodamine (ROX)-labeled Abl substrate peptide 13 (ROX-EAIYAAPFAKKK, ROX-13) was used (see Figure S4, lane 8 in the Supporting Information). Furthermore, no fluorophore labeling was observed with heat-denatured CK2 (see Figure S4, lane 7 in the Supporting Information). This result indicates that a kinase active site is required for cross-linking. As a final critical control, the cross-linked product was treated with acid, which was expected to cleave the phosphoramidate bond present in the cross-linked complex (Scheme 1b) and release the fluorophore label from CK2. As expected, no fluorescence was observed after treatment with acid (Figure 1a, lane 7; see Figure S4, lane 3 in the Supporting Information); thus, incubation with an acid is a means of liberating the kinase and the substrate. This series of experiments affirms that cross-linking is dependent on kinase-catalyzed labeling. The results indicate that kinase–substrate cross-linking is phosphorylation-dependent and requires active kinase, ATP-ArN3 (3), and UV irradiation.
Figure 1.
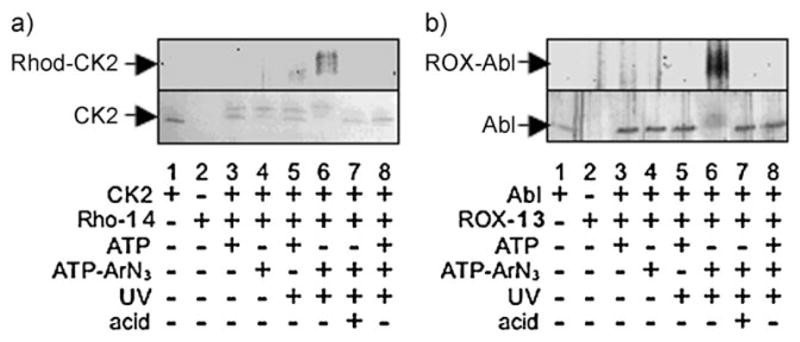
Kinase-catalyzed photo-cross-linking with peptide substrates. a) CK2 or b) Abl kinase was incubated with the rhodamine-labeled CK2 substrate peptide 14 (Rho-14) or the ROX-labeled Abl substrate peptide 13 (ROX-13), respectively, in the presence of ATP (1) or ATP-ArN3 (3). The resulting mixtures were separated by SDS-PAGE and visualized by in-gel fluorescence scanning (a and b, top), Coomassie staining (a, bottom), or silver staining (b, bottom). Reaction mixtures were incubated either with or without exposure to UV light (365 nm). TFA was added to a final concentration of 50% after cross-linking (lane 7). The appearance of CK2 as a double band is probably due to limited proteolysis of the catalytic subunit.[18] The gels are representative of at least three independent experiments.
To validate the compatibility of phosphorylation-dependent substrate cross-linking with multiple kinases, we tested the reaction of the Abl kinase with the ROX-labeled Abl peptide substrate ROX-13. As seen with CK2, Abl was fluorophore-labeled only after UV-mediated cross-linking with ATP-ArN3 and ROX-13 (Figure 1b, lanes 3–6). Further-more, the observation that fluorophore labeling was lost after incubation with an acid (Figure 1b, lane 7) confirmed the presence of the phosphoramidate bond. The results indicate that phosphorylation-dependent kinase–substrate cross-linking is compatible with multiple kinases.
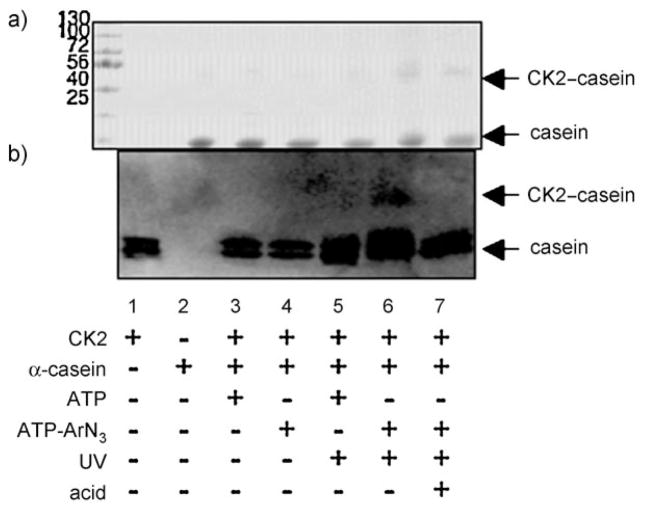
Having established phosphorylation-dependent cross-linking between kinases and peptide substrates, we next tested cross-linking with a full-length protein substrate. The α-casein protein has been widely used as a model CK2 substrate in phosphoprotein analysis.[15] Cross-linking was carried out by incubating a mixture of CK2 and α-casein with ATP-ArN3 (3) under UV light. The products were separated by SDS-PAGE and analyzed by Coomassie staining (Figure 2a) and western blotting (Figure 2b). Coomassie staining showed an increase in the intensity of a band at roughly 68 kD (Figure 2a, lane 6), which corresponds to the expected cross-linked product of the catalytic α subunit of CK2 (45 kD)[16,17] and α-casein (23 kD). Importantly, immunoblotting showed the presence of CK2 in the 68 kDa complex (Figure 2b, lane 6). The cross-linked complex was absent in reactions performed without UV light or with ATP (Figure 2b, lanes 3–5). Furthermore, the CK2-cross-linked complex was lost after acid treatment (Figure 2b, lane 7), a result consistent with cleavage of the phosphoramidate bond and degradation of the α-casein–CK2 complex. These experiments demonstrate that a full-length protein substrate is compatible with phosphorylation-dependent kinase–substrate cross-linking.
Figure 2.
Kinase-catalyzed photo-cross-linking with full-length α-casein. CK2 kinase was incubated with full-length α-casein in the presence of ATP (1) or ATP-ArN3 (3), and the resulting mixtures were separated by SDS-PAGE and visualized by a) Coomassie staining or b) western blotting with a CK2 antibody. The expected 68 kDa cross-linked complex is indicated as CK2–casein. The components of each reaction mixture are indicated below each lane (see Figure 1 legend). The gels are representative of at least three independent experiments.
In conclusion, we have demonstrated the first phosphorylation-dependent kinase–substrate-cross-linking strategy. The results indicate that cross-linking requires a photoactivable ATP analogue, such as ATP-ArN3, UV irradiation, and an active kinase. Because phosphate transfer is required, a powerful feature of ATP-ArN3-mediated cross-linking is that a kinase substrate is necessarily present in the cross-linked product. When coupled with mass spectrometric analysis, phosphorylation-dependent kinase–substrate cross-linking can be used to determine the sites of phosphorylation as well as the effector kinase. Importantly, the cross-linking approach is appropriate for studies with cell-lysate or homogenized-tissue samples, since the natural kinase and substrate are utilized. We expect phosphorylation-dependent kinase–substrate cross-linking to aid in the characterization of cell-signaling networks.
Experimental Section
ATP-ArN3 (3) was synthesized as described in the Supporting Information and characterized by 1H, 13C, and 31P NMR spectroscopy, UV spectroscopy, and HRMS. The CK2 and Abl substrate peptides 12 and 13 were purchased from New England Biolabs (NEB). The PKA substrate peptide 11 and fluorophore-labeled peptides 14 and 13 were synthesized by 9-fluorenylmethoxycarbonyl-based solid-phase peptide synthesis, as described previously.[8,14] PKA, CK2, and Abl were purchased from NEB. For kinase-catalyzed labeling, each reaction mixture contained either ATP (1) or ATP-ArN3 (3; 1.3 mM) along with the substrate peptide (33.3 μM). PKA (26.6 units μL−1), CK2 (9 units μL−1), or Abl (9 units μL−1) was added to each reaction mixture, along with the buffer supplied by the manufacturer. The reaction mixtures were incubated for 2 h at 30°C and then subjected to quantitative MALDI-TOF MS analysis (Bruker Daltonics), as described previously.[8,9,13,14] For the photo-cross-linking experiments, CK2 (50 units μL−1, 0.7 μM) was incubated with either Rho-14 (200 μM) or full-length α-casein (100 μM, Sigma), and Abl (20 units μL−1, 44.4 nM) was incubated with ROX-13 (200 μM). All reaction mixtures also contained ATP (1) or ATP-ArN3 (3; 1.3 mM) and the kinase buffer supplied by the manufacturer. Photo-cross-linking was initiated by irradiation with a handheld UV lamp at 365 nm for 2 h at 30°C. The phosphoramidate bond in the cross-linked products was cleaved by adding trifluoroacetic acid (TFA) to a final concentration of 50% and incubating the mixture at 16°C for 3 h. The reaction products were separated by SDS-PAGE and visualized by Coomassie staining, silver staining, in-gel fluorescence imaging, or western blotting with a CK2 antibody (Millipore) after transfer onto a polyvinylidene difluoride membrane (Immobilon-PSQ, Millipore). Additional experimental details and data are available as Supporting Information.
Supplementary Material
Footnotes
We thank the National Institutes of Health (GM079529) and Wayne State University for funding.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200905244.
References
- 1.Statsuk AV, Maly DJ, Seeliger MA, Fabian MA, Biggs WH, Lockhart DJ, Zarrinkar PP, Kuriyan J, Shokat KM. J Am Chem Soc. 2008;130:17568. doi: 10.1021/ja807066f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu K, Kalesh KA, Ong LB, Yao SQ. ChemBioChem. 2008;9:1883. doi: 10.1002/cbic.200800212. [DOI] [PubMed] [Google Scholar]
- 3.Maly DJ, Allen JA, Shokat KM. J Am Chem Soc. 2004;126:9160. doi: 10.1021/ja048659i. [DOI] [PubMed] [Google Scholar]
- 4.Parang K, Kohn JA, Saldanha SA, Cole PA. FEBS Lett. 2002;520:156. doi: 10.1016/s0014-5793(02)02778-3. [DOI] [PubMed] [Google Scholar]
- 5.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. Science. 2002;298:1912. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 6.Eblen S, Kumar N, Shah K, Henderson M, Watts C, Shokat K, Weber M. J Biol Chem. 2003;278:14926. doi: 10.1074/jbc.M300485200. [DOI] [PubMed] [Google Scholar]
- 7.Blethrow JD, Glavy JS, Morgan DO, Shokat KM. Proc Natl Acad Sci USA. 2008;105:1442. doi: 10.1073/pnas.0708966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green KD, Pflum MKH. ChemBioChem. 2009;10:234. doi: 10.1002/cbic.200800393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green KD, Pflum MKH. J Am Chem Soc. 2007;129:10. doi: 10.1021/ja066828o. [DOI] [PubMed] [Google Scholar]
- 10.Brunner J. Annu Rev Biochem. 1993;62:483. doi: 10.1146/annurev.bi.62.070193.002411. [DOI] [PubMed] [Google Scholar]
- 11.Xiong Y, Bernardi D, Bratton S, Ward MD, Battaglia E, Finel M, Drake RR, Radominska-Pandya A. Biochemistry. 2006;45:2322. doi: 10.1021/bi0519001. [DOI] [PubMed] [Google Scholar]
- 12.Yarbrough LR, Schlageck JG, Baughman M. J Biol Chem. 1979;254:12069. [PubMed] [Google Scholar]
- 13.Tao WA, Wollscheid B, O3Brien R, Eng JK, Li X-j, Bodenmiller B, Watts JD, Hood L, Aebersold R. Nat Methods. 2005;2:591. doi: 10.1038/nmeth776. [DOI] [PubMed] [Google Scholar]
- 14.Warthaka M, Karwowska-Desaulniers P, Pflum MKH. ACS Chem Biol. 2006;1:697. doi: 10.1021/cb6003564. [DOI] [PubMed] [Google Scholar]
- 15.Mackinlay AG, West DW, Manson W. Eur J BIochem. 1977;76:233. doi: 10.1111/j.1432-1033.1977.tb11588.x. [DOI] [PubMed] [Google Scholar]
- 16.Allende J, Allende C. FASEB J. 1995;9:313. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 17.Litchfield DW. Biochem J. 2003;369:1. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarno S, Vaglio P, Meggio F, Issinger OG, Pinna LA. J Biol Chem. 1996;271:10595. doi: 10.1074/jbc.271.18.10595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.