Abstract
Forty nondemented older adults who were divided into two groups on the basis of their cognitive status (MCI: n=20; Normal Control: n=20) underwent diffusion tensor imaging, and estimates of fractional anisotropy (FA) and mean diffusivity (MD) were obtained for the genu and splenium of the corpus callosum. Results demonstrated the following: (1) group comparisons revealed that splenium FA was significantly lower in MCI participants than in NC participants, despite no differences in gross morphometry or hippocampal volumes; (2) in the overall sample, higher stroke risk was associated with lower white matter integrity, particularly in the genu; (3) increased stroke risk was more strongly associated with poorer splenium FA in those with MCI than in normal elderly; and (4) splenium FA significantly predicted performance on verbal memory (adjusting for the effects of age, education, and whole brain volume). Findings demonstrate a relationship between increased vascular burden and white matter changes, and they support the possibility that posterior white matter pathology may contribute to the development of MCI-related cognitive changes.
Keywords: MCI, mild cognitive impairment, stroke risk, Framingham, aging, white matter, diffusion tensor imaging, DTI
1-Introduction
Over the past two decades, there has been considerable evidence of gray matter alterations in mild cognitive impairment (MCI), a clinical construct that describes individuals with mildly impaired performance on objective neuropsychological tests but relatively intact global cognition and daily functioning (Petersen et al., 1999, 2001). It is accepted that MCI, which has been validated as qualitatively different from both normal aging and dementia (Petersen, 2004; Smith & Ivnik, 2003), is a well-known risk factor for the development of dementia, particularly Alzheimer's disease (AD). Research has shown that gray matter changes are widespread throughout the cortex and are particularly striking in mesial temporal lobe (MTL) structures such as the entorhinal cortex (deToledo-Morrell et al., 2000; Dickerson et al., 2001) and hippocampal formation (Callen et al., 2001; deToledo-Morrell et al., 2004; Du et al., 2001), brain areas known to be especially important for memory formation and pathologically involved early in the AD process. In addition to degenerative changes in the MTL, research suggests that there is increased gray matter pathology in posterior brain regions relative to anterior regions in early AD (Arnold et al., 1991; Braak & Braak, 1995). While these gray matter alterations have been well typified and described in the literature, the exact mechanisms underlying these brain changes have not yet been fully elucidated and understood.
Recently, there has been a growing body of research in the literature to suggest that white matter pathology may contribute to age-related cognitive impairment and possibly potentiate the development of dementia (Raz & Rodrigue, 2006; Sullivan & Pfefferbaum, 2006). In general, numerous post-mortem and volumetric studies have shown that macroscopic white matter alterations such as demyelination, white matter lesion (WML) pathology, and degeneration occur in both normal aging (Raz, 2000; Jernigan et al., 2001) and dementia (Barber et al., 2000; Gurol et al., 2006). Although these studies have advanced our knowledge considerably, the majority have employed conventional magnetic resonance imaging (MRI) which is limited in its ability to reflect white matter microstructural integrity, and thus it is not clear if the extent and pattern of white matter changes differ in aging and dementia, or whether these changes play a role in the evolution and expression of dementia such as AD (Wozniak & Lim, 2006). More recently, diffusion tensor imaging (DTI)—an MRI technique that produces contrast between healthy and pathologic tissue (Basser & Pierpaoli, 1996)—has shown particular promise in helping to elucidate the nature and pattern of white matter changes that occur across the aging spectrum (see Minati, Grisoli, & Bruzzone, 2007 for review).
To date, only a few studies have employed DTI to examine early white matter changes in older adults who are at high risk for the development of dementia due to the presence of Mild Cognitive Impairment (MCI), Most studies that have examined white matter changes in MCI have focused primarily on the cingulum (Rose et al., 2000; Takahashi et al., 2002) and medial temporal lobe regions because they are known to be affected early in the course of AD (Kantarci et al., 2001; Fellgiebel et al., 2006). In contrast, studies of normal aging have typically investigated changes in the corpus callosum (Pfefferbaum et al., 2000; Salat et al., 2005; Hasan et al., 2004) and have shown that white matter integrity decreases with increasing age, particularly in frontal regions (Head et al., 2004; Ota et al., 2006). Although several studies have shown greater posterior callosal white matter degradation in MCI (Naggara et al., 2006; Cho et al., 2008; Medina et al., 2006; Ukmar et al., 2007), other studies have not found white matter changes in the splenium in this population (Fellgiebel et al., 2004; Zhang et al., 2007; Stahl et al., 2007). In general, little is known about changes in the tissue characteristics of callosal subregions in MCI and how any observed changes may be associated with the presence and severity of either vascular risk or neuropsychological functioning.
The present study used DTI to compare and contrast white matter changes in older adults with MCI and age-matched normal control (NC) participants. Subregions of the corpus callosum (i.e., the genu and splenium) were selected given the heterogeneity of its microstructure (Aboitz et al., 1996) as well as its heterotopic nature (i.e., anteroposterior cortical connectivity) (Huang et al., 2005). We hypothesized that more widespread, global white matter changes would be evident in MCI participants (i.e., poorer integrity of both callosal regions). In addition, given the predilection for frontal white matter alterations in aging (Head et al., 2004; 2005) and the sensitivity of DTI to early hypoxic-ischemic injury, we expected that anterior white matter (i.e., genu) would be primarily affected in normal aging and that integrity of this white matter bundle would be inversely associated with stroke risk. Finally, we expected that regional DTI indices of white matter integrity would be associated with cognition such that temporoparietal white matter (i.e., splenium) would be related to memory whereas frontal white matter (i.e., genu) would be associated with executive functions. To our knowledge, the present study is the first to relate DTI indices to stroke risk and neuropsychological functioning in a sample diagnosed with MCI.
2 - Methods and Materials
2.1 - Participants
This study included 40 age- and education-matched nondemented participants (MCI: n = 20, NC = 20; see Table 1 for demographic comparisons). This cohort was recruited solely for research purposes, and they were subsequently drawn from a larger pool of 115 individuals enrolled in a longitudinal study of aging. Participants were consecutively accrued and selected because they had undergone both neuroimaging as well as a comprehensive neuropsychological evaluation. The determination of cognitive status was based upon clinical examination by a staff neurologist as well as an extensive medical, laboratory, and neuropsychological evaluation. Participants were free from the following exclusionary conditions: (1) past history of head injury with loss of consciousness or serious neurologic disorder (e.g., multiple sclerosis, Parkinson's disease, epilepsy); (2) significant history of alcohol or drug abuse; (3) history of learning disability; (4) history of stroke; (5) current/past history of severe psychiatric illness or (7) MRI contraindications (e.g., claustrophobia, pacemakers, or metal implants). The appropriate institutional review boards approved this study and all participants provided written informed consent.
Table 1.
Demographic, clinical, global cognitive, and brain MRI morphometric characteristics of normal control (NC) participants and patients with mild cognitive impairment (MCI).
| Groups | p-value | ||
|---|---|---|---|
| MCI Mean SD | NC Mean SD | ||
| Age (years) | 78.00 (7.32) | 78.14 (7.06) | .88 |
| Sex (women/men)° | 9/20 | 11/20 | .36 |
| Education (years) | 16.11 (2.64) | 16.48 (2.21) | .63 |
| Stroke Risk (FSRP) | 17.6 (7.54) | 15.1 (10.38) | .39 |
| Depression (GDS) | 6.57 (4.79) | 5.79 (4.96) | .53 |
| Total DRS Score | 138.11 (5.36) | 141.9 (2.79) | .007 |
| Activities of Daily Living† | 0.09 (0.68) | −0.08 (1.05) | .63 |
| Hippocampal Volume (mm3) | 5889.4 | 5942.9 | .97 |
| Whole Brain Volume (cm3) | 1312.8 (148.6) | 1288.2 (109.9) | .46 |
| Gray Matter Volume (cm3) | 511.7 (77.2) | 518.6 (51.2) | .77 |
| White Matter Volume (cm3) | 470.6 (77.5) | 443.9 (70.2) | .26 |
| CSF Volume (cm3) | 330.5 (32.0) | 319.8 (46.2) | .43 |
| Gray Matter Proportion (%) | 38.91 (3.04) | 40.56 (3.75) | .20 |
| White Matter Proportion (%) | 35.75 (3.62) | 34.45 (2.97) | .22 |
| CSF Volume Proportion (%) | 25.33 (2.84) | 24.98 (3.26) | .72 |
Note: FSRP = Framingham Stroke Risk Profile (D 'Agostino et al., 1994); GDS = Geriatric Depression Scale; DRS = Dementia Rating Scale; CSF = Cerebrospinal fluid.
Chi-square test with continuity correction
Activities of Daily Living = composite score representing Money Management and Health and Safety subscales of the Independent Living Scales (ILS).
2.2 - Diagnosis of Mild Cognitive Impairment
Criteria used to diagnose MCI were based on an adaptation of those recently outlined by Petersen and Morris (2005). Specifically, a diagnosis of MCI was based on the following: 1) normal activities of daily living; 2) absence of dementia; and 3) mild quantifiable cognitive impairment within one or more domains (i.e., attention, language, memory, executive function, visuospatial function). Given that the utility of the Mini-Mental Status Examination (MMSE; Folstein, Folstein, & McHugh, 1975) has been shown to decrease for use with impaired populations (particularly in highly educated samples) and does not appear to distinguish MCI from normal aging (Diniz, 2007), we chose to use the Mattis Dementia Rating Scale (DRS) which has recently been shown to be a more sensitive screening tool for MCI (Yang et al., 2006). We used a cut-off score of 127/144 points possible, a cut-off that was selected statistically to ensure that no participant scored more than 1.5 SD below the published normative mean for this test. Group differences on the DRS (means and standard deviations) are shown in Table 1. Functional ability was assessed using the Independent Living Scales (ILS; Loeb, 1996), an ecologically valid performance-based measure designed to assess the ability to independently complete activities of daily living. Subjective memory complaints were not included in the diagnostic determination of MCI given that this criterion has been heavily questioned in the literature. For example, it was recently shown that subjective memory complains do not precede an AD diagnosis in at least half of all cases (Palmer et al., 2008).
Although no standard cut-off criterion for defining impairment in MCI has yet been delineated, Petersen et al. (1999; Petersen & Morris, 2005) have suggested employing a 1.5 standard deviation (SD) cut-off, although Busse et al. (2006) demonstrated that a more liberal cut-off (i.e., 1 SD below the mean) is optimal since it offers higher sensitivity than the traditional cut-off of 1.5 SD. Thus, given our aim to be broadly inclusive, we compromised between the recommendations of Petersen and colleagues (Petersen et al., 1999; Petersen & Morris, 2005) and Busse et al. (2006) by using a cut-off of 1.2 SD (after applying norms adjusted for age, education, and gender), signifying a level of performance worse than 88.5% of the population (indicative of mild to moderate impairment) (Delano-Wood et al., 2008). All neuropsychological scores were standardized with a z-score transformation on the basis of normative data of the neuropsychological tests (Welsh et al., 1994). Scores that reflected number of errors or response times were multiplied by −1, so that negative z-scores consistently reflected poor performance. All participants were categorized into one of four subgroups (single-domain amnestic MCI [n = 7]; single-domain nonamnestic MCI [n = 5]; multiple-domain amnestic-MCI [n = 4]; and multiple-domain nonamnestic-MCI [n = 4]) based on neuropsychological test scores (Petersen & Morris, 2005). Examination of specific MCI subtypes (e.g., single- vs. multi-domain, amnestic vs. non-amnestic) could not adequately be carried out due to the relatively small number of individuals within each cell. However, for descriptive purposes, we collapsed MCI subtypes to reflect two general groups for post hoc analyses: 1) amnestic MCI (single- and multiple-domain amnestic MCI participants) and 2) non-amnestic MCI (single- and multiple-domain amnestic MCI participants).
2.3 Stroke risk assessment
The Framingham Stroke Risk Profile (FSRP; D'Agostino et al., 1994), the most widely used sex-specific clinical estimate of cerebrovascular risk burden, was administered to each participant. The FSRP was developed to predict a 10-year probability for risk of stroke and is based on the following risk factors identified from 36 years of longitudinal study within the Framingham Heart Study: age, systolic blood pressure (SBP), antihypertensive medication, diabetes, cigarette smoking, history of cardiovascular disease (CVD), atrial fibrillation (AF), and left ventricular hypertrophy (LVH) as determined by ECG. The FSRP has been advocated by the American Stroke Association for risk assessment and has been shown to be predictive of stroke in aging populations (Truelsen et al., 1994).
2.4 - Neuropsychological Assessment
All participants were administered a cognitive battery that has been described previously (Salmon & Butters, 1992). Specific measures included tests of global cognitive function (Mattis Dementia Rating Scale), attention [Wechsler Adult Intelligence Scale-Revised (WAIS-R) Digit Span subtest], language (WAIS-R Vocabulary Subtest, American National Adult Reading Test, Boston Naming Test, Verbal Fluency), memory (California Verbal Learning Test; Wechsler Memory Scale – Revised Logical Memory), visuospatial functioning (WAIS-R Block Design and Digit Symbol subtests; Clock Drawing test), simple motor functioning (Grooved Pegboard), and executive functioning (Tower, Color-Word Interference, Sorting, and Letter-Number Sequencing subtests from the Delis-Kaplan Executive Function System [D-KEFS; Delis et al., 2001a, 2001b).
2.5 - MR Acquisition and Processing
All images were acquired on a GE Signa LX 1.5 Tesla scanner. High-resolution T1-weighted anatomic images were collected with an SPGR sequence (124 slices acquired in the sagittal plane; 1.2 mm slice thickness; 256×256 matrix; field of view [FOV]=250 mm; resulting in a 1 mm2 in-plane resolution). For the DTI sequence, 12 axial images through the corpus callosum were acquired with a high-angular resolution diffusion encoding sequence using spiral acquisition (Frank, 2001). Diffusion was encoded with an SE preparation along 42 diffusion directions with b = 1990 s/mm2. DTI quantification was preceded by eddy current correction based on the inversion recovery images, using a six-parameter affine correction on a slice-by-slice basis (Frank, 2001). Image parameters for the DTI sequence were: TE=100ms, 64×64 image matrix, slice thickness = 3.8mm, FOV=24cm2, TR=2.5 sec, and NEX=7. The images were reconstructed onto a 128×128×12 grid with a voxel size of 1.875×1.875×3.8 mm3.
Image processing prior to ROI analyses included several image registration steps which resulted in co-registered structural and diffusion-weighted data resampled to 1 mm3 voxels in Talairach atlas space (Talairach & Tournoux, 1988). Solving the six independent equations with respect to MDxx, MDxy, etc., yielded the elements of the diffusion tensor. The general diffusion tensor was then diagonalized, yielding eigenvalues λ1, λ2, λ3, as well as eigenvectors that defined the predominant diffusion direction. The transformation was then applied to the DTI images before calculation of fractional anisotropy (FA), a measure which reflects orientation coherence and myelin content, and mean diffusivity (MD), an index of the overall magnitude of water diffusion within the white matter.
2.6 - Region of Interest (ROI) and Hippocampal Volumetric Analyses
All analyses were conducted with Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). Per Pfefferbaum and Sullivan (2003), regions of interest (ROIs) were not defined on the FA maps themselves and, instead, were constructed using a T1-weighted structural image to avoid using the dependent variable to define itself and thus preclude accurate examination of abnormal white matter. Individualized, manually-outlined versus Talairach daemon-defined ROIs were chosen, in part, to minimize partial volume effects and more accurately reflect individual variation in the corpus callosum.
Each ROI was manually outlined by two operators who were blind to group (see below for reliability information). The callosal subregions were outlined separately on sagittal T1 images using the following classification scheme based on cortical connectivity information obtained via DTI tractography (Hofer & Frahm, 2006): The callosum was divided into five specific arithmetic divisions and the perpendicular extending through the anterior most point of the inner convexity of the anterior callosum was used to define the genu (including the rostrum; anterior one-sixth of the corpus callosum); the splenium was defined as the posterior one-fourth region of the callosum. Measurement took place on every slice in both hemispheres in which both the subregions were fully volumed and clearly visible. Interrater reliability was computed for 10 randomly chosen subjects, which yielded interrater intraclass correlations of r = 0.91 for FA and r = .94 for ADC in the genu and of r = 0.93 for FA and r = 0.95 for ADC in the splenium. Following completion of individual tracings, the ROI masks were overlaid onto the FA and trace images to obtain average FA and MD values for the two callosal subregions for each subject.
Hippocampal volumes were obtained bilaterally via inspection and manual outlining performed in the coronal plane. Images were realigned perpendicular to the anterior-posterior commissure line; however, they were not transformed into standard space coordinates. Regions of interest were defined using AFNI software and completed by an experienced operator (A.J.J), who was blind to participant identity and group membership. High levels of intra- and inter-rater reliability for the procedure have been previously established (intraclass correlation coefficients > 0.90; Jak et al., 2007). Hippocampal regions of interest were delineated using a stereotactic approach adapted from methods published previously (Nagel et al., 2004; Jak et al., 2007). Briefly, the anterior bound of the hippocampus was chosen as the coronal slice through the fullest portion of the mammillary bodies, and the posterior boundary was traced on the last coronal slice on which the superior colliculi could be visualized.
2.7 - Segmentation of Structural Images
Whole brain images were skull-stripped and segmented into gray matter, white matter, and cerebrospinal fluid (CSF) compartments. Following N3 bias correction of field inhomogeneities (Sled, Zijdenbos, & Evans, 1998), each scan was processed with one or both of the following automated methods to most fully remove all non-brain material: FreeSurfer's Hybrid Watershed Algorithm and/or Brain Surface Extractor (Version 3.3) (Shattuck et al., 2001), which have been demonstrated to be effective for processing images from older adults (Fennema-Notestine et al., 2006). When necessary, scans were manually edited to remove any residual non-brain material (e.g., skull). Tissue segmentation was performed using FSL's FAST (FMRIB's Automated Segmentation Tool) (Zhang, Brady, & Smith, 2001), whole brain volume was derived, and total gray matter, white matter, and CSF volumes were normalized (Bigler & Tate, 2001). Specifically, each compartment was normalized by dividing the respective structure volume by the whole brain volume and multiplying by 100 to correct for intersubject differences in overall brain size.
2.8 - Statistical Analyses
Composite scores were computed for memory (recall and recognition), executive functioning, attention, and visuospatial skills, and significant correlations between the tests that comprise each composite score supported this approach. Raw scores for each of the measures listed below were z-transformed using the means and standard deviations of the control group. Next, the z-scores for the individual tests were averaged to create a summary score for each cognitive domain. The specific measures that comprise each domain are as follows: (1) Memory (Recall) was assessed with the Wechsler Memory Scale – Revised (Wechsler, 1987) Logical Memory subtests (WMS-R; Immediate and Delayed Free Recall) and the California Verbal Learning Test (CVLT; Trials 1–5 Total Recall and Long Delay Free Recall; Delis, Kramer, Kaplan, & Ober, 1987); (2) Memory (Recognition) was measured with WMS-R Logical Memory Total Recognition Correct subtest and CVLT Total Recognition Correct subtest; (3) Attention was measured with the Attention subscale of the DRS and the Digit Span subtest of the Wechsler Adult Intelligence Scale – Revised (WAIS-R; Wechsler, 1981); (4) Visuospatial skills were assessed with the Block Design subtest of the Wechsler Intelligence Scale for Children – Revised (WISC-R; Wechsler, 1974) and the Construction subscale of the DRS; and (5) Executive functioning was measured with several DKEFS subtests (Trails, Number-Letter Sequencing, Tower, and Verbal Fluency [Letter and Category]).
Group comparisons (MCI vs. NC) were performed with t-tests or Chi-Square tests, as appropriate. Group by callosal region mixed-model analyses of variance (ANOVAs) were conducted to examine the main effects of group and white matter integrity of callosal subregions as well as their possible interaction. Hierarchical multiple regression and ANCOVA were used to determine the relationship between DTI indices and the interaction between stroke risk and diagnostic group, adjusting for age and whole brain volume. In addition, Pearson product-moment correlations examined associations between DTI indices and cognitive variables of interest. Finally, where appropriate, multiple comparisons were adjusted for in the reported analyses (adjustment of alpha level by number of tests performed). All analyses were conducted in SPSS (Version 14.0).
3- Results
3.1 - Regional Differences in Morphometry and Callosal DTI Indices by Group
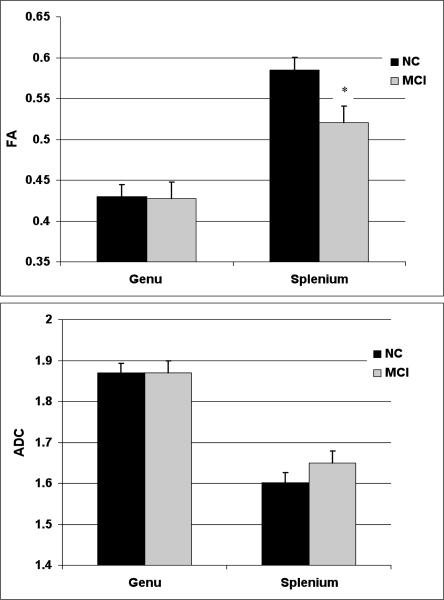
As shown in Table 1, there were no significant differences between MCI and NC participants on structural MR whole-brain segmentation volumetric measurements or in total hippocampal volumes (all p-values > .20). For FA measurements, a group (MCI vs. NC) by callosal subregion (genu vs. splenium) mixed-model ANOVA demonstrated an expected significant main effect of region (F(1, 38) = 139.76, p < .001, ηp2 = .84) indicating that splenium FA was higher than genu FA when collapsed across groups. Although there was no main effect of group (F < 1, p = .68, ηp2 = .04), there was a significant interaction between group and callosal subregion (F(1, 38) = 4.81, p = .01, ηp2 = .21). Follow-up independent samples t-tests demonstrated that splenium FA was significantly lower in the MCI group than in the NC group (t(38) = 5.53, p < .001), whereas the groups did not differ significantly in genu FA (t < 1, p = .94). A similar analysis for MD measurements showed a significant main effect of region (F(1, 38) = 38.62, p < .001, ηp2 = .52) indicating that splenium MD was lower than genu MD, but there was no main effect of group (F < 1) and no interaction between group and region (F < 1). Figure 1 presents mean FA and MD values for the genu and splenium across groups.
Figure 1.
Mean fractional anisotropy (FA) and mean diffusivity (MD) values for the genu and splenium in normal control (NC) participants and mild cognitive impairment (MCI) patients.
Note: Lower FA and higher MD values reflect poorer microstructural integrity of the white matter. Bars indicate one standard error of the mean. * p < .001
As stated earlier, it was not possible to investigate each of the four MCI subtypes due to a lack of power (i.e., low sample sizes within each cell); however, exploratory analyses were conducted to examine for differences between amnestic and non-amnestic MCI groups (single- and multiple-domain collapsed across group). In comparison to the non-amnestic MCI group, the amnestic MCI group demonstrated significantly smaller hippcampi (t(15) = 3.35, p = .004), and there was a trend indicating lower stroke risk (t(18) = −1.90, p = .07). In terms of group differences on DTI indices, results demonstrated that the non-amnestic MCI group showed poorer FA of the splenium when compared to the amnestic group (t(18) = 2.89, p = .01). Further, findings from ANCOVA showed that the non-amnestic MCI group showed poorer splenium FA (F(5,40) = 8.26, p = .015) but not genu FA (adjusted for age, education, and whole brain volume). Neither amnestic nor non-amnestic MCI status was associated with any other DTI index in this sample.
3.2 - Associations Between Stroke Risk and Callosal DTI Indices by Group
As shown in Table 1, the MCI and NC groups did not differ significantly on mean stroke risk (p = .39). Collapsed across group, elevated stroke risk was significantly associated with advancing age (r = .40, p = .01), decreased FA in both callosal regions (genu FA: r = −.51, p = .001; splenium FA: r = −.46, p = .003), and MD in the genu (r = .48, p = .002) but not the splenium (r = .29, p = .069).
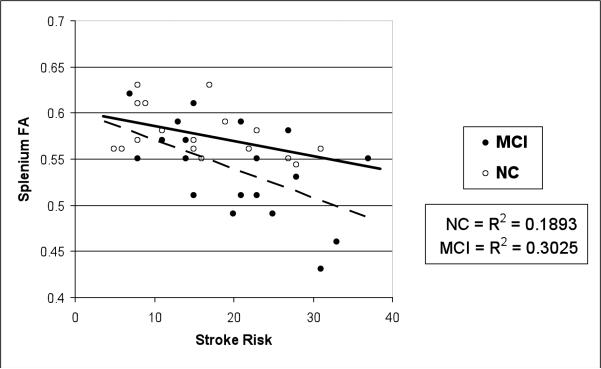
A series of hierarchical multiple regression analyses demonstrated that higher stroke risk across the entire sample strongly predicted poorer genu FA and MD above and beyond the effects of age and total brain volume (genu FA: β = −.48, ΔR2 = .23, p = .001; genu MD: β = .45, ΔR2 = .20, p = .002). In addition, the same model was significant for splenium FA (β = −.38, ΔR2 = .12, p = .02) but not for splenium MD (β = .14, ΔR2 = .02, p = .40). To determine whether the MCI and NC groups differed by stroke risk and DTI indices, a group by stroke risk interaction term was added to the model. Results revealed that, over and above the effects of age, total brain volume, MCI status, and stroke risk, the interaction term (MCI status × stroke risk) was statistically significant for the prediction of FA of the splenium (β = −.51, ΔR2 = .14, p = .02) and a trend was observed for the prediction of genu FA (β = −.31, ΔR2 = .06, p = .06). Using the same model, the MCI status × stroke risk interaction term did not predict MD of either subregion (genu MD, p = .32; splenium MD, p = .32). Figure 2 illustrates the group by stroke risk interaction on mean splenium FA.
Figure 2.
Group by stroke risk interaction of mean splenium fractional anisotropy (FA) values.
Note. Stroke risk was based on the mean Framingham Stroke Risk Profile (D'Agostino et al., 1994).
3.3 - Associations Between Group Membership, Cognition, and Regional DTI Indices
Means and standard deviations by group (MCI vs. NC) for each neuropsychological variable of interest are shown in Table 2. After controlling for multiple comparisons (adjusted alpha level to account for number of tests performed), independent samples t-tests demonstrated that the MCI group performed significantly more poorly than the NC group on the following two memory composite variables: Memory Recall (t(36) = 4.33, p < .001) and Memory Recognition (t(35)= 2.84; p = .008). Secondary analyses were conducted to examine individual tests within those factors that were significant. All memory variables contributing to the Memory Recall composite variable were significant: DRS Memory; t(35) = 3.22; p < .001); WMS-R LM-I (t(36) = 3.63; p = .001; LM-II (t(36) = 4.28; p < .001); CVLT Learning (Trials 1–5; t(36) = 2.93, p = .006); and CVLT LD Free Recall (t(36) = 2.51; p = .017). Both measures contributing to the Memory Recognition composite variable were also significant: WMS-R LM Total Recognition Correct (t(35) = 2.84; p = .008) and CVLT Total Recognition Correct (t(36) = 2.71; p = .007).
Table 2.
Means and standard deviations of neuropsychological variables by group
| Cognitive Variable | Cognitively Normal (n = 20) | MCI (n=20) |
|---|---|---|
| Attention† | .05 (.82) | .01 (.61) |
| Executive Functioning† | .10 (.61)* | −.82 (.65)* |
| Memory (Recall)† | .00 (1.5)*** | −1.90 (.83)*** |
| Memory (Recognition)† | −.06 (1.00)** | −1.24 (1.71)** |
| Visuospatial Ability† | .01 (.52)* | −1.02 (.62)* |
| Processing Speed (Trails A Total Time) | .00 (1.0) | .23 (.81) |
| Confrontation Naming (BNT Total Correct) | .00 (1.0) | −.40 (1.11) |
Note: All scores z-transformed.
p < .05
p < .01
p<.001
BNT = Boston Naming Test
indicates composite scores for cognitive domain.
Across the entire sample, neuropsychological variables of interest (DRS Total Score; all composite variables, and individual subtests assessing confrontation naming [BNT] and processing speed [Trails A]) were correlated with genu and splenium FA. Results demonstrated only trends between genu FA and cognitive tasks associated with the executive functioning composite variable (r = .31, p = .058) and naming (BNT; r = .30, p = .061). However, splenium FA was significantly positively associated with global cognition (DRS; r = .39, p = .01) as well as the Recall Memory composite variable (r = .35, p = .02). Separate secondary analyses showed that the following individual tests comprising the Recall Memory composite variable were significantly positively associated with splenium FA: WMS-R Logical Memory [Delayed Recall: r = .38, p = .01]; CVLT [List A Trials 1–5; r = .31, p = .047 and Long Delay Free Recall: r = .37, p = .01]). Results of a hierarchical regression demonstrated that lower splenium FA significantly predicted poorer verbal memory recall performance, above and beyond the effects of age, education, and whole brain volume (β = .51, ΔR2 = .21, p = .003). The results of the regression are depicted in Table 3.
Table 3.
Hierarchical regression for splenium FA as a predictor of verbal memory (recall)
| Step and Predictors | Statistics for Step | Statistics for Predictors | |||||
|---|---|---|---|---|---|---|---|
| R2 | Df | ΔR2 | Δdf | ΔF | β | T | |
| Step 1 | .028 | 3 | – – | – – | .331 | ||
| Age | .33 | −2.54* | |||||
| Education | .15 | 1.97 | |||||
| Whole Brain Volume | −.16 | 0.94 | |||||
| Step2 | .240 | 4 | .212 | 1 | 2.57* | ||
| Splenium FA | .51 | 3.24** | |||||
Notes:
p < .05
p = .003.
Verbal memory (recall) is a composite score represented by the following cognitive subtests: WMS-R Logical Memory (Immediate and Delayed Recall) and CVLTLearning (List A Trials 1–5 and Long Delay Free Recall).
4 - Discussion
The present study used DTI to investigate differences in regional callosal white matter integrity between normally aging older adults and those with MCI. The relationship between callosal white matter integrity, stroke risk, and neuropsychological functioning was also examined. Results demonstrated that older adults with MCI had diminished white matter integrity in the splenium relative to cognitively normal older adults. Across the entire sample, elevated stroke risk was associated with decreased white matter integrity, particularly in the genu. Furthermore, although there were no significant associations in our sample between genu FA and tasks of executive functioning, decreased FA in the splenium was associated with poorer performance on global cognitive functioning as well as several tests of verbal memory. Interestingly, recall but not recognition memory was positively related to posterior white matter integrity.
Across the sample as a whole, stroke risk was inversely associated with white matter integrity in both callosal subregions measured. Consistent with the notion that frontal white matter is most vulnerable to age-related ischemic changes, results showed a particularly strong relationship between increased stroke risk and decreased white matter integrity in the anterior callosal region (i.e., genu). In contrast to normal elderly, those with MCI demonstrated selective posterior callosal white matter vulnerability, and these findings are particularly notable given that the groups did not differ in terms of age, stroke risk, or global brain changes (i.e., whole brain, gray matter, white matter, or CSF volumes). These findings are supported by other studies that have shown splenium but not genu FA changes in MCI samples (Naggara et al., 2006; Cho et al., 2008; Medina et al., 2006; Ukmar et al., 2007). However, the literature is not consistent, as other studies have not found white matter changes in any subregion of the corpus callosum (Fellgiebel et al., 2004; Zhang et al., 2007; Stahl et al., 2007). Although these studies had slightly lower sample sizes that may have precluded an ability to detect group differences, in general, trends were reported that mirror our findings (i.e., no association between MCI status and genu FA; lower splenium FA in MCI). Overall, it is possible that the splenium is particularly susceptible to pathological insult given that it contains numerous small diameter fibers thought to be more vulnerable than large diameter fibers (Pantoni, Garcia, & Gutierrez, 1996). Early degeneration of these smaller white matter fibers in the splenium may interrupt neuronal transmission and impair cortical function, and this possibility is supported by our observed associations between splenium FA and neuropsychological performance.
Identifying mechanisms contributing to poorer posterior white matter integrity and associated cognitive impairment in MCI is particularly difficult and challenging. For example, although our groups did not differ with respect to overall vascular burden (i.e., stroke risk), MCI-related microstructural changes in the splenium may reflect cumulative ischemic pathology stemming from a primarily vascular etiology. This theory is supported by our post hoc finding of poorer white matter integrity (especially within the posterior region of the corpus callosum) in non-amnestic versus amnestic MCI. Indeed, there have been observations that non-amnestic MCI is associated with increased WML burden (Mariani et al., 2007) and hypertension (Reitz et al., 2007), and Mariani et al. (2007) suggest that non-amnestic MCI might itself be considered a vascular cognitive disorder. Alternatively, it may be that incipient pathological processes associated with a dementia syndrome such as AD underlie the early, degenerative white matter changes we observed in the posterior brain regions of our MCI participants. However, it is notable that our MCI sample did not differ significantly from our normal control participants on hippocampal volume, and this finding suggests that Wallerian degeneration may not be a primary contributor to the poorer posterior white matter integrity demonstrated in our MCI group. Nevertheless, microvasculature changes often occur in AD (Kalaria, 2000), and vascular risk factors such as hypertension and atherosclerosis increase AD risk (Kalaria, 2002) and are associated with AD-related neuropathological changes (increased neuritic plaques and neurofibrillary tangles) (Borroni et al., 2007; Zhu et al., 2007). It is thus plausible that the interaction between early AD and vascular pathology may increase the likelihood of expressing cognitive impairment or hasten the rate of decline (Chui et al., 2006; Esiri et al., 1999). Finally, the differential relationship between stroke risk and posterior microstructural changes in MCI and normal aging may be related to the concept of cognitive reserve (Stern, 2006). Specifically, the existence of subclinical vascular disease could influence the presentation of MCI by reducing the threshold for cognitive dysfunction in AD (Esiri et al., 1999). These early neuropathological changes may impair the brain's ability to compensate for considerable stroke risk, increase the burden of pathology, and enhance the relationship between stroke risk and reduced white matter anisotropy.
Very few studies have used DTI to examine the relationship between cognition and white matter integrity across the aging spectrum. Although trends were observed between genu FA and cognition (i.e, executive functioning and confrontation naming) in our sample, we did not fully replicate O'Sullivan et al. (2001) results which showed that executive functioning (Wisconsin Card Sorting Test) was related to DTI indices in anterior white matter. Discrepant findings across studies may be attributable to differences in sample size, DTI parameters, and/or specific ROI methodology. Additionally, it may have been difficult to demonstrate associations with executive functioning given that this particular cognitive domain is complex and multi-modal, and thus heavily dependent on several cognitive abilities (e.g., processing speed, working memory, attention). Other studies have shown that DTI indices of white matter integrity are associated with global cognitive functioning in patients with dementia (Rose et al., 2000; Bozzali et al., 2002); however, to our knowledge, the present study is the first to show a positive relationship between splenium FA and verbal memory ability in a nondemented sample of older adults.
Interestingly, although, the MCI and normal control groups differed significantly on both recall and recognition memory abilities, results indicated a dissociation between recall and recognition memory and our DTI measures of white matter integrity. Specifically, although no relationship was found between our memory variables and genu FA, recall memory—but not recognition memory—was negatively associated with splenium FA. It is thought that active recall is more effort-demanding than recognition, and thus perhaps our finding implies that underlying brain mechanisms involved in recall may be more vulnerable than those underlying recognition in the prodromal phase of AD. This finding is supported by a recent study which demonstrated that 13 of 14 verbal recall measures significantly differed across normal aging, MCI, and AD groups, and that learning and recall—but not recognition—best discriminated patients with MCI from AD and normal aging (Greenaway et al., 2006). The authors suggest that their findings support the notion that these memory indices may be the most sensitive predictors of later progression to AD (Bondi et al., 1999; Albert et al., 2001). Also, Tsivilis et al. (2008) recently showed that mammillary body volume significantly correlated with tests of episodic memory recall but poorly correlated with recognition memory, supporting models that posit that limbic-diencephalic memory mechanisms require hippocampal inputs for recall but not for recognition. Thus, it has been suggested that the neuroanatomical substrates for recognition memory rely on more diffuse, distributed networks and are thus dependent on multiple brain pathways than the substrates underlying recall memory. Within this framework, it is thus conceivable that circuits supporting recognition memory are not affected until later in the AD prodrome.
To our knowledge, the present study is the first to show that changes in white matter microstructural integrity in sub-regions of the corpus callosum are differentially associated with increased stroke risk and decreased neuropsychological test performance in older adults with MCI. Results indicate that white matter changes are evident in at-risk older adults and further validate the use of DTI to capture subtle, early white matter changes before significant atrophy is present. In addition, our results show that DTI indices of white matter integrity are associated with various aspects of neuropsychological function. Given that studies relating cognition and traditional MR-derived measures of white matter integrity (e.g., visual ratings of white matter lesions) have not been consistent, DTI indices of abnormal white matter may ultimately prove to be better predictors of cognitive functioning across the aging spectrum.
Despite these novel findings, it is important to highlight that we sampled a relatively small number of MCI participants and results presented here need to be replicated in larger samples, particularly since data gleaned from MCI subtypes is critically important in order to elucidate the possible differing etiologies of each subtype. In addition, the current study is cross-sectional in its design and therefore limited in assessing the role of stroke risk and posterior white matter degeneration in the subsequent development of dementia (e.g., vascular dementia, AD). Longitudinal follow-up of our samples will be essential to determine whether the cross-sectional findings found in this study predict progression to dementia. Finally, despite being one of the most commonly employed rating scales of vascular risk, the FSRP may not have fully captured the possible underlying cerebrovascular disease burden. Direct measurement of cerebrovascular integrity or reactivity via MR imaging techniques such as perfusion or angiography studies is needed.
In conclusion, the present results suggest that regional callosal white matter changes, independent of age and brain volume, are associated with specific deficits in neuropsychological performance in participants with MCI. In addition, stroke risk appears to be inversely related to white matter integrity, and results indicate that individuals with MCI may be more vulnerable to the influence of stroke risk than healthy aging individuals. The observation that stroke risk may differentially modify posterior white matter integrity in MCI highlights the possible complex relationship between AD- and vascular-related pathology in the development of dementia. Finally, results suggest that selective posterior white matter degeneration may play a particular role in MCI-related cognitive changes.
Acknowledgements
This work was supported by grants from the National Institutes of Health (K24 AG026431, R01 AG012674, R01 MH64729, R01 MH75870, and P50 AG05131), by a Career Development Award and Merit Review Research Program from the Department of Veterans Affairs, and by Investigator-Initiated and New Investigator Research Grants from the Alzheimer's Association. The authors gratefully acknowledge the assistance of staff, patients, and volunteers of the UCSD Alzheimer's Disease Research Center, and the UCSD Laboratory of Cognitive Imaging. Disclosures: Dr. Dean Delis, a co-author on this manuscript, has a financial interest in some of the neuropsychological measures administered as part of this study. He is the lead author of the Delis-Kaplan Executive Function System (D-KEFS), published by The Psychological Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitz F, Rodriguez E, Olivares R, Zaidel E. Age-related changes in fibre composition of the human corpus callosum: sex differences. Neuroreport. 1996;7:1761–1764. doi: 10.1097/00001756-199607290-00013. [DOI] [PubMed] [Google Scholar]
- Albert M, Moss M, Tanzi R, et al. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1(1):103–16. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, O'Brien JT. MRI volumetric correlates of white matter lesions in dementia with Lewy bodies and Alzheimer's disease. Int J Geriatr Psychiatry. 2000;15(10):911–916. doi: 10.1002/1099-1166(200010)15:10<911::aid-gps217>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstuctural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Resonon B. 1996;111:209–216. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Tate DF. Brain volume, intracranial volume, and dementia. Invest Radiol. 2001;36(9):539–546. doi: 10.1097/00004424-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and Apolipoprotein E genotype in the preclinical detection of Alzheimer's disease. Psychology and Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Borroni B, Archetti S, Ferrari M, Cesana BM, Padovani A, Regan CE, Katona C, Walker Z, Livingston G. Relationship of vascular risk to the progression of Alzheimer's disease. Neurology. 2007;68(13):1083–1084. doi: 10.1212/01.wnl.0000260435.20698.77. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, Comi G, Filippi M. White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2002;72:742–746. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16(3):271–8. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: Long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- Callen DJ, Black SE, Gao F, Caldwell CB, Szalai JP. Beyond the hippocampus: MRI volumetry confirms widespread limbic atrophy in AD. Neurology. 2001;57(9):1669–74. doi: 10.1212/wnl.57.9.1669. [DOI] [PubMed] [Google Scholar]
- Cho H, Yang DW, Shon YM, Kim BS, Kim\ YI, Choi YB, Lee KS, Shim YS, Yoon B, Kim W, Ahn KJ. Abnormal integrity of corticocortical tracts in Mild Cognitive Impairment: A diffusion tensor imaging study. J Korean Med Sci. 2008;23:477–483. doi: 10.3346/jkms.2008.23.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, Mungas D, Reed BR, Kramer JH, DeCarli CC, Weiner MW, Vinters HV. Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol. 2006;60(6):677–687. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R,W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25(1):40–3. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- Delano-Wood L, Abeles N, Sacco J, Wierenga CE, Horne NR, Bozoki A. Regional white matter pathology in Mild Cognitive Impairment: Differential influence of lesion type on neuropsychological functioning. Stroke. 2008;39:794–800. doi: 10.1161/STROKEAHA.107.502534. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System (D-KEFS) The Psychological Corporation; San Antonio, Texas: 2001a. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System-technical manual. The Psychological Corporation; New York: 2001b. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. Psychological Corporation; New York: 1987. [Google Scholar]
- deToledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer's disease: In vivo detection of entorhinal cortex atrophy. Ann NY Acad Sci. 2000;11:240–753. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, Leurgans S, Wuu J, Turner DA. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004;25(9):1197–203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, deToledo-Morrell L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol Aging. 2001;22(5):747–54. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Du Z, Ma S, Lin RC, Dodel F, Gao KR, Bales LC, Triarhou E, Chernet KW, Perry DL, Nelson S, Luecke LA, Phebus FP, Bymaster S, Paul M. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- Fellgiebel S, Wille P, Muller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P. Ultrastructural hippocampal and white matter alterations in Mild Cognitive Impairment: A diffusion tensor imaging study. Demen Geriatr Cog Dis. 2004;18:101–108. doi: 10.1159/000077817. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Dellani PR, Greverus D, Scheurich A, Stoeter P, Muller MJ. Predicting conversion to dementia in mild cognitive impairment by volumetric and diffusivity measurements of the hippocampus. Psychiatry Research Neuroimaging. 2006;146:283–287. doi: 10.1016/j.pscychresns.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Ozyurt IB, Brown GG, Clark CP, Morris S, Bischoff-Grethe A, Bondi MW, Jernigan TL, Fischl B, Segonne F, Shattuck DW, Leahy RM, Rex DE, Toga AW, Smith SM. The Human Brain Morphometry BIRN. Quantitative evaluation of automated skull-stripping methods applied to contemporary and legacy images: Effects of diagnosis, bias correction, and slice location. Hum Brain Map. 2006;27:99–113. doi: 10.1002/hbm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. Journal Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frank LR. Anisotropy in high angular resolution diffusion-weighted MRI. Magnetic Resonance Med. 2001;45(6):935–939. doi: 10.1002/mrm.1125. [DOI] [PubMed] [Google Scholar]
- Greenaway MC, Lacritz LH, Binegar D, Weiner MF, Lipton A, Cullum MC. Patterns of verbal memory performance in mild cognitive impairment, Alzheimer's disease, and normal aging. Cognitive and Behavioral Neurology. 2006;19(2):79–84. doi: 10.1097/01.wnn.0000208290.57370.a3. [DOI] [PubMed] [Google Scholar]
- Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Battiglieri T. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66(1):6–7. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Gupta RK, Santos RM, Wolinsky JS, Narayana PA. Diffusion tensor fractional anisotropy of the normal-appearing seven segments of the corpus callosum in healthy adults and relapsing-remitting multiple sclerosis patients. J Magn Reson Imaging. 2004;21:735–743. doi: 10.1002/jmri.20296. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo E, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer's type: Evidence form diffusion tensor imaging. Cerebral Cortex. 2004;14(4):410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer's disease. Cerebral Cortex. 2005;15:732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited—Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32(3):989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, Jiang S, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PCM, Hillis AE, Wytik R, Mori S. DTI tractography based parcellation of white matter: application of the mid-sagittal morphology of corpus callosum. NeuroImage. 2005;26:295–305. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Corey-Bloom J, Nagel BJ, Bondi MW. Differential cross-sectional and longitudianal impact of APOE genotype on hippocampal volume in nondemented older adults. Dem Geriat Cog Dis. 2007;23:282–289. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging. 2000;21(2):321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. Small vessel disease and Alzheimer's dementia: pathological considerations. Cerebrovasc Dis. 2002;13(Suppl.2):48–52. doi: 10.1159/000049150. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CR, Xu YC, Campeau NG, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Mild cognitive impairment and Alzheimer disease: regional diffusivity of water. Radiology. 2001;219:101–107. doi: 10.1148/radiology.219.1.r01ap14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb PA. Independent Living Scales Manual. The Psychological Corporation; San Antonio, Texas: 1996. [Google Scholar]
- Mariani E, Monastero R, Ercolani S, Mangialasche F, Caputo M, Feliziani FT, Vitale DF, Senin U, Mecocci P, ReGAI Study Group Vascular risk factors in mild cognitive impairment subtypes. Findings from the ReGAI project. Dement Geriatr Cogn Disord. 2007;24(6):448–456. doi: 10.1159/000110653. [DOI] [PubMed] [Google Scholar]
- Medina D, deToledo-Morrell L, Urresta F, Gabrieli JDE, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobio Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Minati L, Grisoli M, Bruzzone MG. MR spectroscopy, functional MRI, and diffusion-tensor imaging int eh aging brain: A conceptual review. J Geriatr Psychaitry Neurol. 2007;20(1):3–21. doi: 10.1177/0891988706297089. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Palmer SL, Reddick WE, Glass JO, Helton KJ, Wu S, Xiong X, Kun LE, Gajjar A, Mulhern RK. Abnormal hippocampal development in children with medulloblastoma treated with resk-adapted irradiation. Am J Neuroradiol. 2004;25:1575–1582. [PMC free article] [PubMed] [Google Scholar]
- Naggara O, Oppenheim C, Rieu D, Raoux N, Rodrigo S, Dalla Barba G, Meder JF. Diffusion tensor imaging in early Alzheimer's disease. Psychiatry Res. 2006;146(3):243–9. doi: 10.1016/j.pscychresns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. NeuroImage. 2006;31:1445–1452. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. Evidence for cortical `disconnection' as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Palmer K, Bäckman L, Winblad B, Fratiglioni L. Early symptoms and signs of cognitive deficits might not always be detectable in persons who develop Alzheimer's disease. Int Psychogeriatr. 2008;20(2):252–258. doi: 10.1017/S1041610207006564. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1647. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity (key symposium) J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magn Reson Med. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition. vol. 2. Erlbaum; New Jersey: 2000. pp. 1–90. [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Tang MX, Manly J, Mayeuz R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neruol. 2007;64(12):1734–1740. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SE, Chen F, Chalk JB, Zelaya FO, Strugnell WE, Benson M, Semple J, Doddrell DM. Loss of connectivity in Alzheimer's disease: an evaluation of white matter tract integrity with colour coded MR diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2000;69(4):528–530. doi: 10.1136/jnnp.69.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Butters N. Neuropsychological assessment of dementia in the elderly. In: Katzman R, Rowe JW, editors. Principles of geriatric neurology. F.A. Davis Company, London distributors: Williams & Wilkins, Ltd; Philadelphia, Pennsylvania: 1992. pp. 144–163. [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. NeuroImage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith GE, Ivnik RJ. Normative neuropsychology. In: Petersen RC, editor. Mild Cognitive Impairment: Aging to Alzheimer's Disease. Oxford University Press Inc.; New York: 2003. pp. 63–88. [Google Scholar]
- Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg ST. White matter damage in Alzheimer disease and Mild Cognitive Impairment: Assessment with diffusion-tensor MR imaging nad parallel imaging techniques. Radiology. 2007;243(2):483–492. doi: 10.1148/radiol.2432051714. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer's disease. Alz Dis Assoc Dis. 2006;20(3 Suppl 2):S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30(6):749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yonezawa H, Takahashi J, Kudo M, Inoue T, Tohgi H. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neurosci Lett. 2002;332:45–48. doi: 10.1016/s0304-3940(02)00914-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Truelsen T, Lindenstrom E, Boyesen G. Comparison of probability of stroke between the Copenhagen city heart study and the Framingham study. Stroke. 1994;25:802–807. doi: 10.1161/01.str.25.4.802. [DOI] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11(7):834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Ukmar M, Makuc E, Onor ML, Garbin G, Trevisiol M, Cova MA. Evaluation of white matter damage in patients with Alzhiemer's disease and in patients with mild cogniteive impairment by udsing diffusion tensor imaing. Neuroradiology. 2007;14:201–208. doi: 10.1007/s11547-008-0286-1. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children – Revised. Psychological Corporation; New York: 1974. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Revised Manual. The Psychological Corporation; San Antonio: 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale – Revised. Psychological Corporation; New York: 1987. [Google Scholar]
- Welsh K, Butters N, Mohs RC. CERAD Part V: A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Lim KO. Advances in white matter imaging: A review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neuroscience and Biobehavioral Reviews. 2006;30:762–774. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Lai CL, Lin RT, Tai CT, Liu CK. Cut-off values of blessed dementia rating scale and its clinical application in elderly Taiwanese. Kaohsiung J Med Sci. 2006;22(8):377–84. doi: 10.1016/S1607-551X(09)70326-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov randomfield model and the expectation-maximization algorithm. IEEE Transactions on Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Hahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe MD, Chui H, Jagust WJ, Miller BL, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Smith MA, Honda K, Aliev G, Moreira PI, Nunomura A, Casadesus G, Harris PL, Siedlak SL, Perry G. Vascular oxidative stress in Alzheimer's disease. J Neurol Sci. 2007;257(1–2):240–246. doi: 10.1016/j.jns.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]