Abstract
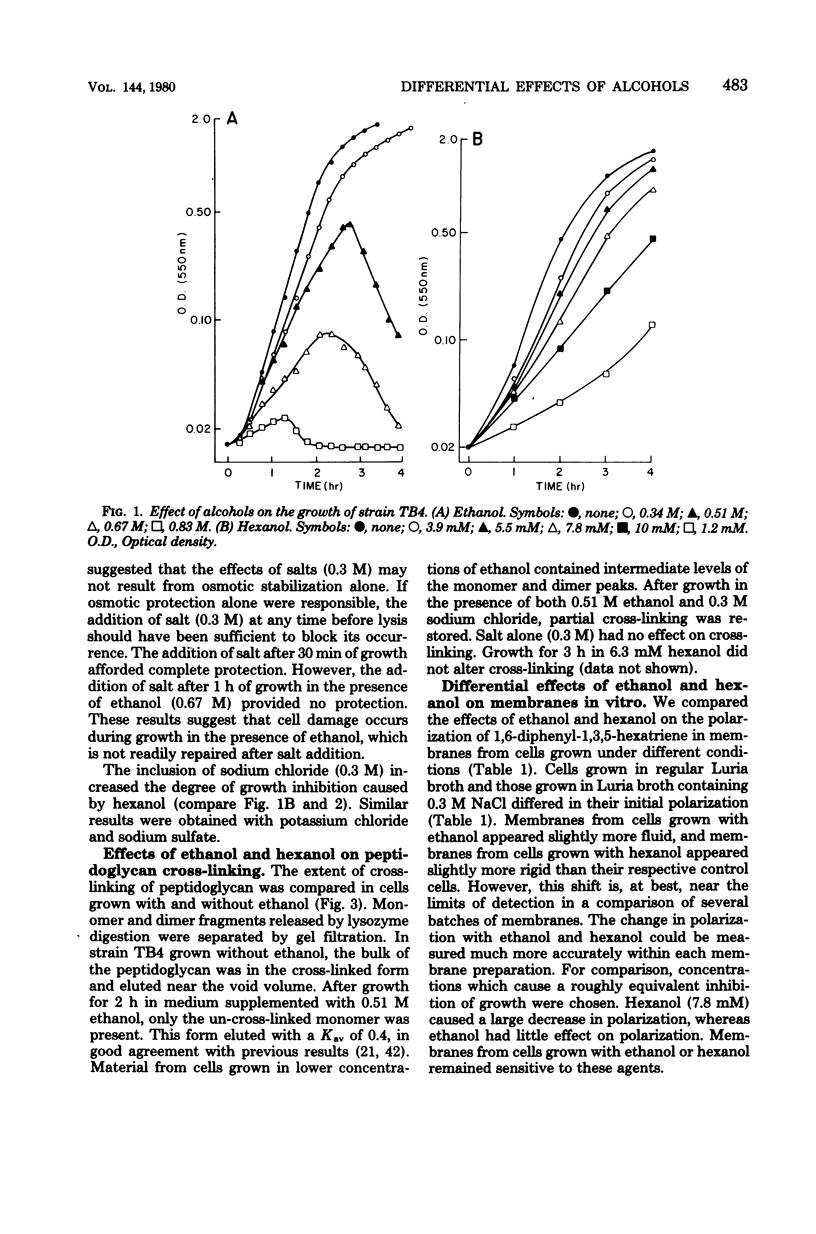
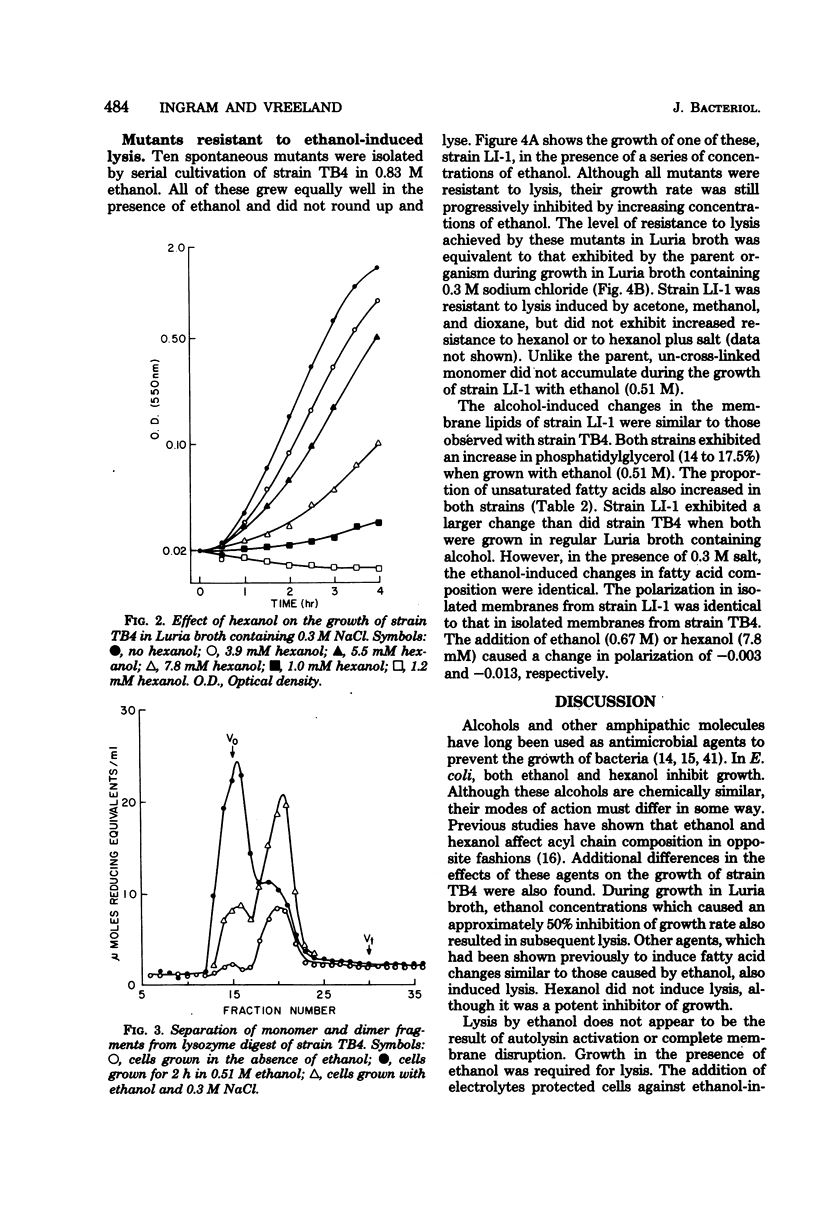
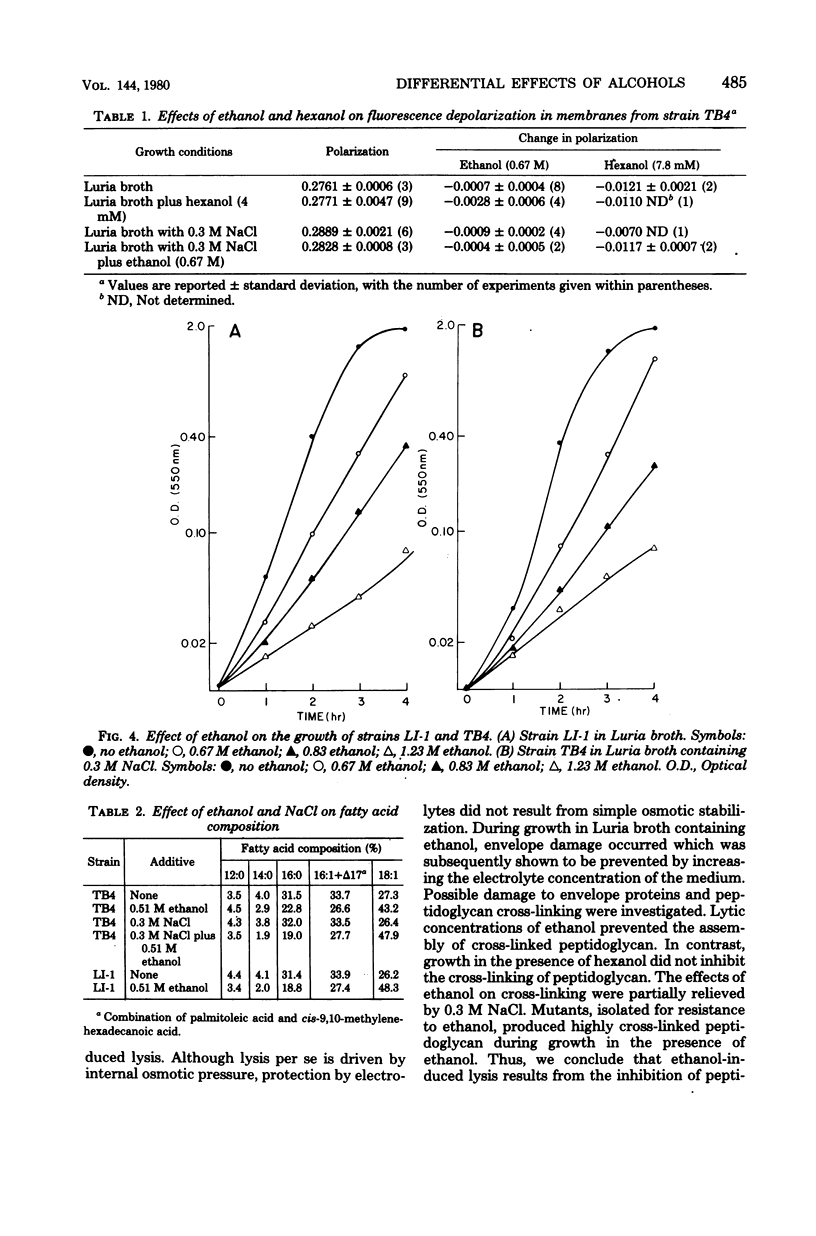
Both ethanol and hexanol inhibited the growth of Escherichia coli, but their effects on the organization and composition of the cell envelope were quite different. Hexanol (7.8 x 10(-3) mM) increased membrane fluidity, whereas ethanol (0.67 M) had little effect. During growth in the presence of ethanol, the proportion of unsaturated fatty acids increased. The opposite change was induced by hexanol. Unlike hexanol, growth in the presence of ethanol resulted in the production of un-cross-linked peptidoglycan with subsequent lysis. Salt (0.3 M) protected cells against ethanol-induced lysis but potentiated growth inhibition by hexanol. Mutants isolated for resistance to ethanol-induced lysis synthesized cross-linked peptidoglycan during growth in the presence of ethanol but remained sensitive to hexanol. A general hypothesis was presented to explain the differential effects of ethanol and hexanol. All alcohols are viewed as similar in having both an apolar chain capable of interacting with hydrophobic environments and a hydroxyl function capable of hydrogen bonding. The differential effects of short-chain alcohols may represent effects due to the high molar concentrations of hydrogen bonding groups with an apolar end within the environment. These may replace bound water in some cases. With longer-chain alcohols such as hexanol, the effects of the acyl chain would dominate, and limitations of solubility and cellular integrity would mask these hydroxyl effects.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger B., Carty C. E., Ingram L. O. Alcohol-induced changes in the phospholipid molecular species of Escherichia coli. J Bacteriol. 1980 Jun;142(3):1040–1044. doi: 10.1128/jb.142.3.1040-1044.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttke T. M., Ingram L. O. Mechanism of ethanol-induced changes in lipid composition of Escherichia coli: inhibition of saturated fatty acid synthesis in vivo. Biochemistry. 1978 Feb 21;17(4):637–644. doi: 10.1021/bi00597a012. [DOI] [PubMed] [Google Scholar]
- CHEN R. F., BOWMAN R. L. FLUORESCENCE POLARIZATION: MEASUREMENT WITH ULTRAVIOLET-POLARIZING FILTERS IN A SPECTROPHOTOFLUOROMETER. Science. 1965 Feb 12;147(3659):729–732. doi: 10.1126/science.147.3659.729. [DOI] [PubMed] [Google Scholar]
- Chesbro W., Evans T., Eifert R. Very slow growth of Escherichia coli. J Bacteriol. 1979 Aug;139(2):625–638. doi: 10.1128/jb.139.2.625-638.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. H., Goldstein D. B. Effects of low concentrations of ethanol on the fluidity of spin-labeled erythrocyte and brain membranes. Mol Pharmacol. 1977 May;13(3):435–441. [PubMed] [Google Scholar]
- Clark D. P., Beard J. P. Altered phospholipid composition in mutants of Escherichia coli sensitive or resistant to organic solvents. J Gen Microbiol. 1979 Aug;113(2):267–274. doi: 10.1099/00221287-113-2-267. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., DAWES E. A., MORRISON G. A. Inhibition of growth of Aerobacter aerogenes; the mode of action of phenols, alcohols, acetone, and ethyl acetate. J Bacteriol. 1950 Oct;60(4):369–379. doi: 10.1128/jb.60.4.369-379.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Inouye M. Lipid fluidity-dependent biosynthesis and assembly of the outer membrane proteins of E. coli. Cell. 1979 May;17(1):155–161. doi: 10.1016/0092-8674(79)90303-9. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Gilmore J. R., Glaser M. Use of a fluorescent probe to determine the viscosity of LM cell membranes with altered phospholipid compositions. Biochemistry. 1977 May 3;16(9):1881–1890. doi: 10.1021/bi00628a019. [DOI] [PubMed] [Google Scholar]
- Fried V. A., Novick A. Organic solvents as probes for the structure and function of the bacterial membrane: effects of ethanol on the wild type and an ethanol-resistant mutant of Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):239–248. doi: 10.1128/jb.114.1.239-248.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo W. B. The mode of action of antibacterial agents. J Appl Bacteriol. 1967 Apr;30(1):17–50. doi: 10.1111/j.1365-2672.1967.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Ingram L. O. Adaptation of membrane lipids to alcohols. J Bacteriol. 1976 Feb;125(2):670–678. doi: 10.1128/jb.125.2.670-678.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Changes in lipid composition of Escherichia coli resulting from growth with organic solvents and with food additives. Appl Environ Microbiol. 1977 May;33(5):1233–1236. doi: 10.1128/aem.33.5.1233-1236.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Dickens B. F., Buttke T. M. Reversible effects of ethanol on E. coli. Adv Exp Med Biol. 1980;126:299–337. doi: 10.1007/978-1-4684-3632-7_24. [DOI] [PubMed] [Google Scholar]
- Ingram L. O. Preferential inhibition of phosphatidyl ethanolamine synthesis in E. coli by alcohols. Can J Microbiol. 1977 Jun;23(6):779–789. doi: 10.1139/m77-115. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Thurston E. L., Van Baalen C. Effects of selected inhibitors on growth and cell division in Agmenellum. Arch Mikrobiol. 1972;81(1):1–12. doi: 10.1007/BF00715019. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Van Baalen C., Fisher W. D. Cell division mutations in the blue-green bacterium Agmenellum quadruplicatum strain BG1: a comparison of the cell wall. J Bacteriol. 1972 Aug;111(2):614–621. doi: 10.1128/jb.111.2.614-621.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M. K., Gleeson J., Upreti A., Upreti G. C. Intrinsic perturbing ability of alkanols in lipid bilayers. Biochim Biophys Acta. 1978 May 4;509(1):1–8. doi: 10.1016/0005-2736(78)90002-0. [DOI] [PubMed] [Google Scholar]
- Kozarich J. W., Strominger J. L. A membrane enzyme from Staphylococcus aureus which catalyzes transpeptidase, carboxypeptidase, and penicillinase activities. J Biol Chem. 1978 Feb 25;253(4):1272–1278. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee A. G. Interactions between anesthetics and lipid mixtures. Normal alcohols. Biochemistry. 1976 Jun 1;15(11):2448–2454. doi: 10.1021/bi00656a031. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald A. G. A dilatometric investigation of the effects of general anaesthetics, alcohols and hydrostatic pressure on the phase transition in smectic mesophases of dipalmitoyl phosphatidylcholine. Biochim Biophys Acta. 1978 Feb 2;507(1):26–37. doi: 10.1016/0005-2736(78)90371-1. [DOI] [PubMed] [Google Scholar]
- Nunn W. D. The inhibition of phospholipid synthesis in escherichia coli by phenethyl alcohol. Biochim Biophys Acta. 1975 Mar 24;380(3):403–413. doi: 10.1016/0005-2760(75)90108-3. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Paterson S. J., Butler K. W., Huang P., Labelle J., Smith I. C., Schneider H. The effects of alcohols on lipid bilayers: a spin label study. Biochim Biophys Acta. 1972 Jun 20;266(3):597–602. doi: 10.1016/0006-3002(72)90003-0. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Silbert D. F. Arrangement of fatty acyl groups in phosphatidylethanolamine from a fatty acid auxotroph of Escherichia coli. Biochemistry. 1970 Sep 1;9(18):3631–3640. doi: 10.1021/bi00820a021. [DOI] [PubMed] [Google Scholar]
- Stárka J., Moravová J. Cellular division of penicillin-induced filaments of Escherichia coli. Folia Microbiol (Praha) 1967;12(3):240–247. doi: 10.1007/BF02868738. [DOI] [PubMed] [Google Scholar]
- Sullivan K. H., Hegeman G. D., Cordes E. H. Alteration of the fatty acid composition of Escherichia coli by growth in the presence of normal alcohols. J Bacteriol. 1979 Apr;138(1):133–138. doi: 10.1128/jb.138.1.133-138.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. H., Jain M. K., Koch A. L. Activation of the beta-galactoside transport system in Escherichia coli ML-308 by n-alkanols. Modification of lipid-protein interaction by a change in bilayer fluidity. Biochim Biophys Acta. 1974 Jun 13;352(2):287–297. doi: 10.1016/0005-2736(74)90220-x. [DOI] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Tilley F. W., Schaffer J. M. RELATION BETWEEN THE CHEMICAL CONSTITUTION AND GERMICIDAL ACTIVITY OF THE MONOHYDRIC ALCOHOLS AND PHENOLS. J Bacteriol. 1926 Nov;12(5):303–309. doi: 10.1128/jb.12.5.303-309.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XII. Inhibition of cross-linking by penicillins and cephalosporins: studies in Staphylococcus aureus in vivo. J Biol Chem. 1968 Jun 10;243(11):3169–3179. [PubMed] [Google Scholar]



