Abstract
Tissue engineering holds great promise for regeneration and repair of diseased tissues, making the development of tissue engineering scaffolds a topic of great interest in biomedical research. Because of their biocompatibility and similarities to native extracellular matrix, hydrogels have emerged as leading candidates for engineered tissue scaffolds. However, precise control of hydrogel properties, such as porosity, remains a challenge. Traditional techniques for creating bulk porosity in polymers have demonstrated success in hydrogels for tissue engineering; however, often the conditions are incompatible with direct cell encapsulation. Emerging technologies have demonstrated the ability to control porosity and the microarchitectural features in hydrogels, creating engineered tissues with structure and function similar to native tissues. In this review, we explore the various technologies for controlling the porosity and microarchitecture within hydrogels, and demonstrate successful applications of combining these techniques.
Introduction
In most scaffolding materials, the porosity of the scaffolds plays an important role in directing tissue formation and function.1–3 A substantial amount of scaffold porosity is often necessary to allow for homogeneous cell distribution and interconnection throughout engineered tissues. In addition, increased porosity can have a beneficial effect on the diffusion of nutrients and oxygen, especially in the absence of a functional vascular system.2 Hydrogels have been used as scaffolds for tissue engineering applications because of their similarities with extracellular matrix (ECM), excellent biological performance, and inherent cellular interaction capability. Hydrogels are crosslinked macromolecular networks formed by hydrophilic polymers swollen in water or biological fluids.4 Upon implantation, hydrogel porosity allows for local angiogenesis to occur, which is a key requirement for vascularized tissues. The degree of porosity will also have a substantial effect on the mechanical properties, with the stiffness of the scaffold decreasing as porosity increases,5 and the mechanical characteristics varying greatly with fluid flux caused by deformation.6 The porosity and pore architecture in terms of porosity and pore interconnectivity play a significant role in cell survival, proliferation, and migration to fabricate functional hydrogel, and secrete ECM.7,8 The pore interconnectivity allows for cell ingrowth, vascularization, and nutrient diffusion for cell survival.9–11 The extent of ECM secretion also increases by increasing the pore size.8 It was found that in genipin crosslinked gelatin hydrogels with smaller pores, the tendency was tilted toward cell growth rather than of ECM secretion,8 resulting in overconfluence during the middle and late stages of differentiation; consequently, the extent of ECM secretion decreased compared to that within gelatin hydrogels with larger pores.8 The average pore size of the hydrogels greatly affects the growth and penetration of cells in the 3D structure of hydrogels. Without using an intrinsic capillary network, the maximal thickness of engineered tissue is approximately 150–200 μm because of insufficient oxygen and nutrient transport within the deeper compartments of the biomaterial.12 In addition, mean pore size has been shown to impact the amount of contraction a graft will undergo after implantation. An average pore diameter of 20–125 μm was required for contraction-inhibiting activity to be observed in collagen–glycosaminoglycan graft copolymers used for dermal repair.13 The effect of implant pore size on tissue regeneration is emphasized by experiments demonstrating the optimum pore size of 5 μm for neovascularization, 5–15 μm for fibroblast ingrowth, 20–125 μm for regeneration of adult mammalian skin, 100–350 μm for regeneration of bone, 40–100 μm for osteoid ingrowth, and 20 μm for the ingrowth of hepatocytes.14 Fibrovascular tissues also require pore sizes greater than 500 μm for rapid vascularization and survival transplanted cells.15 The microscale features of individual and clusters of pores combine to create the hydrogel microarchitecture, controlling many aspects of cellular orientation, aggregation, and function.2,16 Thus, control of scaffold porosity and microarchitecture plays a key role in regulating engineered tissue properties.17
Control of these intricate hydrogel features is important toward guiding the development of the resulting engineered tissues. Techniques to control the overall porosity of hydrogels include solvent casting/particle leaching, freeze-drying, gas foaming, and electrospinning. Combinations of these methods have been used to fabricate porous hydrogels for many tissue engineering applications. In addition, more advanced control of specific pore features and microarchitecture has been achieved through various micropatterning18 and micromolding19–22 techniques. With these techniques it is possible not only to specifically control individual and group pore architecture, but also to take the next step to create microvascular features to improve integration within host tissues. In this review we will describe the potential and limitations of these methods to control bulk porosity as well as the microarchitectural features of channels and capillary networks.
Macroscale Porosity and Microarchitecture
Solvent casting/particle leaching
Solvent casting/particle leaching begins with the dispersion of a porogen with controlled particle size into a polymer solution. The appropriate technique is used to solidify the polymer, producing a polymer–porogen network.23,24 The solute particles are subsequently leached, or dissolved away by immersing the material in a selective solvent, resulting in the formation of a porous network.
A wide variety of porogens have been employed for this technique depending on the hydrogel and application. Historically, salt particles are most commonly used because they are inexpensive, widely available, easy to handle, and stable under an assortment of processing conditions. Alternative porogen materials, including sugars,25 paraffin,26 and gelatin,27 have also been employed with hydrogels. The nature of the porogen has been demonstrated to play an important role in the interconnectivity of pores and in turn the cell–scaffold interactions after seeding.28 Porogen geometry has also been shown to influence the structure of the porous network formed using particle leaching.29 In particular, it has been demonstrated that spherical particles result in more interconnected pores than cubic particles at the same final porosity.29
Using salt leaching, poly(ethylene glycol) (PEG)–poly(ɛ-caprolactone) (PCL)–based hydrogels were produced using NaCl as a porogen (pore size distribution: 180–400 μm) and dimethyl sulfoxide as a solvent.30 After leaching with distilled water, a highly porous and interconnected matrix with enhanced swelling properties was formed and was demonstrated to be capable of facilitating efficient cell seeding of rabbit chondrocytes.30 Similar processing techniques using NaCl have recently been applied to oligo[(PEG) fumarate],31 alginate-g-poly(N-isopropylacrylamide),32 poly(2-hydroxyethyl methacrylate),33 and other hydrogel systems.
In another variation, salt leaching has been used to create polyester scaffold templates that can be utilized for fabrication of macroporous hydrogels. In one study, a PEG-poly(lysine) prepolymer was cast and cured around a salt-leached poly(lactic acid-co-glycolic acid) (PLGA) scaffold.34 The PLGA network was degraded using sodium hydroxide (NaOH), leaving behind a macroporous PEG-poly(lysine) hydrogel.34 Endothelial cells were seeded and shown to establish tubules with dimensions that were consistent with the pore size, suggesting that the scaffold structure provided physical direction for vessel formation.34
Particle leaching has recently been used to fabricate porous components in microfluidic devices.35 A living radical photopolymer system and salt leaching technique were combined to generate porous polymer networks in microfluidic channels. The microchannel was produced by living radical photopolymer, whereas porosity was generated after salt leaching.35 Valve systems were fabricated and it was shown that porous valves will swell and close much more rapidly than nonporous valves made with similar materials.35
A major advantage of the solvent casting method is that pore size and overall porosity can be tuned by changing the particle size and concentration within the prepolymer, respectively. Additionally, this technique can be performed feasibly on a small scale, making it widely available and well suited for use during the development stages of a new hydrogel system. However, using the solvent casting method there is little control over the orientation and the degree of interconnectivity of pores. Owing to limitations associated with removing solid particles from the hydrogel, this technique is usually restricted to the fabrication of commonly thin hydrogel sheets (typically less than 500 μm) that must later be assembled into a larger construct. It should be noted that many particle leaching methods rely on the use of cytotoxic organic solvents that require lengthy drying times to assure complete removal and are not compatible with cell viability.
Freeze-drying
Freeze-drying, also known as lyophilization, has been used extensively for the fabrication of porous hydrogels for tissue engineering. This method uses rapid cooling to produce thermodynamic instability within a system and cause phase separation. The solvent is then removed by sublimation under vacuum36 leaving behind voids in the regions it previously occupied.
This technique was applied to produce porous collagen–chitosan hydrogels.37 After preparation of a 9:1 collagen:chitosan blend, glutaraldehyde solution was added to crosslink the mixture before freeze-drying. The freezing temperature before lyophilization was shown to have an impact on the characteristics of the resulting hydrogel. In particular, samples that were frozen at −20°C and −80°C resulted in open pore structures after lyophilization, whereas parallel sheet structure was obtained at −196°C (in liquid N2).37 Moreover, the swelling ratio diminished from 4400% to 2000% as the temperature decreased from −20°C to −196°C due to the reduction of contact surface area.37 Both in vitro and in vivo characterizations confirmed that the fabricated hydrogels seeded by preadipocytes cells were biocompatible, induced vascularization, and formed adipose tissue.37
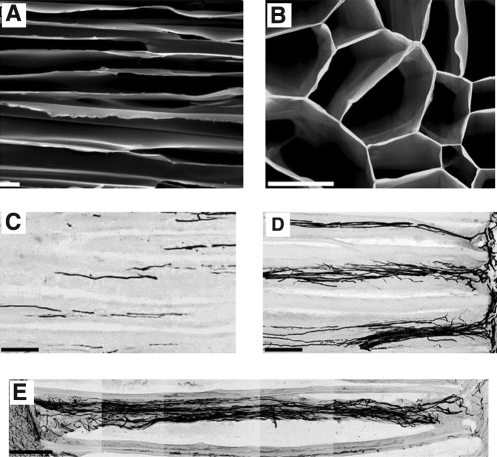
A modified freeze-drying procedure has been used to fabricate agarose hydrogels with linear pores (Fig. 1A, B).38 This method utilizes a freezing step that involves exposing only one end of a pillar of agarose to a block of dry ice immersed within a pool of liquid nitrogen.38 The resulting uniaxial temperature gradient caused ice crystals to form that were oriented in the direction of the gradient.38 Upon removal of water by lyophilization, a highly linear network of porous channels was formed with dimensions suitable for cell infiltration.38 Scaffolds fabricated with this method were able to guide axonal regeneration in a spinal cord injury model (Fig. 1C–E).39
FIG. 1.
Morphology and in vivo performance of freeze-dried agarose hydrogel scaffolds. Scanning electron microscopy (SEM) images of longitudinal (A) and cross-sectional (B) orientations show linear porosity. Axonal penetration after 1 month in vivo in a spinal cord injury model in the absence (C) and presence (D) of brain-derived neurotrophic factor. The best sample attained shows axons completely crossing the construct (E). Scale bar: 100 μm. Adapted with permission from Stokols et al.39
Since the quenching procedure used in freeze-drying techniques is generally rapid, the phase separation occurring during the initial freezing may not be complete, resulting in the formation of a polymer-lean phase that may still contain suboptimal amounts of polymer.40 To circumvent this problem, an alternate method involving the use of repeated freeze–thaw cycles may be employed. Upon repeating the freezing process, further phase separation of the polymer-lean phase within the pores occurs, forming a new, more diluted, polymer-lean phase and a more concentrated polymer-rich phase allowing the formation of larger pores. This technique was used to fabricate poly(vinyl alcohol) (PVA) hydrogels using a cycle consisting of a 20 h freeze step at −22°C followed by a 4h thawing step at 25°C.40
Freeze-drying techniques are suitable for a wide range of hydrogel materials, including natural41–43 and synthetic hydrophilic polymers,44,45 to produce interconnected porous structures. However, these methods encounter difficulty in precisely tuning pore size since the hydrogel architecture formed using this method are extremely sensitive to the kinetics of the thermal quenching process. Other issues associated with this technique are the low structural stability and generally weak mechanical properties of the fabricated materials. The freeze-drying process often results in the formation of a surface skin because the matrix may collapse at the scaffold–air interface due to the interfacial tension caused by solvent evaporation.46 In addition, freeze-drying is energy intensive and requires a relatively long processing time for complete removal of solvent.23
To address these issues, an alternative freeze gelation process has been utilized to produce porous hydrogels for chitosan and alginate, respectively.46 In this study, a frozen chitosan solution (−20°C) was immersed in a precooled NaOH/ethanol solution to adjust its pH to allow for gelation of chitosan below its freezing point.46 Since the polymer was already gelled in the frozen state, the solvent could be removed with drying at room temperature without the formation of a surface skin. A highly interconnected porous hydrogel with the pores that ranged from 60 to 150 μm and porosity of 90% was produced using this technique.46 In vitro studies indicated that seeded rat osteoblast-like cells were able to attach, spread, and proliferate both inside and on the surface of the resulting hydrogel.46 Compared with conventional freeze-drying, this method has improved energy efficiency, and is amenable to scale up.46
Gas foaming
Conventional gas foaming
Gas foaming utilizes the nucleation and growth of gas bubbles dispersed throughout a polymer to generate a porous structure.47 The gas bubbles can either be formed by foaming/blowing agent via chemical reaction,48 or be released from a presaturated gas–polymer mixture at a high pressure.47 A foaming/blowing agent is a substance that is mixed into the prepolymer and generates a gas when it chemically decomposes. The most commonly used foaming agent for fabricating porous hydrogels is sodium bicarbonate owing to its ability to generate CO2 in mildly acidic solutions. As an example, sodium bicarbonate has been used to fabricate an acrylic acid–acryl amide porous hydrogel by crosslinking acrylic acid and acryl amide with N,N′-methylenebisacrylamide.49 The resultant hydrogel showed an interconnected structure with pore size ranging from 100 to 250 μm and 100% equilibrium swelling ratio.49
Sodium bicarbonate has recently been used as a blowing agent to create macroporous hydrogels from photocrosslinkable PEG diacrylate covalently linked to the peptide sequence RGD to promote cell adhesion on this otherwise cell-repellant hydrogel material. This scaffold was found to support the adhesion and long-term viability of human mesenchymal stem cells and facilitated mineralization when exposed to osteogenic medium.50 Interestingly, human mesenchymal stem cells were also capable of binding to porous PEG-based gels even in the absence of the RGD adhesion sequence, suggesting that structure of the scaffold was sufficient for cell attachment.50
Ammonium bicarbonate is another gas blowing agent that has been used to produce interconnected pores inside hydrogels via its decomposition to CO2 and NH3. As an example, ammonium bicarbonate was added during the irradiation of carboxymethylcellulose–sodium and polyacrylamide to induce porosity51 and was shown to enhance the swelling ratio in comparison with hydrogels prepared in the absence of a blowing agent.
Owing to the wide availability of the most common blowing agents, this method provides an inexpensive platform to introduce porosity into hydrogel materials. The blowing agents used are generally cell friendly, and this technique can be employed without the use of organic solvent, making it well suited for tissue engineering applications.
Gas foaming with dense gas CO2
A dense gas is a fluid above or close to its critical temperature and pressure, which demonstrates physical properties intermediate to those of a true gas or liquid phase. Dense gas CO2 has a relatively low critical temperature (Tc: 31°C) and is an attractive candidate for biomaterial processing because it is inert, nontoxic, and inexpensive.23,52 This gas has been widely used as a foaming agent to induce porosity in the structure of several common hydrophobic polymers such as poly(lactic acid) (PLA), PLGA, and PCL.23,52–55 However, dense gas CO2 generally has low solubility in hydrophilic polymers. Various techniques such as CO2–water emulsion templating56–60 or the use of a cosolvent system have been developed to improve the ability of a dense gas to diffuse into a hydrophilic polymer and produce porosity.61,62 Using dense gas CO2 to generate porosity eliminates the use of surfactant or foam stabilizer that is required in conventional gas foaming techniques.50,51
Emulsion templating involves forming a high internal phase emulsion (HIPE) that is composed of an external phase consisting of a curable polymer and an internal phase made of minute droplets. The structure of the external phase is locked in by reaction-induced phase separation such as sol–gel chemistry or free-radical polymerization.60 Subsequent removal of the emulsion droplets allows highly porous and interconnected materials (polyHIPES) to be obtained.60 Conventional emulsion templating techniques require large amounts of organic solvent to generate the internal phase (more than 75%), which may be difficult to remove after curing,56 resulting in cell cytotoxicity. CO2–water HIPEs, using supercritical CO2 as the internal droplet phase and an aqueous solution as the external phase, have been considered to produce emulsion-templated materials without the use of an organic solvent.59 Supercritical CO2–water emulsion templating techniques have been used to produce highly porous crosslinked hydrogels using many naturally occurring biopolymers such as dextran,56 chitosan,59 and alginate57 and synthetic polymers, including PVA, blended PVA/PEG,59 and CaCO3/polyacrylamide composites.60
CO2–water emulsion polymerization templating has been used with the polysaccharide dextran to form highly interconnected and thin-walled porous hydrogels.56 In this process, perfluoropolyether was employed as a surfactant to stabilize the supercritical CO2–water emulsion and potassium peroxydisulfate as an initiator for radical polymerization to produce an interconnected porous dextran hydrogel.56 Variation of the volume fraction of CO2 did not have a significant effect on pore size.56 It was found that as the volume fraction of CO2 increased, the walls between adjacent pores became thinner and the pores had a more polyhedrical shape. An increase in the surfactant concentration led to more open interconnected structure. The use of nonbiodegradable surfactant and a mean pore size less than 26 μm may not allow this technique to be used for cell culture applications.
Physically crosslinked alginate hydrogels have been produced using the CO2–water emulsion templating technique.57 In this method, supercritical CO2 simultaneously served as the templating agent as well as inducing acidity, which caused the release of calcium ions from their chelated form, leading to crosslinking of the alginate and formation of a porous hydrogel.57 The fabricated alginate hydrogels displayed an open and interconnected pore network in the range of 23.9–250 μm depending on the surfactant concentration and CO2 fraction.57
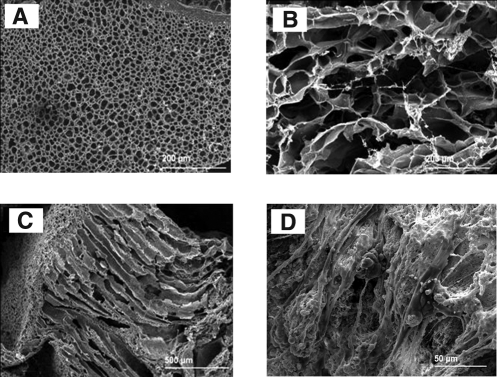
A dense gas hydrogel formation process was designed to fabricate highly porous biopolymeric hydrogels such as elastin-based hydrogels using different crosslinking agents, including glutaraldehyde63 and hexamethylene diisocyanate.64 In the latter process, α-elastin solution in phosphate-buffered saline or dimethyl sulfoxide was mixed with the crosslinking agent and pressurized with CO2 to 60 bar for at least 30 min to crosslink.64 Subsequent depressurization induced large channels in the 3D structures of the hydrogels (Fig. 2C). The average pore size of hydrogels fabricated by using 2% (v/v) hexamethylene diisocyanate increased from 3.9 ± 0.8 to 79.8 ± 54.8 μm when pressure was increased from 1 bar to 60 bar (Fig. 2A, C). The α-elastin hydrogels facilitated fibroblast growth and proliferation in the 3D structures (Fig. 2D).63
FIG. 2.
α-Elastin hydrogels fabricated using high-pressure CO2. SEM images of α-elastin hydrogels fabricated at atmospheric pressure (A) and 60 bar (B, C) [top surface (B), cross section (C)]. Fibroblast cells were shown to attach on the scaffold surface (D). Adapted with permission from Annabi et al.64
A cosolvent is often used to improve the diffusion of a dense gas into a hydrophilic polymer to produce porous hydrogels. In a recent study, a biodegradable polymer such as collagen and gelatin as well as a solvent such as ethanol or diluted acid were placed in a high pressure chamber.62 The vessel was then pressurized with a supercritical fluid at a predetermined temperature and pressure to allow the supercritical fluid to dissolve into the polymer with the aid of the solvent.62 Finally, the pressure was released and a porous structure was obtained. The size and morphology of the porous hydrogel can be controlled by adjusting the operating pressure and temperature.62
The use of supercritical CO2 allows low temperatures to be maintained within the polymer, which is favorable if temperature-sensitive growth factors are to be directly incorporated into the material. The requirement of a reactor that can handle high pressures and access to supercritical CO2 limits wide access to this processing technique. It is critical to determine the effect of high-pressure CO2 on the biopolymers such as proteins. High-pressure CO2 has been used in micronization, impregnation, and fabrication of hydrogels using proteins such as insulin,65,66 catalase,67 recombinant human deoxyribonuclease,68 elastin,69 trypsin, and lysozymes.65,70 The biological activity and structural perturbations induced during the dense gas process were found to be protein specific. The biochemical integrity of proteins such as elastin,69 lysozyme, and insulin65 was preserved upon exposure to dense gas CO2. The biological activity of proteins with isoelectric points above 3 such as recombinant human deoxyribonuclease68 and β-galactosidase71 was a function of CO2 operating conditions.
Electrospinning
Electrospinning has garnered significant interest in the field of tissue engineering because of its ability to fabricate interconnected porous scaffolds. The basic premise of electrospinning is the use of an externally applied electric field to draw fibers from a charged polymer solution held by surface tension at the end of a capillary tube. The polymer is charged by applying a high voltage and is then drawn as a thin jet toward an oppositely charged collector plate by electrostatic force. As the jet travels through the air, the solvent evaporates and jet diameter decreases substantially.72 The fibers are typically collected on a stationary ground plate, but a rotating drum can be used as the collector to achieve a preferred orientation.73,74 The resulting material properties such as fiber diameter, porosity, and morphology can be controlled by various parameters such as applied voltage, viscosity, solution conductivity, and temperature.75 This technique can be used to produce fibers in the micro- and nanometer range.
Electrospinning has been used to fabricate porous hydrogels of PVA and polyacrylic acid.76 Using this method, a PVA and polyacrylic acid solution mixture at different ratios was placed in a glass capillary with the 0.4 mm inner diameter tip, which was tilted downward at an angle between 0° and 30° depending on the solution viscosity.76 An ultrafine (submicron) fibrous hydrogel was formed after the electrospinning process. The majority of the interfiber pores were connected, which is desirable for tissue engineering applications to allow cell–cell interaction and migration.76 The swelling behavior of the fibrous hydrogel membrane was found to be dependent on the environmental pH and could be enhanced by application of an electric field.76
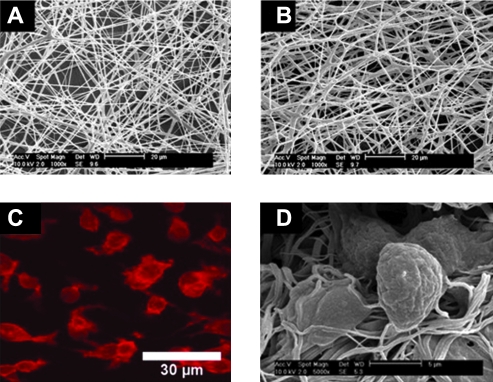
The techniques of electrospinning and salt-leaching were combined to form a macroporous hyaluronic acid (HA)–collagen hydrogel with fibers on the nanometer scale.77 In this process, HA and collagen were dissolved in a NaOH/N,N-dimethyl formamide solvent mixture.77 During the electrospinning process, NaCl particles were simultaneously deposited into the electrospun fibers to induce interfiber porosity. After subsequent chemical crosslinking and salt leaching, a porous HA-based hydrogel was produced.77 In vitro studies demonstrated that the hydrogel could support adhesion, proliferation, and retention of in vivo morphology of bovine chondrocyte cells (Fig. 3).77
FIG. 3.
Morphology and in vitro assessment of hyaluronic acid (HA)–based scaffolds. SEM images of electrospun salt-leached HA-based scaffolds at 95:5 (A) and 80:20 (B) ratios of HA:collagen using sodium hydroxide and N,N-dimethyl formamide as a mixed solvent. Laser scanning confocal microscopy (C) and SEM (D) images showed that chondrocytes maintained their typical rounded morphology on an 80:20 HA:collagen scaffold after 3 days. Adapted with permission from Kim et al.77 Color images available online at www.liebertonline.com/ten.
Many natural polymers such as collagen, silk fibroin, and fibrinogen have been processed using electrospinning into fine nonwoven mats with fibers in the micro- and nanometer range for tissue engineering applications.74,78–82 Various cell types have been reported to attach, proliferate, and differentiate within these matrices, demonstrating their utility in tissue engineering.73 However, significant challenges that still exist in using this technique with hydrogels include an inability to fabricate complex 3D hydrogel shapes, poor mechanical properties, and limited control over the porosity and pore size.83
Traditional methods for hydrogel fabrication commonly involve procedures or chemicals that are not desirable for cell viability. Low pressure and dehydration in freeze-drying techniques, the use of surfactants in dense gas templating techniques, organic solvents, and cytotoxic crosslinkers are examples of these abrasive conditions. Typically, cells can be seeded into these materials after creation; however, the inability to encapsulate the cells during the initial fabrication could diminish the ability to achieve heterogeneous cell distribution.84 For example, if the pores are too small for cells to penetrate, or if the pores are insufficiently interconnected, it may not be possible to seed cells throughout a hydrogel-based scaffold after formation, whereas this is readily achievable if the fabrication technique is cytocompatible. Direct encapsulation is possible in hydrogels that are created through such techniques as temperature change,22 change in ion concentration,85,86 or UV crosslinking.21 Although cell viability can be maintained long-term after creation using these methods, often these techniques do not allow for control over pore size and distribution as compared with more cytotoxic techniques. A balance must be reached in terms of heterogeneous cell seeding, pore size and distribution, and the hydrogel physical properties for each intended application.
Inducing porosity in hydrophilic–hydrophobic hybrid hydrogels
A common challenge in many of the techniques described previously for the fabrication of porosity in hydrogels is to improve the mechanical stability of the 3D structure. Hydrophilic hydrogels often exhibit poor mechanical properties, especially in their swollen state, which can be an obstacle for their broad applications in tissue regeneration. One technique is the addition of biodegradable hydrophobic polymers to enhance the mechanical properties of purely hydrophilic hydrogels. For example, the mechanical properties of collagen, elastin, and gelatin hydrogels fabricated by electrospinning were dramatically increased with the addition of 10% PCL without use of any chemical crosslinker.87
One of the major issues in the fabrication of porosity in hybrid hydrogels is the phase separation caused by intrinsic immiscibility of hydrophilic and hydrophobic polymers. Before the creation of porosity by several methods such as solvent casting/particulate leaching, freeze-drying, gas foaming, and electrospinning, a homogeneous mixture of the polymer components must be formed. Methods including intimate mixing and interpenetrating polymer network (IPN) via sequential or simultaneous reaction of both polymers have been used to mix the two phases.
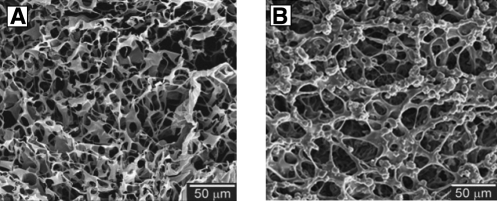
Mechanical stirring can be used for intimate mixing of the polymer solutions. A homogeneous single phase is readily acquired by simple mechanical stirring when both compounds are soluble in one solvent. Using this technique, chitosan/PCL porous hydrogels were prepared by dissolving both compounds in an acidic solutions followed by freeze-drying (Fig. 4A, B).88 The hydrogel exhibited excellent support for cellular activity in both 2D and 3D. Mechanical stirring and an emulsifier have been used to create emulsions of two immiscible phases before the fabrication of porosity in hydrogels. Porous PVA-PCL hybrid hydrogels with enhanced mechanical properties were successfully fabricated by creating an emulsion followed by freeze-drying and crosslinking.88 In this study, PVA and PCL solutions were well mixed by using mechanical stirring to create homogeneous foam. The foam was immediately frozen at −70°C and lyophilized at −85°C for 24 h to acquire a highly porous 3D hydrogel with open and interconnected pores ranging from 30 to 300 μm.88 The compressive loading of this hydrogel was enhanced at least twofold by increasing the concentration of PCL from 30 to 50 wt%. Fibroblasts and chondrocyte cells were shown to grow well in 3D porous composite hydrogels fabricated from PVA-PCL (1:1 weight ratio), underlining their excellent properties for tissue regeneration applications.89
FIG. 4.
SEM images of chitosan-poly(ɛ-caprolactone) hydrogel scaffold formed by freeze-drying. Hydrogels of pure chitosan (A) and chitosan blended with 50 wt% poly(ɛ-caprolactone) (B). Solutions in 25 vol.% acetic acid were frozen at −196°C and lyophilized at −80°C. Adapted with permission from Sarasam et al.87
The creation of an IPN is an efficient method to reinforce porous hydrogels. In one study, nanosized particles of PLA was added to a solution of N-isopropyl acrylamide and a crosslinker to form a porous hydrogel by in situ polymerization and crosslinking.89 PLA nanospheres were attached to the network matrix of poly N-isopropyl acrylamide hydrogels and generated enlarged porous structure. The presence of these large pores enhanced the swelling ratio at room temperature.90 An IPN structure of porous-hybrid hydrogel of poly(ethyl methacrylate) as a hydrophobic and poly(2-hydroxyethyl acrylate) as a hydrophilic network was fabricated by using sequential polymerization.90 A hydrophobic poly(ethyl methacrylate) network was prepared by simultaneous polymerization and crosslinking of ethyl methacrylate (EMA) monomers in ethanol, followed by polymerization and crosslinking of 2-hydroxyethyl acrylate (HEA) monomers. Herein ethanol served as both a solvent and a porogen to induce porosity in this hybrid hydrogel.91,92
The combination of solvent casting/particulate leaching and freeze-drying has been used to produce porous hybrid hydrogels. A hybrid method for the fabrication of a homogenous mixture of porous collagen hydrogel and biodegradable polymers such as poly(glycolic acid), PLA, and PLGA has been reported.91,92 A porous structure of hydrophobic polymer was first formed by solvent casting/particulate leaching technique and then immersed in a collagen solution. The mixture was placed under vacuum to fill the pores with collagen solution and then freeze-dried to fabricate microporosity into the matrix followed by crosslinking.22 The integration of these techniques was used to fabricate a highly porous hybrid hydrogel with well-defined 3D structures.
Creating an interconnected, highly porous hydrogel structure with excellent mechanical strength and the desired pore size is critical for tissue engineering. Hybrid hydrogels can be used to tune the desired properties of hydrogel for a specific application, making them desirable for use in many engineered tissues. The average pore sizes that can be achieved by using different techniques to induce porosity in hydrogels are compared in Table 1.
Table 1.
Pore Size Achieved Using Different Processes for Hydrogel Fabrication
| Process | Polymer | Pore size (μm) | Ref. |
|---|---|---|---|
| Conventional gas foaming | AAm | 100–250 | 49 |
| PEGDA | 100–600 | 50 | |
| CO2–water emulsion templating | Dextran | 6.25–7 | 56 |
| Chitosan, PVA, PVA/PEG | 3–15 | 59 | |
| Alginate | 23.9–250 | 57 | |
| CaCO3/PAM | 4.7–4.9 | 60 | |
| Dense gas CO2+crosslinker | Elastin | 80 | 64 |
| Dense gas CO2+cosolvent | Gelatin | 80–120 | 62 |
| Porogen leaching | PEG/PCL | 180–400 | 30 |
| OPF | 100–500 | 31 | |
| Alginate-g-poly(N-isopropylacrylamide) | 100–300 | 32 | |
| PHEMA | 200–500 | 33 | |
| 45–106 | 35 | ||
| PEG-poly(lysine) | 250–500 | 34 | |
| Freeze-drying | Collagen/chitosan | 50 | 37 |
| Agarose | 71–187 | 38 | |
| Chitosan, alginate | 60–150 | 46 | |
| Gelatin | 40–500 | 8,43 | |
| PVA/PCL | 30–300 | 88 | |
| Chitosan/PCL | 10–100 | 87 | |
| Electrospinning | Gelatin/PCL | 20–80 | 83 |
AAm, acryl amide; OPF, oligo[(polyethylene glycol) fumarate]; PAM, polyacrylamide; PCL, poly(ɛ-caprolactone); PEG, poly(ethylene glycol); PEGDA, poly(ethylene glycol) diacrylate; PHEMA, poly(2-hydroxyethyl methacrylate); PVA, poly(vinyl alcohol).
Microscale Control of Porosity and Microarchitecture
Fabrication of microchannels
To further control and improve diffusion and transport in hydrogels, researchers have used multiple techniques to create microchannels within hydrogel structures. Although hydrogels often have a high degree of hydration with diffusion properties similar to that in the bulk surrounding fluid, microchannels have been shown to be effective in further improving the mass transport capabilities of hydrogels.22,93 A common method for producing microfluidic channels within hydrogels is the use of soft lithography micromolding.94 Briefly, a photomask containing the desired pattern is printed and used in combination with an SU-8 photoresist-coated silicon wafer to create a template. Polydimethylsiloxane (PDMS) is then poured onto the SU-8 pattern, cured, and removed to generate a PDMS mold. The SU-8 template can typically be used multiple times to create multiple PDMS molds or stamps. Some polymers can be molded directly on the SU-8 master, avoiding the production of a PDMS replica. Otherwise, the polymer of interest is poured onto the PDMS stamp and cured. For example, PEG diacrylate on a PDMS mold may be polymerized using UV light in the presence of a photoinitiator. The PEG-PDMS combination can then be dissociated with hydration and mild agitation. These surface channel patterns can be made into microchannels by curing another solid hydrogel layer on top of the patterned surface to enclose the channels. Microscale channels have been successfully created using PDMS micromolding with PEG-DA and methacrylated HA,22 whereas channels have been created directly on SU-8 masters using thermal gelation of agarose.22
Creation of microchannels in cell-laden hydrogels has been shown to improve cell viability as well as orientation and alignment. Diffusion studies demonstrated that cell viability and function were improved in cells that were closer to the perfused channels, presumably because of increased nutrient exchange.95 The combination of controlled pore size throughout a poly(2-hydroxyethyl methacrylate) hydrogel, created with spherical sacrificial elements, with photopatterned microchannels led to improved elongation, spreading, and fibrillar formation of C2C12 myoblasts.96 Similarly, focused photoablation achieved using pulsed lasers in PEGylated fibrinogen hydrogels successfully created microchannels of controlled size that were shown to drive the directional growth of neurites.97 Creation of microchannels to control both nutrient transport and cellular alignment, combined with controlled pore size in the bulk material, brings hydrogel-based tissues closer to the appearance and function of native tissues.
Rapid prototyping or solid free-form fabrication is another family of techniques that has recently been demonstrated as potentially useful for the production of patterned hydrogels for tissue engineering. A detailed consideration of this technology is beyond the scope of this review; however, the reader is directed to a review of this technology and its advantages published elsewhere.3,98 These approaches use computer-aided design to automatically generate 3D structures using a variety of methods.98 A rapid prototyping technique that has found favor in the fabrication of hydrogels containing microchannels is stereolithography. Briefly, stereolithography is a liquid-based technique that utilizes layer-by-layer curing of a photosensitive prepolymer solution.99 A laser scanner is used to photopolymerize a thin layer of liquid prepolymer, which is located above a computer-controlled stage. After 2D photopatterning of a single polymer layer, the stage is moved downward to cover the top of the cured polymer with fresh liquid prepolymer, and the process is repeated to generate a 3D object layer-by-layer.
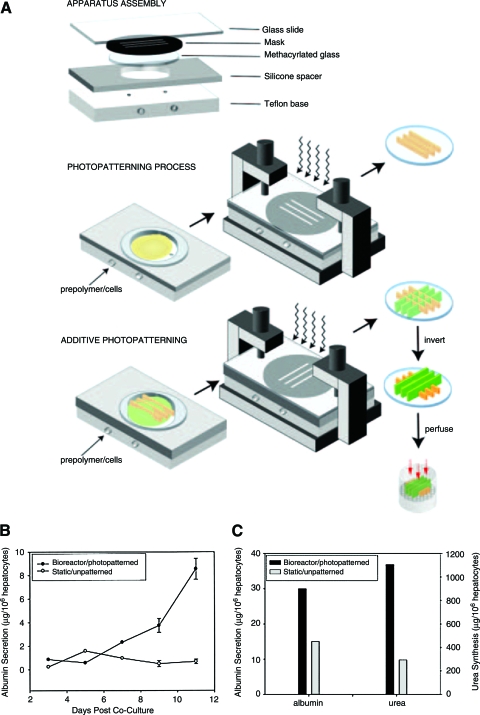
As a proof of principle, this technique was used to create cell-laden methacrylated-PEG hydrogels with simple ring shapes.99 It was shown that high cell viability could be maintained even when 2–3 min were needed to fabricate each layer.100 Others have extended this work to generate complex shapes at high resolution.100 Imbedded channels were fabricated with multiple bifurcations that could potentially serve as artificial microvasculature for tissue-engineered constructs.100 Multiple lumen conduit structures were formed with the aim of guiding nerve regeneration (Fig. 5A, B). It was demonstrated that microbeads could be precisely placed within the fabricated structures, demonstrating the ability to generate single constructs from multiple materials containing different growth factors or cell types (Fig. 5C, D).101,102
FIG. 5.
Multilumen poly(ethylene glycol) hydrogel conduits fabricated using stereolithography. Isometric (A, C) and top (B, D) views of a poly(ethylene glycol) hydrogel channels with potential in nerve regeneration. The conduits in (C) and (D) contain green and blue fluorescent particles in the outer and inner region, respectively, demonstrating the ability to fabricate constructs from multiple materials. Scale bars represent 1 mm. Reprinted with permission from Arcaute et al.100 Color images available online at www.liebertonline.com/ten.
Stereolithography is capable of delivering high-resolution features that are limited primarily by the diameter of the laser used. It also allows the direct incorporation of cells during the fabrication of scaffolds, which is advantageous as it allows for a uniform cell distribution within the construct. The relatively high cost of equipment necessary for stereolithography has limited its use among researchers. Additionally, this technique is also only compatible with photocrosslinkable polymers limiting the potential material selection. Layer-by-layer assembly using this method is relatively time consuming, potentially limiting the technique to more robust cell types.
Interconnected microvascular networks
Although individual microchannels can improve nutrient transport and tissue function, the greater goal is to create intact microvascular networks in hydrogel-based tissues to improve tissue function and integration with the host vasculature. Synthetic materials such as self-assembling peptide gels,103,104 as well as natural hydrogels such as collagen105,106 have been used to study in vitro capillary morphogenesis.
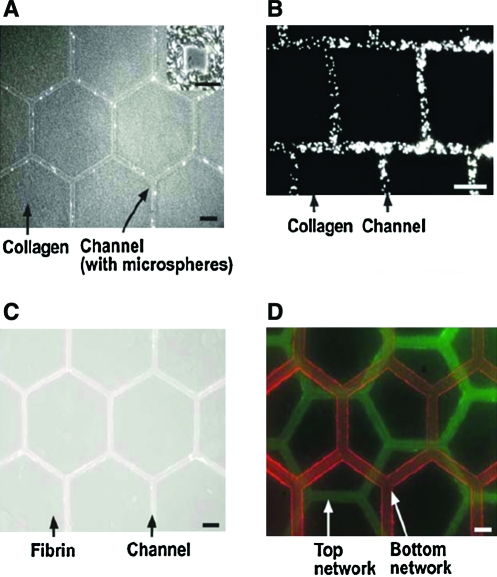
Recent work has used micromolded gelatin channels as a sacrificial element to create perfusable microvascular networks in collagen and fibrin hydrogels.16 The 3D gelatin channels were micromolded in PDMS, then encapsulated in collagen or fibrin, and placed in a standard 37°C incubator, causing the gelatin to melt away, leaving behind a series of connected microchannels ranging from 6 to 50 μm in diameter (Fig. 6). Subsequent experiments determined that seeded endothelial cells would line the channels and provide typical barrier function against perfused particles as expected in vivo, making this technique useful as an in vitro model of microvascular function, as well as a potential technique for creating vascularized, hydrogel-based engineered tissues.
FIG. 6.
Use of sacrificial gelatin to create perfusable microvascular networks. Gelatin patterns are cast in polydimethylsiloxane molds and then encapsulated by collagen (A, B) or fibrin (C, D), and the gelatin is removed via melting at 37°C. Perfusable, one-layer cell-laden structures were created using collagen (A, B) and fibrin (C), and multilayer structures were also demonstrated (D). Reprinted with permission from Golden and Tien.105 Scale bars are 200 μm for panels A, C, D, 25 μm for panel B, and 50 μm for panel A inset. Color images available online at www.liebertonline.com/ten.
Control of microarchitectural features
One ultimate goal of microengineering techniques for tissue fabrication is the precise control of not only porous structures, but of the microarchitectural features within the construct as well. By recreating specific microarchitectural motifs, tissue engineers aim to optimize cell viability, morphology, and function.107,108 Using basic micromolding techniques, researchers have demonstrated the ability to create complex structures with microscale pore and tissue structures.109 Agarose was formed into rods, tori, and honeycomb, or multiple connected tori, and H35 hematoma or human fibroblasts were seeded on these hydrogel structures and allowed to self-assemble within the hydrogels. The resulting structures demonstrated the ability to allow cells to form their own structures as dictated by the prescribed microarchitectural features and pores.
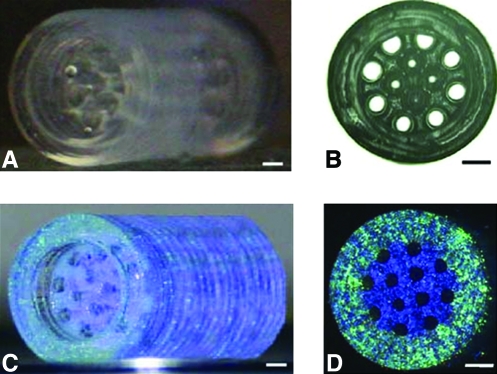
Using microfabrication techniques researchers have also demonstrated the ability to recapitulate native micro- and macroscale features to create engineered tissues with biomimetic appearance and function. For example, it has been demonstrated that the subsequent polymerization of cell-laden hydrogels, using different photomasks, can be used to build tissues layer by layer (Fig. 7A). Individual hepatic features are photopolymerized sequentially to create dual-layered tissues, surrounded by an ultrastructural honeycomb structure similar to the native sinusoid of the liver. Viability and hepatic cell function were improved in micropatterned constructs as compared to unpatterned controls (Fig. 7B, C), demonstrating the importance of recreating the native microarchitectural features. This technique demonstrates how the combination of dictating the microarchitectural features and pore structure simultaneously can lead to improved cell viability and function.
FIG. 7.
Schematic of layer-by-layer hepatic tissue formation. Using incremental spacers combined with alternate photomasks allows for the layer-by-layer creation of cell-laden tissues in specific microarchitectural organization (A). Perfusion of micropatterned hepatic tissues demonstrated significant improvements in albumin and urea secretion over unpatterned controls (B, C). Reprinted with permission from Tsang et al.109 Color images available online at www.liebertonline.com/ten.
Conclusions and Future Directions
Hydrogels hold substantial promise for creating functional engineered tissues, providing a significant need to control hydrogel porosity and microarchitecture. Traditional polymer-processing techniques, such as porogen leaching and gas foaming, have demonstrated the ability to create hydrogels with uniform porosity throughout the scaffold with high accuracy. Combining these traditional processes with more recent microfabrication techniques has brought the field closer to the ultimate goal of complete control over microarchitecture and porosity in engineered tissues. With continued research in these and other advanced techniques such as cell and organ printing, hydrogel-based tissue engineering will continue to make advances toward clinical restoration of tissue function. There are considerable challenges to overcome before clinical application of complex ex vivo–engineered tissues can become a reality, such as control over mechanical properties, cell alignment, and behavior and integration with the host vasculature. However, with the techniques presented here, and new techniques in the future, the improved ability to control the porosity and microarchitecture of hydrogels will drive the research closer to these goals.
Acknowledgments
The Khademhosseini group is funded by the U.S. Army Engineer Research and Development Center, the Institute for Soldier Nanotechnology, the National Science Foundation, and the National Institute of Health grants (HL092836, EB009196, and DE019024). The authors also acknowledge the financial support from the Australian Research Council (Grant No. DP0988545).
Disclosure Statement
No competing financial interests exist.
References
- 1.Peppas N. Hilt J.Z. Khademhosseini A. Langer R. Hydrogels in biology and medicine. Adv Mater Deerfield. 2006;18:1. [Google Scholar]
- 2.Khademhosseini A. Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007;28:5087. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Nichol J.W. Khademhosseini A. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter. 2009;5:1312. doi: 10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppas N.A. Hydrogels in Medicine and Pharmacy. Boca Raton, FL: CRC Press; 1987. [Google Scholar]
- 5.Gerecht S. Townsend S.A. Pressler H. Zhu H. Nijst C.L. Bruggeman J.P. Nichol J.W. Langer R. A porous photocurable elastomer for cell encapsulation and culture. Biomaterials. 2007;28:4826. doi: 10.1016/j.biomaterials.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 6.Martin I. Obradovic B. Treppo S. Grodzinsky A.J. Langer R. Freed L.E. Vunjak-Novakovic G. Modulation of the mechanical properties of tissue engineered cartilage. Biorheology. 2000;37:141. [PubMed] [Google Scholar]
- 7.Mandal B. Kundu S. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials. 2009;30:2956. doi: 10.1016/j.biomaterials.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Lien S.M. Ko L.Y. Huang T.J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009;5:670. doi: 10.1016/j.actbio.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Griffon D.J. Sedighi M.R. Schaeffer David V. Eurell Jo A. Johnson Ann L. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2:313. doi: 10.1016/j.actbio.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Kim H.J. Kim U.J. Vunjak-Novakovic G. Min B.-M. Kaplan D.L. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials. 2005;26:4442. doi: 10.1016/j.biomaterials.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Roy T.D. Simon J.L. Ricci J.L. Rekow E.D. Thompson V.P. Parsons J.R. Performance of degradable composite bone repair products made via three-dimensional fabrication techniques. J Biomed Mater Res. 2003;66:283. doi: 10.1002/jbm.a.10582. [DOI] [PubMed] [Google Scholar]
- 12.Fidkowski C. Kaazempur-Mofrad M.R. Borenstein J. Vacanti J. Langer R. Wang Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11:302. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 13.Yannas I.V. Lee E. Orgill D.P. Skrabut E.M. Murphy G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA. 1989;86:933. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whang K. Healy K.E. Elenz D.R. Nam E.K. Tsai D.C. Thomas C.H. Nuber G. Glorieux R. Travers R. Sprague S.M. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 1999;5:35. doi: 10.1089/ten.1999.5.35. [DOI] [PubMed] [Google Scholar]
- 15.Wake M.C. Patrick C.W., Jr. Mikos Antonios G. Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant. 1994;3:339. doi: 10.1177/096368979400300411. [DOI] [PubMed] [Google Scholar]
- 16.Khademhosseini A. Langer R. Borenstein J. Vacanti J.P. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA. 2006;103:2480. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khademhosseini A. Bettinger C. Karp J.M. Yeh J. Ling Y. Borenstein J. Fukuda J. Langer R. Interplay of biomaterials and micro-scale technologies for advancing biomedical applications. J Biomater Sci Polym Ed. 2006;17:1221. doi: 10.1163/156856206778667488. [DOI] [PubMed] [Google Scholar]
- 18.Du Y. Lo E. Ali S. Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc Natl Acad Sci USA. 2008;105:9522. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khademhosseini A. Eng G. Yeh J. Fukuda J. Blumling J., 3rd Langer R. Burdick J.A. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J Biomed Mater Res A. 2006;79:522. doi: 10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- 20.Khademhosseini A. Yeh J. Jon S. Eng G. Suh K.Y. Burdick J.A. Langer R. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip. 2004;4:425. doi: 10.1039/b404842c. [DOI] [PubMed] [Google Scholar]
- 21.Yeh J. Ling Y. Karp J.M. Gantz J. Chandawarkar A. Eng G. Blumling J., 3rd Langer R. Khademhosseini A. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27:5391. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Ling Y. Rubin J. Deng Y. Huang C. Demirci U. Karp J.M. Khademhosseini A. A cell-laden microfluidic hydrogel. Lab Chip. 2007;7:756. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 23.Quirk R.A. France R.M. Shakesheff K.M. Howdle S.M. Supercritical fluid technologies and tissue engineering scaffolds. Curr Opin Solid State Mater Sci. 2005;8:313. [Google Scholar]
- 24.Sheridan M.H. Shea L.D. Peters M.C. Mooney D.J. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J Control Release. 2000;64:91. doi: 10.1016/s0168-3659(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 25.Horák D. Kroupová J. Slouf M. Dvorák P. Poly (2-hydroxyethyl methacrylate)-based slabs as a mouse embryonic stem cell support. Biomaterials. 2004;25:5249. doi: 10.1016/j.biomaterials.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Draghi L. Resta S. Pirozzolo M. Tanzi M. Microspheres leaching for scaffold porosity control. J Mater Sci Mater Med. 2005;16:1093. doi: 10.1007/s10856-005-4711-x. [DOI] [PubMed] [Google Scholar]
- 27.Gong Y. Zhou Q. Gao C. Shen J. In vitro and in vivo degradability and cytocompatibility of poly (l-lactic acid) scaffold fabricated by a gelatin particle leaching method. Acta Biomater. 2007;3:531. doi: 10.1016/j.actbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Suh S.W. Shin J.Y. Kim J. Kim J. Beak C.H. Kim D.-I. Kim H. Jeon S.S. Choo I.-W. Effect of different particles on cell proliferation in polymer scaffolds using a solvent-casting and particulate leaching technique. ASAIO J. 2002;48:460. doi: 10.1097/00002480-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J. Wu L. Jing D. Ding J. A comparative study of porous scaffolds with cubic and spherical macropores. Polymer. 2005;46:4979. [Google Scholar]
- 30.Park J.S. Woo D.G. Sun B.K. Chung H.-M. Im S.J. Choi Y.M. Park K. Huh K.M. Park K.-H. In vitro and in vivo test of polyethylene glycol/poly e-caprolactone-based hydrogel scaffold for cell delivery application. J Control Release. 2007;124:51. doi: 10.1016/j.jconrel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Dadsetan M. Hefferan T.E. Szatkowski Jan P. Mishra P.K. Macura S.I. Lu L. Yaszemski Michael J. Effect of hydrogel porosity on marrow stromal cell phenotypic expression. Biomaterials. 2008;29:2193. doi: 10.1016/j.biomaterials.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J.H. Lee S.B. Kim S.J. Lee Y.M. Rapid temperature/pH response of porous alginate-g-poly(N-isopropylacrylamide) hydrogels. Polymer. 2002;43:7549. [Google Scholar]
- 33.Horák D. Hlídková H. Hradil J. Lapcíková M. Slouf M. Superporous poly(2-hydroxyethyl methacrylate) based scaffolds: preparation and characterization. Polymer. 2008;49:2046. [Google Scholar]
- 34.Ford M.C. Betrtram J.P. Hynes S.R. Michaud M. Li Q. Young M. Segal S.S. Madri J.A. Lavik E.B. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. Proc Natl Acad Sci USA. 2006;103:8. doi: 10.1073/pnas.0506020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simms H.M. Brotherton C.M. Good B.T. Davis R.H. Anseth K.S. Bowman C.N. In situ fabrication of macroporous polymer networks within microfluidic devices by living radical photopolymerization and leaching. Lab Chip. 2005;5:151. doi: 10.1039/b412589d. [DOI] [PubMed] [Google Scholar]
- 36.Thomson R.C. Wake M.C. Yaszemski M.J. Mikos A.G. Biodegradable polymer scaffolds to regenerate organs. Adv Polym Sci. 1995;122:245. doi: 10.1163/156856295x00805. [DOI] [PubMed] [Google Scholar]
- 37.Wu X. Black L. Santacana-Laffitte G. Patrick C.W., Jr. Preparation and assessment of glutaraldehyde-crosslinked collagen-chitosan hydrogels for adipose tissue engineering. J Biomed Mater Res Part A. 2007;81:59. doi: 10.1002/jbm.a.31003. [DOI] [PubMed] [Google Scholar]
- 38.Stokols S. Tuszynski M.H. The fabrication and characterization of linearly oriented nerve guidance scaffolds for spinal cord injury. Biomaterials. 2004;25:5839. doi: 10.1016/j.biomaterials.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 39.Stokols S. Tuszynski M.H. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials. 2006;27:443. doi: 10.1016/j.biomaterials.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 40.Ricciardi R. D'Errico G. Auriemma F. Ducouret G. Tedeschi A.M. De Rosa C. Laupretre F. Lafuma F. Short time dynamics of solvent molecules and supramolecular organization of poly (vinyl alcohol) hydrogels obtained by freeze/thaw techniques. Macromolecules. 2005;38:6629. [Google Scholar]
- 41.Jin R. Moreira Teixeira L.S. Dijkstra P.J. Karperien M. Zhong Z. Feijen J. Fast in-situ formation of dextran-tyramine hydrogels for in vitro chondrocyte culturing. J Control Release. 2008;132:24. [Google Scholar]
- 42.Lv Q. Hu K. Feng Q. Cui F. Fibroin/collagen hybrid hydrogels with crosslinking method: preparation, properties, and cytocompatibility. J Biomed Mater Res A. 2008;84:198. doi: 10.1002/jbm.a.31366. [DOI] [PubMed] [Google Scholar]
- 43.Kang H.W. Tabata Y. Ikada Y. Fabriction of porous gelatin scaffolds for tissue engineering. Biomaterials. 1999;20:1339. doi: 10.1016/s0142-9612(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 44.Lee Y.-G. Kang H.-S. Kim M.-S. Son T.-I. Thermally crosslinked anionic hydrogels composed of poly (vinyl alcohol) and poly (gamma-glutamic acid): preparation, characterization, and drug permeation behavior. J Appl Polym Sci Symp. 2008;109:3768. [Google Scholar]
- 45.Lin W.C. Yu D.G. Yang M.C. Blood compatibility of novel poly([gamma]-glutamic acid)/polyvinyl alcohol hydrogels. Colloids Surf B Biointerfaces. 2006;47:43. doi: 10.1016/j.colsurfb.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Ho M.-H. Kuo P.-Y. Hsieh H.-J. Hsien T.-Y. Hou L.-T. Lai J.-Y. Wang D.-M. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials. 2004;25:129. doi: 10.1016/s0142-9612(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 47.Lips P.A.M. Velthoen I.W. Dijkstra P.J. Wessling M. Feijen J. Gas foaming of segmented poly(ester amide) films. Polymer. 2005;46:12737. [Google Scholar]
- 48.Caykara T. Kucuktepe S. Turan E. Swelling characteristics of thermo-sensitive poly[(2-diethylaminoethyl methacrylate)-co-(N,N-dimethylacrylamide)] porous hydrogels. Polym Int. 2007;56:532. [Google Scholar]
- 49.Huh K.M. Baek N. Park K. Enhanced swelling rate of poly(ethylene glycol)-grafted superporous hydrogels. J Bioact Compat Polym. 2005;20:231. [Google Scholar]
- 50.Keskar V. Marion N.W. Mao J.J. Gemeinhart R.A. In vitro evaluation of macroporous hydrogels to fabricate stem cell infiltration, growth, and mineralization. Tissue Eng Part A. 2009;15:1695. doi: 10.1089/ten.tea.2008.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abd El-Rehim H.A. Hegazy E.-S.A. Diaa D.A. Characterization of super-absorbent material based on carboxymethylcellulose sodium salt prepared by electron beam irradiation. J Macromol Sci Pure. 2006;43:101. [Google Scholar]
- 52.Tai H. Popov V.K. Shakesheff K.M. Howdle S.M. Putting the fizz into chemistry: applications of supercritical carbon dioxide in tissue engineering, drug delivery and synthesis of novel block copolymers. Biochem Soc Trans. 2007;35:516. doi: 10.1042/BST0350516. [DOI] [PubMed] [Google Scholar]
- 53.Barry J.J.A. Silva M.M.C.G. Popov V.K. Shakesheff K.M. Howdle S.M. Supercritical carbon dioxide: putting the fizz into biomaterials. Philos Trans A Math Phys Eng Sci. 2006;364:249. doi: 10.1098/rsta.2005.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barry J.J.A. Gidda H.S. Scotchford C.A. Howdle S.M. Porous methacrylate scaffolds: supercritical fluid fabrication and in vitro chondrocyte responses. Biomaterials. 2004;25:3559. doi: 10.1016/j.biomaterials.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 55.Cansell F. Aymonier C. Loppinet-Serani A. Review on materials science and supercritical fluids. Curr Opin Solid State Mater Sci. 2003;7:331. [Google Scholar]
- 56.Palocci C. Barbetta A. La Grotta A. Dentini M. Porous biomaterials obtained using supercritical CO2-water emulsions. Langmuir. 2007;23:8243. doi: 10.1021/la700947g. [DOI] [PubMed] [Google Scholar]
- 57.Partap S. Rehman I. Jones J.R. Darr J.A. Supercritical carbon dioxide in water emulsion-templated synthesis of porous calcium alginate hydrogels. Adv Mater Deerfield. 2006;18:501. [Google Scholar]
- 58.Tan B. Lee J.-Y. Cooper A.I. Synthesis of emulsion-templated poly(acrylamide) using CO2-in-water emulsions and poly (vinyl acetate)-based block copolymer surfactants. Macromolecules. 2007;40:1945. [Google Scholar]
- 59.Lee J.-Y. Tan B. Cooper A.I. CO2-in-water emulsion-templated poly(vinyl alcohol) hydrogels using poly(vinyl acetate)-based surfactants. Macromolecules. 2007;40:1955. [Google Scholar]
- 60.Bing Z. Lee J.Y. Choi S.W. Kim J.H. Preparation of porous CaCO3/PAM composites by CO2 in water emulsion templating method. Eur Polym J. 2007;43:4814. [Google Scholar]
- 61.Chen C.-F. Chang C.-S. Chen Y.-P. Lin T.-S. Su C.-Y. Lee S.-Y. Applications of supercritical fluid in alloplastic bone graft: a novel method and in vitro tests. Ind Eng Chem Res. 2006;45:3400. [Google Scholar]
- 62.Shih H.-h. Lee K.-r. Lai H.-m. Tsai C.-c. Chang Y.-c. Method of making porous biodegradable polymers, US 6673286. 2004.
- 63.Annabi N. Mithieux S.M. Weiss A.S. Dehghani F. The fabrication of elastin-based hydrogels using high pressure CO2. Biomaterials. 2009;30:1. doi: 10.1016/j.biomaterials.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 64.Annabi N. Mithieux S.M. Boughton E.A. Ruys A.J. Weiss A.S. Dehghani F. Synthesis of highly porous crosslinked elastin hydrogels and their interaction with fibroblasts in vitro. Biomaterials. 2009;30:4550. doi: 10.1016/j.biomaterials.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 65.Winters M.A. Knutson B.L. Debenedetti P.G. Sparks H.G. Przybycien T.M. Stevenson C.L. Prestrelski S.J. Precipitation of proteins in supercritical carbon dioxide. J Pharm Sci. 1996;85:586. doi: 10.1021/js950482q. [DOI] [PubMed] [Google Scholar]
- 66.Yeo S.D. Debenedetti P.G. Patro S.Y. Przybycien T.M. Secondary structure characterization of microparticulate insulin powders. J Pharm Sci. 1994;83:1651. doi: 10.1002/jps.2600831203. [DOI] [PubMed] [Google Scholar]
- 67.Yeo S.D. Lim G.B. Debenedetti P.G. Bernstein H. Formation of microparticulate protein powders using a supercritical fluid antisolvent. Biotechnol Bioeng. 1993;41:341. doi: 10.1002/bit.260410308. [DOI] [PubMed] [Google Scholar]
- 68.Bustami R.T. Chan H.K. Sweeney T. Dehghani F. Foster N.R. Generation of fine powders of recombinant human deoxyribonuclease using the aerosol solvent extraction system. Pharm Res. 2003;20:2028. doi: 10.1023/b:pham.0000008053.69903.c1. [DOI] [PubMed] [Google Scholar]
- 69.Dehghani F. Annabi N. Valtchev P. Mithieux S.M. Weiss A.S. Kazarian S.G. Tay F.H. Effect of dense gas CO2 on the coacervation of elastin. Biomacromolecules. 2008;9:1100. doi: 10.1021/bm700891b. [DOI] [PubMed] [Google Scholar]
- 70.Striolo A. Favaro A. Elvassore N. Bertucco A. Noto V.D. Evidence of conformational changes for protein films exposed to high-pressure CO2 by FT-IR spectroscopy. J Supercrit Fluids. 2003;27:283. [Google Scholar]
- 71.LeClair Ellis J. Tomasko D.L. Dehghani F. Novel dense CO2 technique for beta-galactosidase immobilization in polystyrene microchannels. Biomacromolecules. 2008;9:1027. doi: 10.1021/bm701343m. [DOI] [PubMed] [Google Scholar]
- 72.Reneker D.H. Chun I. Nanometer diameter fibers of polymer, produced by electrospinning. Nanotechnology. 1996;7:216. [Google Scholar]
- 73.Ma P.X. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matthews J.A. Wnek G.E. Simpson D.G. Bowlin G.L. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 75.Pham Q.P. Sharma U. Mikos A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 76.Li L. Hsieh Y.-L. Ultra-fine polyelectrolyte hydrogel fibers from poly (acrylic acid)/poly (vinyl alcohol) Nanotechnology. 2005;16:2852. [Google Scholar]
- 77.Kim T.G. Chung H.J. Park T.G. Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 2008;4:1611. doi: 10.1016/j.actbio.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Li W.-J. Laurencin C.T. Caterson E.J. Tuan R.S. Ko F.K. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 79.Yang F. Murugan R. Wang S. Ramakrishna S. Electrospinning of nano/micro scale poly (L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 80.Chua K.-N. Lim W.-S. Zhang P. Lu H. Wen J. Ramakrishna S. Leong K.W. Mao H.-Q. Stable immobilization of rat hepatocyte spheroids on galactosylated nanofiber scaffold. Biomaterials. 2005;26:2537. doi: 10.1016/j.biomaterials.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 81.Li W.-j. Danielson K.G. Alexander P.G. Tuan R.S. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(e-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67:1105. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 82.Pham Q.P. Sharma U. Mikos A.G. Electrospun poly (e-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 83.Heydarkhan-Hagvall S. Schenke-Layland K. Dhanasopon A.P. Rofail F. Smith H. Wu B.M. Shemin R. Beygui R.E. Maclellan W.R. Three-dimensional electronspun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials. 2008;29:2907. doi: 10.1016/j.biomaterials.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peppas N.A. Hilt J.Z. Khademhosseini A. Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater Deerfield. 2006;18:1345. [Google Scholar]
- 85.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 86.Nichol J.W. Engelmayr G.C., Jr. Cheng M. Freed L.E. Co-culture induces alignment in engineered cardiac constructs via MMP-2 expression. Biochem Biophys Res Commun. 2008;373:360. doi: 10.1016/j.bbrc.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarasam A.R. Samli A.I. Hess L. Ihnat M.A. Madihally S.V. Blending chitosan with polycaprolactone: porous scaffolds and toxicity. Macromol Biosci. 2007;7:1160. doi: 10.1002/mabi.200700001. [DOI] [PubMed] [Google Scholar]
- 88.Mohan N. Nair P.D. Polyvinyl alcohol-poly (caprolactone) semi IPN scaffold with implication for cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2007;84:584. doi: 10.1002/jbm.b.30906. [DOI] [PubMed] [Google Scholar]
- 89.Zhang X.-Z. Chu C.-C. Zhuo R.-X. Using hydrophobic additive as pore-forming agent to prepare macroporous PNIPAAm hydrogels. J Polym Sci A Polym Chem. 2005;43:5490. [Google Scholar]
- 90.Gallego Ferrer G. Soria Melia J.M. Hernandez Canales J. Meseguer Duenas J.M. Romero Colomer F. Monleon Pradas M. Gomez Ribelles J.L. Pissis P. Polizos G. Poly(2-hydroxyethyl acrylate) hydrogel confined in a hydrophobic porous matrix. Colloid Polym Sci. 2005;283:681. [Google Scholar]
- 91.Chen G. Ushida T. Tateishi T. Hybrid biomaterials for tissue engineering: a preparative method for PLA or PLGA-collagen hybrid sponges. Adv Mater Deerfield. 2000;12:455. [Google Scholar]
- 92.Chen G. Ushida T. Tateishi T. A biodegradable hybrid sponge nested with collagen microsponges. J Biomed Mater Res. 2000;51:273. doi: 10.1002/(sici)1097-4636(200008)51:2<273::aid-jbm16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 93.Brigham M.D. Bick A. Lo E. Bendali A. Burdick J.A. Khademhosseini A. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng Part A. 2008;15:1645. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khademhosseini A. Suh K.Y. Jon S. Eng G. Yeh J. Chen G.J. Langer R. A soft lithographic approach to fabricate patterned microfluidic channels. Anal Chem. 2004;76:3675. doi: 10.1021/ac035415s. [DOI] [PubMed] [Google Scholar]
- 95.Bryant S.J. Cuy J.L. Hauch K.D. Ratner B.D. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials. 2007;28:2978. doi: 10.1016/j.biomaterials.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sarig-Nadir O. Livnat N. Zajdman R. Shoham S. Seliktar D. Laser photoablation of guidance microchannels into hydrogels directs cell growth in three dimensions. Biophys J. 2009;96:4743. doi: 10.1016/j.bpj.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang S. Leong K.-F. Du Z. Chua C.-K. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng. 2002;8:1. doi: 10.1089/107632702753503009. [DOI] [PubMed] [Google Scholar]
- 98.Hollister S.J. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 99.Dhariwala B. Hunt E. Boland T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004;10:1316. doi: 10.1089/ten.2004.10.1316. [DOI] [PubMed] [Google Scholar]
- 100.Arcaute K. Mann B. Wicker R. Stereolithography of three-dimensional bioactive poly (ethylene glycol) constructs with encapsulated cells. Ann Biomed Eng. 2006;34:1429. doi: 10.1007/s10439-006-9156-y. [DOI] [PubMed] [Google Scholar]
- 101.Sieminski A.L. Hebbel R.P. Gooch K.J. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res. 2004;297:574. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 102.Sieminski A.L. Hebbel R.P. Gooch K.J. Improved microvascular network in vitro by human blood outgrowth endothelial cells relative to vessel-derived endothelial cells. Tissue Eng. 2005;11:1332. doi: 10.1089/ten.2005.11.1332. [DOI] [PubMed] [Google Scholar]
- 103.Sieminski A.L. Was A.S. Kim G. Gong H. Kamm R.D. The stiffness of three-dimensional ionic self-assembling peptide gels affects the extent of capillary-like network formation. Cell Biochem Biophys. 2007;49:73. doi: 10.1007/s12013-007-0046-1. [DOI] [PubMed] [Google Scholar]
- 104.Sieminski A.L. Semino C.E. Gong H. Kamm R.D. Primary sequence of ionic self-assembling peptide gels affects endothelial cell adhesion and capillary morphogenesis. J Biomed Mater Res A. 2008;87:494. doi: 10.1002/jbm.a.31785. [DOI] [PubMed] [Google Scholar]
- 105.Golden A.P. Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 106.Chrobak K.M. Potter D.R. Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 107.Dean D.M. Napolitano A.P. Youssef J. Morgan J.R. Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB J. 2007;21:4005. doi: 10.1096/fj.07-8710com. [DOI] [PubMed] [Google Scholar]
- 108.Napolitano A.P. Chai P. Dean D.M. Morgan J.R. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 2007;13:2087. doi: 10.1089/ten.2006.0190. [DOI] [PubMed] [Google Scholar]
- 109.Tsang V.L. Chen A.A. Cho L.M. Jadin K.D. Sah R.L. DeLong S. West J.L. Bhatia S.N. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21:790. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]