Abstract
Background Context
Degenerative spondylolisthesis is a presumed cause of back pain. Previous studies of spondylolisthesis and back pain included only women or combined results for men and women. Comparisons of the frequency of back pain, neurogenic symptoms, and functional limitations specifically among elderly men with and without spondylolisthesis are needed.
Purpose
To determine associations of prevalent spondylolisthesis with back pain symptoms, neurogenic symptoms, and functional limitations among elderly men. Study Design/ Setting: Cross-sectional epidemiologic study conducted within the Osteoporotic Fractures in Men (MrOS) cohort. The MrOS cohort is comprised of 5,995 community dwelling men ages ≥65 years who were recruited at 6 US academic medical centers. Extensive self-reported data and lumbar spine radiographs were obtained for all MrOS participants at baseline.
Patient Sample
For this study, 300 men were selected at random specifically for the evaluation of spondylolisthesis on the baseline spine radiographs.
Outcome Measures
Standardized questionnaires were used to assess self-reported back pain, leg pain (radiculopathy), lower extremity numbness (paresthesias) and lower extremity weakness occurring in the past 12 months, and to ascertain current difficulty with activities of daily living.
Methods
In the present study, radiographic spondylolisthesis was classified as forward slip of ≥5%. Prevalence of back pain, neurogenic symptoms and difficulty with activities of daily living were compared between men with and without spondylolisthesis using chisquare or Fisher’s exact tests.
Results
Spondylolisthesis was present among 92 (31%) men. Among men with and without spondylolisthesis, back pain (63% vs. 67%, p=0.46) and moderate/severe back pain (41% vs. 38%, p=0.76) were reported with similar frequency. Men with spondylolisthesis more often reported radiculopathy (33% vs. 22%, p=0.06), paresthesias (18% vs. 11%, p= 0.10) and weakness (18% vs. 9%, p=0.02) in the lower extremities; as well as difficulty walking 2-3 blocks (21% vs 11%, p=0.03), doing their own shopping (8% vs 2%, p=0.04), and getting in/out of a car (14% vs 6%, p=0.03), compared to men without.
Conclusions
Among elderly men, spondylolisthesis was associated with neurogenic symptoms and lower extremity functional limitations; however, spondylolisthesis was not associated with a higher likelihood of back pain in this population.
Introduction
Spondylolisthesis is defined as an anterior migration, or slip, of a vertebral body in relation to the vertebra located immediately caudad. Five categories of spondylolisthesis have been described including dysplastic, isthmic, traumatic, pathologic, and degenerative.1 Degenerative spondylolisthesis is characterized by an intact vertebral ring,2-3 is presumed to result from degeneration of facet joints and intervertebral discs with aging,1-2 and has traditionally been considered to represent instability of the vertebral segment.2 This condition is common among middle-aged and elderly adults with prevalence estimates in US cohorts ranging from 14%-30%.4-7
The amount of forward slippage is of clinical concern because it can cause spinal stenosis and nerve root compression. Low back pain as well as pain, numbness, or weakness in the legs or lower extremities, are symptoms clinically associated with degenerative spondylolisthesis.8-9 Because of its putative relation to back and leg pain symptoms, degenerative spondylolisthesis is often considered an indication for surgery. In the US, over 300,000 lumbar spine fusion surgeries (or arthrodesis) are performed annually, and one-third of these are for degenerative spondylolisthesis, spinal stenosis or a combination of both.10-11 Numbers of lumbar spine fusion surgeries have steadily risen in the past 20 years, as has the trend toward utilization of instrumentation to achieve fusion.11-13
Despite the amount of surgery performed for degenerative spondylolisthesis, information is lacking about the relation of radiographic spondylolisthesis to back pain symptoms and to physical functioning. Several studies have included only symptomatic individuals with spondylolisthesis,2, 14-16 making it difficult to discern if the frequency of symptoms differs from that among persons without spondylolisthesis. Large cohort studies that are able to compare symptom prevalence among those with and without spondylolisthesis indicate that the frequency of self-reported back pain in the previous 12 months is similar among adults with and without degenerative spondylolisthesis.4-6, 17 However, none of these studies assessed leg symptoms. Therefore, whether or not neurogenic symptoms are more prevalent among adults with degenerative spondylolisthesis compared to those without this condition has not been established. Moreover, although back pain and neurogenic symptoms increase the likelihood of activity limitations among the elderly,18-20 few studies have investigated the effects of spondylolisthesis on physical functioning.4-5
Previous reports about the association of degenerative spondylolisthesis with back pain among community dwelling adults have been limited to only women5 or to men and women combined.4, 17, 21 To investigate this association among men, we conducted a cross-sectional study in a sample of participants in the Osteoporotic Fractures in Men Study (MrOS), a cohort of US men ages 65-100 years. We addressed the following questions. Compared to men without spondylolisthesis, are men with this condition 1) more likely to report back pain, 2) more likely to report neurogenic symptoms, and/or 3) more likely to report difficulty with activities of daily living? A comprehensive assessment of spondylolisthesis prevalence and its association with demographic factors, body size and physical activity is reported elsewhere.7
Methods
Parent cohort
The MrOS Study enrolled 5,995 participants from March 2000 through April 2002 as described elsewhere.22-23 Briefly, recruitment occurred at six US academic medical centers in Birmingham AL, Minneapolis MN, Palo Alto CA, Pittsburgh PA, Portland OR, and San Diego CA. Eligible participants were at least 65 years of age, community-dwelling, able to walk without assistance from another person, and had at least one natural hip for bone density measurement. Eligibility criteria were minimal so that results from the cohort would be applicable to a broad population of community-dwelling men with similar ages.22 Institutional Review Boards at each center approved the study protocol. All participants provided written informed consent.
At baseline, all men completed a comprehensive self-administered questionnaire, and attended a clinic visit where thoracic and lumbar radiographs were obtained using a standardized protocol for the ascertainment of vertebral fracture. Men were placed on their left side in the lateral position with legs flexed and both arms at right angles to the body. The long axis of the spine was set parallel to the table and the mid-axillary (coronal) plane of the body was aligned to the table midline. Images were obtained from T12 to S1. All films were sent to the MrOS San Francisco Coordinating Center (SFCC) for central quality review, processing, digitization, and archiving.
Selection of the study sample
To establish initial data on spinal conditions other than vertebral fracture in the cohort, 300 participants were randomly sampled using a computer generated random number. Baseline films for this sample were transferred from the SFCC to the authors for analysis. Films that were unreadable or showed evidence of spine fusion surgery were excluded, leaving a study sample of 295. A thorough assessment of distributions of baseline characteristics indicated that the study sample was comparable to the entire cohort. For example, means (SD) in the sample and in the cohort respectively were, 74(6) and 74(6) years for age, 174(7) and 174(7) cm for height, and 28(4) and 27(4) kg/m2 for body mass index (BMI). Similar percentages of men in the sample and the cohort were Caucasian (90% vs. 89%), had at least a college education (56% vs. 53%), and ranked their health as good or excellent (83% vs. 86%). Likewise, the prevalence of back pain in the past 12 months (65% vs. 67%), and the neurogenic symptoms in the lower extremities of radiating pain (25% vs. 26%), numbness (14% vs 15%) and weakness (12% vs. 11%), were nearly identical in the sample and in the entire cohort (ascertainment of the symptom variables is described in detail below).
Assessment of spondylolisthesis
The presence of spondylolisthesis was assessed from L1 to S1 on the radiographs without knowledge of any baseline participant information. The magnitude of listhesis was measured by dividing the slip distance by the caudad body width and expressed as a percentage. Slip percentage was categorized according to the Meyerding Grading Scale with Grade 0: no slip, Grade I: 1-25%, Grade II: 26–50%, Grade III: 51–75%, Grade IV: 76–100%, and Grade V: complete slippage.24-25 Slip percentage and Meyerding grade are reliable measures.25 Some researchers have expressed concern about the accuracy of slip of small magnitudes.2, 26-27 Therefore, consistent with the previous work,2 we defined spondylolisthesis as slip percentage ≥5%.
To assess inter-rater agreement of the spondylolisthesis measurements, two of the authors independently evaluated spondylolisthesis on 35 radiographs chosen randomly from the sample of 295 men. The radiographs were labeled with MrOS participant study numbers. We randomly sorted the list of study numbers twice in order to create a separate list for each rater. The raters accessed the radiographs in the order shown on the list on different days. We used this design to minimize the possibility that raters could compare results. To evaluate intra-rater agreement, one rater also repeated measurements on each of the 35 films. For the second assessment, the rater was given the list of study numbers in a new random order. The radiographs were reviewed on different days subsequent to the first assessment. We used the Kappa statistic to determine inter- and intra-rater agreement regarding the presence or absence of spondylolisthesis. Agreement was excellent regarding the presence of any lumbar spondylolisthesis, with Kappa values of 0.89 observed for both inter- and intra- rater agreement. At the vertebral level, we observed excellent agreement at L4/5 (1.0) and good inter-rater agreement at L3/4 (0.65) and L5/S1 (0.65). Intra-rater agreement was excellent with Kappa values being 1.0 at L3/4, 0.84 at L4/5, and 1.0 and L5/S1.
Ascertainment of baseline back pain and functional limitations
History of back pain in the past 12 months was assessed with items from standard back pain questionnaires available at the time MrOS participants were enrolled.28 A body drawing showing the torso from the back of the head to the mid-thigh was included for reference of pain location. Men who responded yes to the question “During the past 12 months have you experienced any back pain?” were further queried about pain severity (mild, moderate, or severe), the frequency of being bothered by back pain (never, rarely, some of the time, most of the time, or all of the time), and the number of days that back pain limited their usual activities. Of those who answered yes to having had back pain in the past 12 months, 92% also answered yes to the following question. “In the past 12 months, have you suffered lower back pain?” Therefore, report of any back pain was used because it represents mostly low back pain. Neurogenic symptoms were assessed with questions regarding back pain that “went down into the buttock, leg or foot” (radicular pain), occurred “with numbness or tingling in the buttock, thigh, lower leg or foot” (paresthesias) or “a feeling of weakness in the leg, ankle or foot” (weakness).
Physical functioning was assessed with two sets of questions. One set asked about difficulty with performing three instrumental activities of daily living (IADL) (meal preparation, doing one’s own shopping, heavy housework) and two physical limitations (walking 2 to 3 blocks outside on level ground, climbing 10 steps) unassisted and without using special aids. Second, using 7 items from the Quebec Pain Disability Scale, participants reported on difficulties with the following activities due to back pain: bending down to pick up lightweight objects, lifting a ten-pound object up from the floor, reaching an object just overhead, putting socks on either foot, getting in or out of the front seat of a car, standing for 2 hours, or sitting for 30 minutes.28
Other baseline measures
Demographic factors examined were age, race/ethnicity, and education level. Body mass index (BMI) (kg/m2) was calculated from weight (kg) directly measured on a balance beam or digital scale and height (cm) measured using a Harpenden stadiometer. Physical activity was assessed with the validated Physical Activity Scale for the Elderly (PASE) questionnaire which yields a score for total activity from reports about leisure, occupational and household activities.29 Men rated their overall health status in comparison to others their own age as ranging from excellent to very poor. Self-report of diagnosed medical conditions included hypertension, angina, myocardial infarction, diabetes, and hip osteoarthritis. Peripheral arterial disease (PAD), which can also manifest with leg pain symptoms, was determined with the ankle-brachial index (ABI). The ABI is computed as the ratio of systolic blood pressure measured with a handheld Doppler stethoscope in the ankle to that in the upper arm. Measurements in MrOS were performed in a standardized manner on the right upper arm and ankle after the participant had rested for five minutes in a recumbent or semi-recumbent position. Presence of PAD was defined as an ABI of <0.90.30
Statistical analysis
Distributions of baseline characteristics according to spondylolisthesis status were performed with t-tests for continuous variables, or with chi-square or Fisher’s exact tests for categorical variables. Responses for severe back pain and always being bothered by back pain were infrequent, so these categories were combined with the adjacent category for analysis. Meal preparation was not evaluated further because only 4 men (all without spondylolisthesis) reported difficulty with this activity. Differences in proportions of back pain symptoms, neurogenic symptoms, IADL difficulty, and functional limitations due to back pain were compared among men with and without spondylolisthesis using chi-square or Fisher’s exact tests. Potential confounding of these associations was evaluated in stratified analyses with the Mantel-Haenszel test, which is appropriate for sparse data.31 Age, height, self-rated health, education level and physical activity were considered as potential confounders, because distributions of these variables differed either according to spondylolisthesis status or report of back pain. Results of the Mantel-Haenszel analyses demonstrated no confounding effects of these factors. Therefore we report the unadjusted results from the chi-square or Fisher’s exact tests. All analyses were conducted using SAS software (SAS Institute, Cary NC, USA).
Results
Participant characteristics
Spondylolisthesis was observed from L3 to S1, and only one vertebral level was involved 96% of time. The percent of slip ranged from 5% -28%, nearly all of which (99%) was Meyerding grade I (data not shown). The prevalence of lumbar spondylolisthesis was 31% (92 men) (Table 1). When compared to men without spondylolisthesis, men with spondylolisthesis were shorter and reported greater levels of leisure time physical activity. Otherwise, distributions of baseline characteristics were comparable between the two groups.
Table 1.
Baseline characteristics according to spondylolisthesis status among men age ≥ 65yrs: The MrOS Study.
| Baseline characteristic | Spondylolisthesis (n=92) | No spondylolisthesis (n=203) | p-value* |
|---|---|---|---|
| Mean(sd) | Mean(sd) | ||
| Age | 75 (6) | 74 (6) | 0.32 |
| Height | 173 (7) | 175 (7) | 0.07 |
| BMI | 27 (3) | 28 (4) | 0.64 |
| Physical Activity Score | 153 (67) | 145 (65) | 0.34 |
| Leisure | 44 (43) | 34 (34) | 0.04 |
| Household | 100 (46) | 96 (41) | 0.44 |
| Number (%) | Number (%) | ||
| Race | |||
| Caucasian | 85 (92%) | 181 (89%) | 0.56** |
| African American | 2 (2%) | 11 (5%) | |
| Other† | 5 (5%) | 11 (5%) | |
| Education level | |||
| High school or less | 47 (51%) | 83 (41%) | 0.10 |
| College or higher | 45 (49%) | 120 (59%) | |
| Self-rated Health | |||
| Excellent | 30 (33%) | 62 (31%) | 0.78 |
| Good | 45 (49%) | 108 (53%) | |
| Fair, poor or very poor | 17 (18%) | 33 (16%) | |
| Medical history | |||
| Diabetes | 13 (14%) | 23 (11%) | 0.50 |
| None | 79 (86%) | 180 (89%) | |
| Hypertension | 48 (52%) | 101 (50%) | 0.70 |
| None | 44 (48%) | 102 (50%) | |
| Angina | 14 (15%) | 37 (18%) | 0.53 |
| None | 78 (85%) | 166 (82%) | |
| Myocardial infarction | 20 (22%) | 33 (16%) | 0.26 |
| None | 72 (78%) | 170 (84%) | |
| Hip osteoarthritis | 8 (9%) | 10 (5%) | 0.21 |
| None | 84 (91%) | 193 (95%) | |
| Peripheral arterial disease‡ | |||
| Yes (ABI < 0.90) | 5 (6%) | 16 (8%) | 0.63** |
| No (ABI ≥0.90) | 83 (94%) | 180 (92%) | |
P-values are computed with t-tests for continuous variables or with chi-square or Fisher’s exact** tests for categorical variables.
Because of small numbers, men of Asian race or who reported ≥ 1 race were combined.
ABI =Ankle brachial index. Data for 11 men were missing.
Spondylolisthesis, back pain and neurogenic symptoms
Any back pain in the past 12 months was reported by about two-thirds of men with and without spondylolisthesis (Table 2). Men with spondylolisthesis were no more likely to report severe back pain, bothersome back pain, or days of activity limitations due to back pain compared to men without spondylolisthesis. However, radicular pain, paresthesias, and weakness were reported with greater frequency among men with spondylolisthesis compared to men without. When back pain was further categorized with or without neurogenic symptoms, 36% of men with spondylolisthesis reported back pain with neurogenic symptoms compared to 24% of their counterparts.
Table 2.
Back pain and neurogenic symptoms in the past 12 months according to spondylolisthesis status among men age ≥ 65yrs: The MrOS Study.
| Back Pain Measure | Spondylolisthesis (n=92) Number (%) | No spondylolisthesis (n=203) Number (%) | p-value |
|---|---|---|---|
| Any back pain | |||
| No | 34 (37%) | 66 (33%) | 0.46 |
| Yes | 58 (63%) | 137 (67%) | |
| Severity of back pain | |||
| No back pain | 34 (37%) | 66 (33%) | 0.76 |
| Mild | 23 (25%) | 54 (27%) | |
| Moderate/Severe | 35 (38%) | 83 (41%) | |
| Frequency of being bothered by back pain | |||
| No back pain | 34 (37%) | 66 (33%) | 0.66 |
| Rarely | 16 (17%) | 34 (17%) | |
| Some of the time | 25 (27%) | 70 (34%) | |
| Most of the time or all the time | 17 (18%) | 33 (16%) | |
| Days that activity was limited due to back pain | |||
| ≤ 10 days | 84 (91%) | 186 (92%) | 0.93 |
| >10 days | 8 (9%) | 17 (8%) | |
| Radicular pain* | |||
| No | 62 (67%) | 158 (78%) | 0.06 |
| Yes | 30 (33%) | 45 (22%) | |
| Paresthesias** | |||
| No | 75 (82%) | 180 (89%) | 0.10 |
| Yes | 17 (18%) | 23 (11%) | |
| Lower extremity weakness† | |||
| No | 75 (82%) | 185 (91%) | 0.02 |
| Yes | 17 (18%) | 18 (9%) | |
| Back pain with any neurogenic symptom | |||
| No back pain | 34 (37%) | 66 (33%) | 0.02 |
| Back pain only | 25 (27%) | 88 (43%) | |
| Both | 33 (36%) | 24 (24%) |
Back pain radiating down into the buttock, hip, leg, or foot.
Back pain with numbness or tingling in the buttock, thigh, lower leg, or foot.
Back pain with a feeling of weakness in the leg, ankle or foot.
P-values for differences in proportions from chi-square tests.
Lower extremity pain, numbness and weakness can also be symptoms of PAD; therefore, it could confound the association of spondylolisthesis and leg symptoms. The prevalence of PAD did not differ among men with and without spondylolisthesis in our sample (Table 1). In analyses repeated after excluding the 21 men with PAD, the prevalence of neurogenic symptoms according to spondylolisthesis status did not materially change: prevalence of radicular pain was 32% vs. 21% (p=0.04), paresthesias was 18% vs. 10% (p=0.06), and lower extremity weakness was 18% vs. 8% (p=0.01).
When spondylolisthesis was separated according to the vertebral level affected, the associations with neurogenic symptoms remained (Table 3), although numbers of men in these categories were small. Men with a slip of ≥15% were significantly more likely to report radicular pain and lower extremity weakness compared to men with a lesser degree of slip.
Table 3.
Neurogenic symptoms in the past 12 months according to vertebral location and degree of slip among older men age ≥ 65 years: the MrOS Study.
| Vertebral level | Radicular Pain* | Lower Extremity Paresthesias** | Lower Extremity Weakness† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | p-value | No | Yes | p-value | No | Yes | p-value | |
| L3/4‡ | |||||||||
| No Slip | 218 (75%) | 71 (25%) | 0.04 | 251 (87%) | 38 (13%) | 0.19 | 257 (89%) | 32 (11%) | 0.02 |
| Slip | 2 (33%) | 4 (67%) | 4 (67%) | 2 (33%) | 3 (50%) | 3 (50%) | |||
| L4/5 | |||||||||
| No Slip | 179 (77%) | 52 (23%) | 0.03 | 203 (88%) | 28 (12%) | 0.17 | 207 (90%) | 24 (10%) | 0.14 |
| Slip | 41 (64%) | 23 (36%) | 52 (81%) | 12 (19%) | 53 (83%) | 11 (17%) | |||
| L5/S1‡ | |||||||||
| No Slip | 198 (74%) | 71 (26%) | 0.34 | 232 (86%) | 37 (14%) | 0.99 | 237 (88%) | 32 (12%) | 0.99 |
| Slip | 22 (85%) | 4 (15%) | 23 (88%) | 3 (12%) | 23 (88%) | 3 (12%) | |||
| Degree of slip‡ | |||||||||
| 0-14% Slip | 213 (76%) | 66 (24%) | 0.01 | 243 (87%) | 36 (13%) | 0.25 | 249 (89%) | 30 (11%) | 0.03 |
| 15-28% Slip | 7 (44%) | 9 (56%) | 12 (75%) | 4 (25%) | 11 (69%) | 5 (31%) | |||
Back pain radiating down into the buttock, hip, leg, or foot.
Back pain with numbness or tingling in the buttock, thigh, lower leg, or foot.
Back pain with a feeling of weakness in the leg, ankle or foot.
P-values for differences in proportions from chi-square or Fisher’s exact tests.
Spondylolisthesis and physical functioning
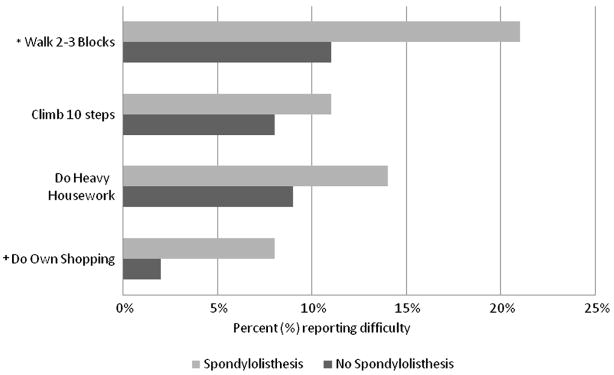
Men with spondylolisthesis were more likely to report difficulty with certain activities (Figure). Compared to their counterparts, they were nearly twice as likely to report difficulty walking 2-3 blocks and about four times as likely to report difficulty with shopping; although the number of men reporting this limitation was small (7 men with spondylolisthesis and 4 men without). Men with and without spondylolisthesis were similarly likely to report difficulty climbing up 10 steps or doing heavy housework.
Fig. 1.
Proportions of men reporting difficulty with physical function and instrumental activities of daily living according to the presence or absence of spondylolisthesis. *P-values for test of differences in proportion are *p=0.03 and +p=0.04.
Of the seven activities which men indicated difficulty performing specifically due to back pain, similar proportions with and without spondylolisthesis reported difficulty with bending down to pick up lightweight objects (13% and 11%, p=0.6), lifting a ten-pound object up from the floor (7% and 6%, p=0.8), reaching an object just overhead (2% and 1%, p=0.7), putting socks on either foot (8% and 8%, p=0.9), standing for 2 hours (17% and 15%, p=0.6), and sitting for 30 minutes (7% v 5%, p=0.6). Only difficulty getting in and out of the front seat of a car was reported with significantly greater frequency among men with spondylolisthesis (14%) compared to men without (6%) (p=0.03).
Discussion
New information about the relation of spondylolisthesis to back pain, neurogenic symptoms, and limitations in physical functioning among elderly community-dwelling men emerged from this study. Although spondylolisthesis and self-reported back pain were common in this cohort, the prevalence of any back pain and several aspects of back pain occurring in the past 12 months was similar among men with and without spondylolisthesis. In contrast, neurogenic symptoms, particularly radicular pain and lower extremity weakness, were reported more frequently by men with spondylolisthesis than by men without. Consistent with this observation, men with spondylolisthesis more often reported difficulty with activities that require lower extremity function including walking 2-3 blocks, shopping, and getting in or out of a car. Thus, spondylolisthesis may have consequences on physical functioning that have not been well described previously.
Our study adds to the accumulating evidence that spondylolisthesis and low back pain are unrelated in general populations. Prevalent spondylolisthesis and back pain were not associated in two cohorts with broad age ranges,17, 21 among primarily elderly adults (average age 79 years),4 or among elderly women.5 Similarly, frequencies of back pain reported during early adult life, mid-life, or later life were similar between adults with and without spondylolisthesis, as was the frequency of persistent or troublesome back pain any time in life.4 However, when queried about recent “pain, aching or stiffness in the back on most days,” those with spondylolisthesis responded affirmatively (32%) more often than those without (19%).4 Future studies could address the possibility that persons with spondylolisthesis experience their symptoms as aching or stiffness rather than pain.
Clinical literature indicates that patients with spondylolisthesis often present with complaints of leg pain, numbness or weakness.3 However, the prevalence of these neurogenic symptoms among those with and without spondylolisthesis is not well documented. Our data indicate about 20%-30% of men with spondylolisthesis reported at least one neurogenic symptom. Radiculopathy, paresthesias and lower extremity weakness were all reported more often by men with spondylolisthesis than by their counterparts. We noted that the occurrence of these symptoms seemed to differ by the vertebral level affected and by the degree of slip. Radicular pain and weakness were both more common among men with L3/4 spondylolisthesis or with slip of ≥15%, although these categories were affected by small numbers. Radicular pain was also more common among those with L4/5 spondylolisthesis.
Our results agree with earlier reports that prevalent spondylolisthesis does not contribute to difficulties performing activities of daily living such as dressing, sitting, climbing stairs or lifting.4-5 However, spondylolisthesis does seem to be adversely associated with lower extremity physical functioning. Others reported that elderly women with anterolisthesis were 1.3 times more likely to report difficulty walking 2-3 blocks compared to women without, although the association was of borderline significance.5 However, no difference was reported in the frequency of having either ‘a lot of difficulty with’ or being ‘unable to walk a half mile’ according to spondylolisthesis status.4 Thus, spondylolisthesis may contribute to limitations in lower extremity physical functioning, rather than to actual inability to perform these functions. The relation of spondylolisthesis to difficulty walking short distances in this cohort, and among elderly women,5 is consistent with the clinical presentation of spondylolisthesis with spinal stenosis.8
Our study has potential limitations. We had access only to recumbent lateral radiographs, which may have underestimated slip distance and led us to misclassify some men with spondylolisthesis as being without this condition. Such misclassification would underestimate the prevalence of spondylolisthesis in the study sample. However, because slip was measured without knowledge of the participants’ baseline data, any misclassification of spondylolisthesis status should be random with respect to symptoms and functional status. Therefore, the associations of spondylolithesis with neurogenic symptoms and physical functioning may be underestimated. Further, we reason our study is unlikely to have missed associations between spondylolisthesis and back pain measures but observed associations with neurogenic symptoms. The two studies that assessed slip magnitude on standing radiographs as compared to recumbent radiographs have yielded equivocal results. One group reported that for 13 of 50 patients with spondylolisthesis or spondylolysis, slip distance of the L5 vertebral body on the sacrum was at least 2mm greater when measured on standing radiographs compared to slip measured on recumbent radiographs.32 However, these authors did not provide any data tables and failed to comment on the remaining 37 patients in whom there was apparently no difference in slip distance on the two types of radiographs.32 In contrast, others reported that the mean (SD) vertebral slip distance of 12.0mm (8.8mm) on recumbent lateral radiographs did not differ significantly from the mean (SD) of 12.3mm (8.6mm) on standing radiographs among patients with spondylolysis.33 Neither of these studies was designed to compare the proportion of patients assessed with spondylolisthesis on each type of radiograph and therefore cannot be used to determine the extent of any misclassification. The lack of flexion-extension radiographs, which were not obtained in the MrOS study, is another possible limitation. Such views may permit the detection of unstable spondylolisthesis,34 although the appropriate diagnostic imaging assessment remains unresolved.35
Our study has several strengths. The MrOS cohort included ambulatory, community dwelling men residing in multiple geographic areas who were not pre-selected for spondylolisthesis, back pain, or other clinical conditions. Men in this sample and in the entire MrOS cohort are comparable in several respects to elderly US men with regard to average height (174.3cm),36 BMI (28.3 kg/m2),36 history of hypertension (46.7%)37 and existing PAD (4.7% with ABI <0.90),38 but differ on certain other health characteristics. Fair-to-poor self-rated health (26.4%), diabetes history (18.1%), difficulty walking a ¼ mile (24.4%), or climbing up 10 steps (17.9%) are more frequently reported among older US men participating in the National Health Interview Survey (NHIS)37 than by men in MrOS. Based on these data, it is likely that the results of this study are broadly applicable to elderly US men who are generally healthy. Nonetheless, because the MrOS study only included older men, the results from this study may not be generalizable to younger men or to women. Responses to the questionnaires should be unbiased because men were unaware of their spondylolisthesis status. Measurement of the ABI allowed us to examine the possible contribution of vascular claudication to leg symptoms. The distribution of neurogenic symptoms did not differ appreciably after exclusion of men with PAD, indicating that the association of spondylolisthesis and neurogenic symptoms could not be attributed to this condition.
The success of surgery for managing degenerative spondylolisthesis with spinal stenosis has been well documented.39-42 Several of these studies concluded that fusion is a necessary part of surgery to treat spondylolisthesis with concomitant spinal stenosis, because outcomes improved compared to decompression alone39 or to a pseudoarthrosis.40-41 Recently, the ‘as treated’ analysis of data from the Spine Patient Outcomes Research Trial (SPORT), the largest trial to date regarding treatment of spondylolisthesis with spinal stenosis, demonstrated improved outcomes at two year follow-up with lumbar decompression and fusion compared to nonsurgical management.42 It is important to note that none of these studies address surgery for spondylolisthesis alone. Despite lack of data regarding the effectiveness of spine surgery for degenerative spinal conditions accompanying axial back pain, many patients are treated with such surgery.43 In the context of this gap in knowledge, the lack of association between spondylolisthesis and back pain observed in MrOS and several other cohort studies4-6, 17 suggests that a decision to perform surgery for spondylolisthesis solely as a treatment for back pain among elderly men should be made with caution. Patients in the SPORT trial who were managed nonoperatively showed moderate improvement on average over the two-year study period without any instances of major neurological worsening.42 These data offers reassurance that patients could elect non-surgical treatments for degenerative spondylolisthesis.
In this cross-sectional study among elderly men, we observed that the prevalence of back pain, back pain severity, and being bothered by back pain in the past year, were not different among men with radiographic degenerative spondylolisthesis compared to those without. However, in comparison with their counterparts, men with spondylolisthesis were more likely to report neurogenic symptoms in the past year and to have experienced recent limitations in performing activities requiring lower extremity function. For the physician treating spondylolisthesis, it is important to monitor neurogenic symptoms and to assess difficulties with activities of daily living that require lower extremity function.
Acknowledgments
Funding The Osteoporotic Fractures in Men (MrOS) Study is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), and the National Center for Research Resources (NCRR) and the NIH Roadmap for Medical Research through grants U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 RR024140.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wiltse LL, Newman PH, Macnab I. Classification of spondylolisis and spondylolisthesis. Clin Orthop Relat Res. 1976;(117):23–29. [PubMed] [Google Scholar]
- 2.Rosenberg NJ. Degenerative spondylolisthesis. Predisposing factors. J Bone Joint Surg Am. 1975;57(4):467–474. [PubMed] [Google Scholar]
- 3.North American Spine S. Clinical Guidelines for Multidisciplinary Spine Care Diagnosis and Treatment of Degenerative Lumbar Spondylolisthesis. Burr Ridge, IL: North American Spine Society; 2008. [Google Scholar]
- 4.Kauppila LI, Eustace S, Kiel DP, Felson DT, Wright AM. Degenerative displacement of lumbar vertebrae. A 25-year follow-up study in Framingham. Spine. 1998;23(17):1868–1873. doi: 10.1097/00007632-199809010-00014. discussion 1873-1864. [DOI] [PubMed] [Google Scholar]
- 5.Vogt MT, Rubin D, Valentin RS, et al. Lumbar olisthesis and lower back symptoms in elderly white women. The Study of Osteoporotic Fractures. Spine. 1998;23(23):2640–2647. doi: 10.1097/00007632-199812010-00020. [DOI] [PubMed] [Google Scholar]
- 6.Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199–205. doi: 10.1097/BRS.0b013e31818edcfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denard PJHK, Miller J, Fink HA, Kado DM, Yoo JU, Marshall LM. Lumbar spondylolisthesis among elderly men: prevalence, correlates and progression. Spine. doi: 10.1097/BRS.0b013e3181bd9e19. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mossaad MM, Herkowitz HN, Dvorak J, Bell G, Nordin M, Grob D. Degenerative Lumbar Spondylolisthesis with Spinal Stenosis: Natural History, Diagnosis, Clinical Presentation, and Nonoperative Treatment. In: Anonymous, editor. The Lumbar Spine Official Publication of the International Society for the Study of the Lumbar Spine. Third. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 514–523. [Google Scholar]
- 9.Vibert BT, Sliva CD, Herkowitz HN. Treatment of instability and spondylolisthesis: surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443:222–227. doi: 10.1097/01.blo.0000200233.99436.ea. [DOI] [PubMed] [Google Scholar]
- 10.Davis H. Increasing rates of cervical and lumbar spine surgery in the United States, 1979-1990. Spine. 1994;19(10):1117–1123. doi: 10.1097/00007632-199405001-00003. discussion 1123-1114. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine. 2005;30(12):1441–1445. doi: 10.1097/01.brs.0000166503.37969.8a. [DOI] [PubMed] [Google Scholar]
- 12.Kuntz KM, Snider RK, Weinstein JN, Pope MH, Katz JN. Cost-effectiveness of fusion with and without instrumentation for patients with degenerative spondylolisthesis and spinal stenosis. Spine. 2000;25(9):1132–1139. doi: 10.1097/00007632-200005010-00015. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992-2003. Spine. 2006;31(23):2707–2714. doi: 10.1097/01.brs.0000248132.15231.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsunaga S, Ijiri K, Hayashi K. Nonsurgically managed patients with degenerative spondylolisthesis: a 10- to 18-year follow-up study. J Neurosurg. 2000;93(2 Suppl):194–198. doi: 10.3171/spi.2000.93.2.0194. [DOI] [PubMed] [Google Scholar]
- 15.Postacchini F, Perugia D. Degenerative lumbar spondylolisthesis. Part I: Etiology, pathogenesis, pathomorphology, and clinical features. Ital J Orthop Traumatol. 1991;17(2):165–173. [PubMed] [Google Scholar]
- 16.Fitzgerald JA, Newman PH. Degenerative spondylolisthesis. J Bone Joint Surg Br. 1976;58(2):184–192. doi: 10.1302/0301-620X.58B2.932080. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen S, Sonne-Holm S, Rovsing H, Monrad H, Gebuhr P. Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine. 2007;32(1):120–125. doi: 10.1097/01.brs.0000250979.12398.96. [DOI] [PubMed] [Google Scholar]
- 18.Vogt MT, Lauerman WC, Chirumbole M, Kuller LH. A community-based study of postmenopausal white women with back and leg pain: health status and limitations in physical activity. J Gerontol A Biol Sci Med Sci. 2002;57(8):M544–550. doi: 10.1093/gerona/57.8.m544. [DOI] [PubMed] [Google Scholar]
- 19.Weiner DK, Haggerty CL, Kritchevsky SB, et al. How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC Cohort and implications for the future. Pain Med. 2003;4(4):311–320. doi: 10.1111/j.1526-4637.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- 20.Hicks GE, Gaines JM, Shardell M, Simonsick EM. Associations of back and leg pain with health status and functional capacity of older adults: findings from the retirement community back pain study. Arthritis Rheum. 2008;59(9):1306–1313. doi: 10.1002/art.24006. [DOI] [PubMed] [Google Scholar]
- 21.Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine (Phila Pa 1976) 2009;34(2):199–205. doi: 10.1097/BRS.0b013e31818edcfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Meyereding H. Spondylolisthesis. Surg Gynecol Obstet. 1932;54:371–379. [Google Scholar]
- 25.Timon SJ, Gardner MJ, Wanich T, et al. Not all spondylolisthesis grading instruments are reliable. Clin Orthop Relat Res. 2005;(434):157–162. doi: 10.1097/01.blo.0000154205.10944.72. [DOI] [PubMed] [Google Scholar]
- 26.Nachemson A. The role of spine fusion Question 8. Spine. 1981;6(3):306–307. [Google Scholar]
- 27.Dupuis PR, Yong-Hing K, Cassidy JD, Kirkaldy-Willis WH. Radiologic diagnosis of degenerative lumbar spinal instability. Spine (Phila Pa 1976) 1985;10(3):262–276. doi: 10.1097/00007632-198504000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Gatchel R. Compendium of outcome instruments for assessment and research of spinal disorders. La Grange, IL: North American Spine Society; 2001. [Google Scholar]
- 29.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 30.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88(3):837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 31.Rothman KJGS. Modern Epidemiology. 2. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 32.Lowe RW, Hayes TD, Kaye J, Bagg RJ, Luekens CA. Standing roentgenograms in spondylolisthesis. Clin Orthop Relat Res. 1976;10(117):80–84. [PubMed] [Google Scholar]
- 33.Saraste H, Brostrom LA, Aparisi T, Axdorph G. Radiographic measurement of the lumbar spine A clinical and experimental study in man. Spine. 1985;10(3):236–241. doi: 10.1097/00007632-198504000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Mossad MM. Degenerative lumbar spondylolisthesis with spinal stenosis: natural history, diagnosis, clinical presentation, and nonoperative treatment. In: Herkowitz HN, Dvorak J, Bell GR, Nordin M, Grob D, editors. The Lumbar Spine. 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 514–523. [Google Scholar]
- 35.Nizard RS, Wybier M, Laredo JD. Radiologic assessment of lumbar intervertebral instability and degenerative spondylolisthesis. Radiol Clin North Am. 2001;39(1):55–71. v–vi. doi: 10.1016/s0033-8389(05)70263-3. [DOI] [PubMed] [Google Scholar]
- 36.McDowell MA, Fryar CD, Hirsch R, Ogden CL. Anthropometric reference data for children and adults: U.S. population, 1999-2002. Adv Data. 2005;(361):1–5. [PubMed] [Google Scholar]
- 37.Schoenborn CA, Vickerie JL, Powell-Griner E. Health characteristics of adults 55 years of age and over: United States, 2000-2003. Adv Data. 2006;(370):1–31. [PubMed] [Google Scholar]
- 38.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 39.Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73(6):802–808. [PubMed] [Google Scholar]
- 40.Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22(24):2807–2812. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 41.Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29(7):726–733. doi: 10.1097/01.brs.0000119398.22620.92. discussion 733-724. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carragee EJ, Deyo RA, Kovacs FM, et al. Clinical research: is the spine field a mine field? Spine (Phila Pa 1976) 2009;34(5):423–430. doi: 10.1097/BRS.0b013e318198c962. [DOI] [PubMed] [Google Scholar]